Catalytic conversion of ethyl lactate to 1,2-propanediol over CuO☆
Song Zhang,Zhibao Huo,Dezhang Ren,Jiang Luo,Jun Fu,Lu Li,Fangming Jin
School of Environmental Science and Engineering,The State Key Laboratory of Metal Matrix Composites,Shanghai Jiao Tong University,Shanghai 200240,China
1.Introduction
Over the past several decades,consumption of fossil fuels and their limited supply have necessitated areduction in our dependenceon petroleum oil.Biomass as a source for producing high value-added chemicals has attracted much attention due to its excellent properties,such as abundant,renewable,and less pollution.A number of efficient methods in the conversion of biomass to chemicals including various alcohols[1],hydroxymethylfurfural[2]and dimethylfuran[3,4]had been reported.
1,2-propanediol(1,2-PDO),a bulk commodity chemical that is mainly used for the production of unsaturated polyester resins[5]and as an antifreeze and de-icing agent and in pharmaceuticals,cosmetics,and foods[6],is industrially produced by the hydration of propylene oxide derived from petrochemical resource[7].With reducing fossil fuel reserves,the development of renewable and green synthetic methods for the 1,2-PDO production is necessary[8,9].Recently,the hydrogenation of biomass-derived ethyl lactate to 1,2-PDO using gaseous hydrogen over transition metal catalysts has attracted much interest,which provides an environmental benign alternative to the petroleum-based process for the 1,2-PDO production[10-14].Ethyl lactate can be easily obtained on alarge amount(120000 t·a-1)by the fermentation of renewable sources such as agricultural crops and biomass streams.Several groups had disclosed that Ru-based catalysts were effective in significantly increasing the activity and selectivity for the conversion of ethyl lactate to 1,2-PDO due to their excellent intrinsic activities.The examples include:Ru B/TiO2[11],RuB/-Al2O3and RuB/SBA-15[12],and Ru/SiO2[14].Li et al.reported vapor-phase hydrogenolysis of ethyl lactate to 1,2-PDO over Co/SiO2and Cu/SiO2,and the 1,2-PDO selectivity was in excess of 98%at 90.2%ethyl lactate conversion[13].How ever,these methods suffer from several drawbacks,such as the use of high-pressure gaseous hydrogen which is not easy to obtain at a reduced energy cost;organic solvents;the use of prepared catalysts,such as Ru-based catalysts supported on some additives(boron,tin or iron,etc.)increased the cost of the preparation and separation process;formation of by-products and longer reaction times.Thus,the development of a cleanly and economically synthetic approach of 1,2-PDO from ethyl lactate is of great importance.
One of the major challenges in the conversion of ethyl lactate to 1,2-PDO is the development of economical hydrogen sources.The previous works showed that the hydrothermal process had a promising potential for environmental benign conversion of biomass into useful chemicals[15-18].Water is not only the most abundant hydrogen resource in the process,but also agreener solvent.Recently,were ported an efficient process for the formation of ethyleneglycol from glycolideover CuOin water,and a high yield of 94%was achieved[19].It occurs to us that the conversion of ethyl lactate to 1,2-PDOmay be implemented in a similar manner.Herein,w e present a highly efficient conversion of ethyl lactate to 1,2-PDO in the presence of CuO in high temperature water(Fig.1).
2.Experimental
2.1.Experimental materials
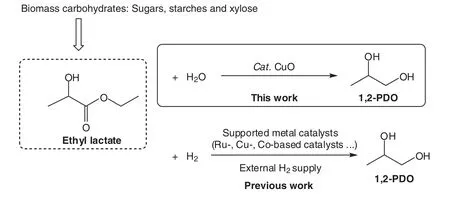
Fig.1.Previous and current process for the formation of 1,2-PDO.
Ethyl lactate(>97%)as the initial reactant was purchased from TCI.1,2-PDO(≥99.8%,chromatographic grade,J&K Scientific Ltd.)was used for quantitative analysis.Zn[200 mesh(0.075 mm),Aladdin]was used as the reductant.Ni,Fe,Al,Mn,Mg,Cu O,Al2O3,Cu O,Cu2O,Fe2O3and Fe3O4[200 mesh(0.075 mm),Sinopharm Chemical Reagent Co.,Ltd.]were also used in the experiment.
2.2.Experimental procedure
All experiments were carried out in a Te flon-lined stainless steel batch reactor with an internal volume of 30 ml.0.47 mmol ethyl lactate was used in all experiments.The typical procedure for the synthesis of 1,2-PDO was as follow s.First,desired ethyl lactate,reductant,catalyst and ultrapure water were loaded into the reactor.Then,nitrogen was charged into the reactor in order to exclude the effect of air and then the sealed reactor was put into a drying oven,it will take about 20 min to be preheated to the desired temperature.After a desired reaction time,the reactor was quickly moved from the drying oven to cool down.Liquid sample was collected and filtered with a 0.45 μm Syringe Filter.Solid sample was collected and washed with deionized water and ethanol several times to remove impurities and dried in the oven at 50°C for 24 h.The schematic of the reactor system was show n in Fig.2.
2.3.Product analysis
After the reaction was quenched,solution samples were collected and filtered for GC-MSanalysis(Agilent GC7890A-MS5975C)equipped with an HP INNOWax polyethylene glycol capillary column with dimensions of 30 m × 250 μm × 0.25 μm,and HPLC analysis(Agilent 1260 serials,Vis detector).Details on the conditions of HPLC and GCMS analyses are available elsew here[20].The total residual organic carbon concentration in liquid samples was also measured with a TOC analyzer(Shimadzu TOC-V).The gas product was detected by a thermal conductivity detector(TCD,Agilent Technologies).Thin layer chromatography(TLC)was performed on aluminum-precoated plates of silica gel 60 with an HSGF254 indicator and visualized under UV light or developed by immersion in the solution of 0.6%KMn O4and 6%K2CO3in water.Solid samples were collected and analyzed by an X-ray diffractometer(Shimadzu XRD-6100)to determine the composition and phase purity.
The yield of 1,2-PDO is calculated as the follow ing equation:

3.Results and Discussion
3.1.Catalyst screening
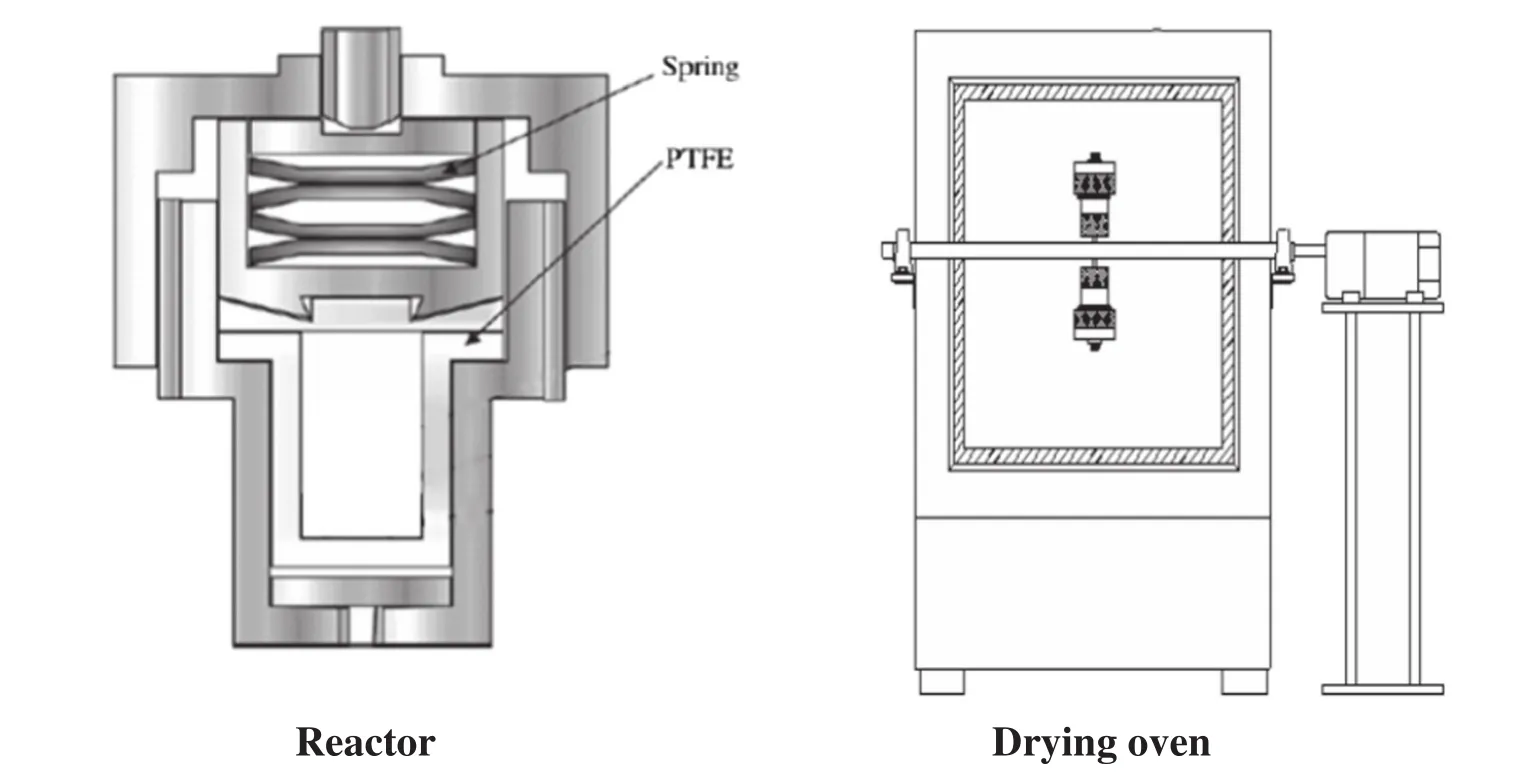
Fig.2.Schematic of batch reactor system.

Fig.3.GC-MS spectrum of the liquid sample.
First of all,aseriesof experimentswerecarried out to investigate the feasibility of ethyl lactate to 1,2-PDO in the presence of 5 mmol Co and 26 mmol Zn with 17%water filling at 250°C for 150 min.The GC-MS image of the liquid product after the reaction clearly show ed that the desired 1,2-PDO was observed(Fig.3).The yield of 37.6%was achieved in the presence of Co(Table 1,entry 1).The solid samples after the reaction were analyzed by XRD[Fig.4(a)],which found that Co still existed in the solid sample,while Zn was oxidized to Zn O,indicating that Zn acted as a reduct ant and Co acted as a catalyst for the conversion of ethyl lactate to 1,2-PDO.Next,w e investigated the performance of other various catalysts,such as CuO,Cu,Fe,MnO2,Zr O2,and Co2O3,and the results were shown in Table 1.The results indicated that CuO,Co and Cu2O exhibited excellent performance for the conversion of ethyl lactate(entries 1,11 and 12),and CuO gave much better results than Co and Cu2O.The yield of 86%was obtained with no surplus initial starting materials observed by thin layer chromatography(TLC)(entry 12).Other catalysts,such as Fe,Cu,Cr,Al2O3,Fe2O3,Ni2O3,Fe3O4,Mn O2,Co2O3,TiO2,ZrO2and Cu Fe2O4were ineffective,and no desired products were observed(entries2-10,13-15).The blank test show ed that no desired 1,2-PDO was obtained,indicating the importance of the combined use of both Zn and CuO in the process(entries 16-18).The XRD of solid samples was show ed in Fig.4(b)when CuO was used as a catalyst.It is noteworthy that Cu rather than CuO was observed in the solid sample,the most likely reason is that CuO was reduced completely to Cu by in situ formed hydrogen by the oxidation of Zn in water[21].In order to con firm this assumption,w e carried out the experiment under the same reaction conditions without the addition of ethyl lactate.Cu was found from XRD,indicating that CuO would be reduced completely to Cu by in situ formed hydrogen during the reaction.

Fig.4.XRD patterns of the solid samples.
Next,we investigated the effect of different amounts of CuO with the remaining other conditions unchanged.The results were show n in Fig.5.The yield of 1,2-PDO increased remarkably from 3 to 5 mmol,and then decreased as the amount of CuO was further increased.The decreasing yield may be because more CuO would lead to more cracking products such as propionic acid.It is obvious that 5 mmol Cu O is the most suitable amount.
3.2.The optimization of the various parameters
To investigate the effect of the reduct ant on the yield of 1,2-PDO,the reactions of ethyl lactate were carried out in the presence of Cu O at 250°C for 150 min.Table 2 show ed that the desired 1,2-PDO was yielded when Zn and Fe were used,while the other reductants,suchas Mg,Ni,Mn,and Al were ineffective and no desired product was obtained at all.Zn is the most efficient reductant as the yield of 1,2-PDO is86%.Thus,Zn was chosen as are ductant in the following experiments.Next,the amount of Zn was optimized in the presence of CuO at 250°C for 150 min.As show n in Fig.6,the results indicated that the most suitable amount is 26 mmol.But the yield of 1,2-PDO decreased significantly when the amount of Zn exceeded 26 mmol,which demonstrated that too much hydrogen pressure may have negative effects in the conversion of ethyl lactate to 1,2-PDO.

Table 1 Effect of catalysts on the yield of 1,2-PDO①
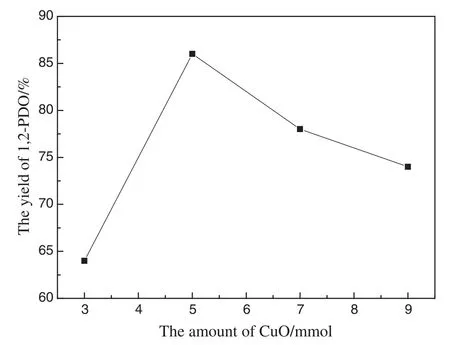
Fig.5.Effect of CuO amount(Zn:26 mmol;time:150 min;temp:250°C;water filling:17%).

Table 2 Effect of reductants on the yield of 1,2-PDO①

Fig.6.Effect of Zn amount(CuO:5 mmol;time:150 min;temp:250°C;water filling:17%).
The change of Zn oxidation to Zn O during the reaction at different times was monitored by XRD.As shown in Fig.4(b),peaks of Zn gradually reduced until it disappeared,whereas peaks of Zn O increased gradually as time increased.How ever,the peak of Cu was also observed,indicating that CuO was reduced gradually as Zn was oxidized to Zn O.These results indicate that ethyl lactate can be converted to 1,2-PDO over CuO by in situ formed hydrogen by the oxidation of Zn in water.
The effect of temperatures from 100 to 250°C on the yields of 1,2-PDO was investigated.As show n in Fig.7,the reaction did not take place at 100°C and no desired 1,2-PDO was observed.The yield of 1,2-PDOim proved gradually as there action temperature increased gradually from 100 °C to 150 °C,and then remarkably increased from 150 to 250°C.The results indicated that high temperature contributed to the conversion of ethyl lactate to 1,2-PDO.How ever,the temperature was limited to 250°Cdue to the limiting temperature of the Teflon material.

Fig.7.Effect of the temperature(Zn:26 mmol;CuO:5 mmol;time:150 min;water filling:17%).
Experiments were performed by changing the reaction time from 1 h to 3 h at 250°C to examine the effect of reaction time on the yield of 1,2-PDO.Fig.8 demonstrated that the yield of 1,2-PDO increased as reaction time increased from 1 h to 2.5 h,and maximum yield was reached w hen reaction time was 2.5 h.How ever,the yield of 1,2-PDO decreased after 2.5 h.This phenomenon may be attributed to the decomposition of produced 1,2-PDO during the reaction.In order to confirm this assumption,an experiment was designed as follow s,1,2-PDO acted as the initial reactant in the presence of 5 mmol Cu and 26 mmol ZnO with 17%water filling at 250°C for 2 h.The HPLC indicated that the initial 120 mmol·L-1concentration of 1,2-PDO was decreased to 100 mmol·L-1,and XRD analysis show ed that Cu and Zn O had not changed.It is obvious that a long reaction time has negative effect on the yield of 1,2-PDO obtained in the presence of Cu and Zn O during the reaction[22].
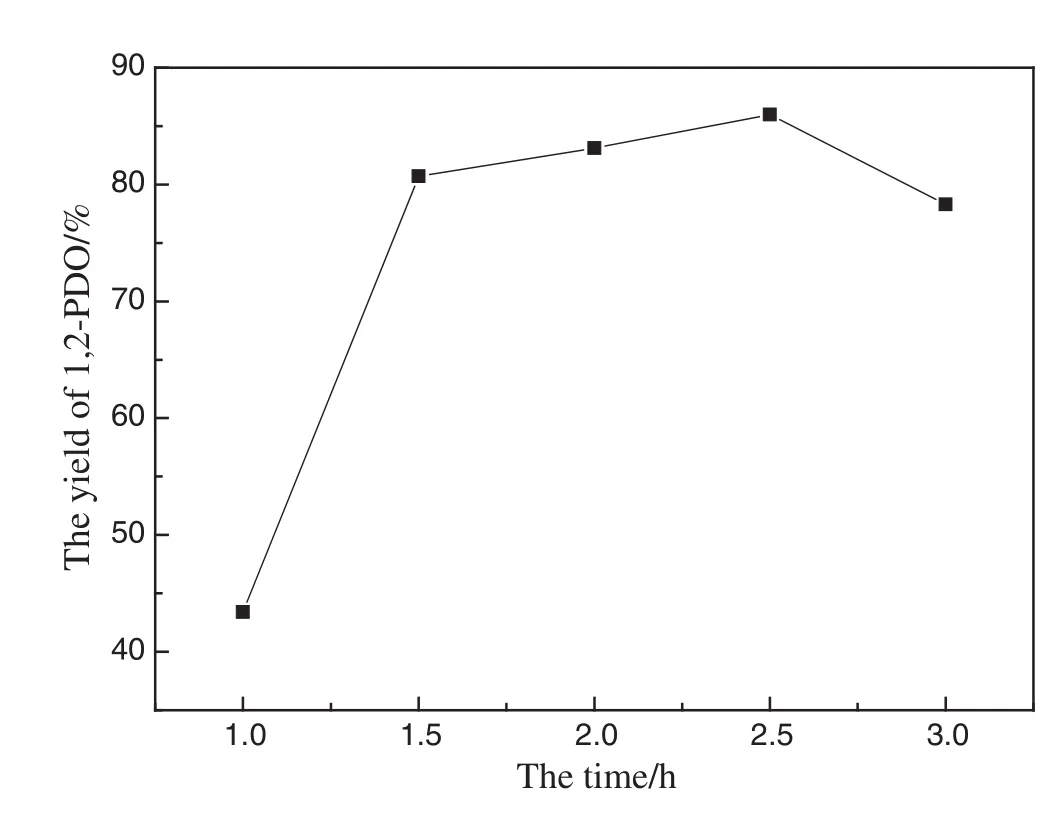
Fig.8.Effect of the time(Zn:26 mmol;CuO:5 mmol;temp:250°C;water filling:17%).
Further study was to optimize suitable water filling from 10%to 45%.Fig.9 indicates that the 1,2-PDO yield first increased significantly when the water filling was changed from 10%to 17%.The yield of 1,2-PDO increased slightly as the water filling increased from 17%to 42%and then decreased after 42%.The optimum water filling was 42%to obtain the highest yield of 93.6%.The decreasing yield at a higher water filling is most likely due to the decomposition of obtained 1,2-PDO with the increase of pressure when water filling increased.
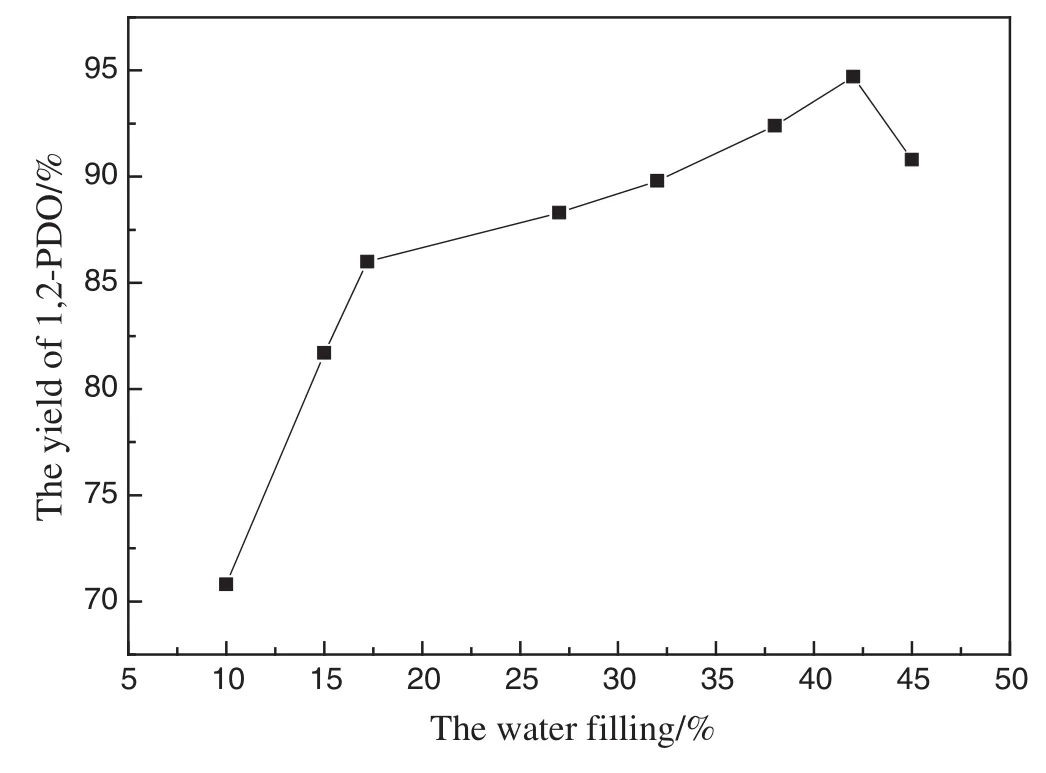
Fig.9.Effect of water filling(Zn:26 mmol;CuO:5 mmol;time:150 min;temp:250°C).
Finally,a carbon balance was conducted at the optimized condition by TOC and found that approximately 97.8%ethyl lactate was converted into liquid products.And only H2as the gaseous product was detected.
3.3.The formation of 1,2-PDO from ethyl lactate using gaseous hydrogen
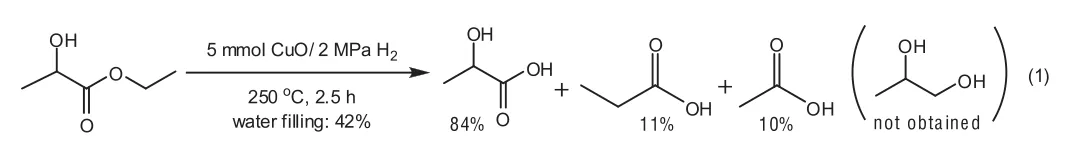
The catalytic hydrogenation of ethyl lactate using gaseous hydrogen instead of in situ hydrogen to 1,2-PDO was also investigated.The reaction was carried out in the presence of 5 mmol Cu O,2 MPa H2,42%water filling at 250°C for 2.5 h.Unfortunately,lactic acid rather than 1,2-PDO was obtained,indicating that gaseous hydrogen was ineffective for the 1,2-PDO production from ethyl lactate[Eq.(1)].
3.4.Mechanistic studies
Based on the previous and current results,the pathway for the conversion of ethyl lactate to 1,2-PDO over CuO was proposed in Fig.10.First,hydrogen was formed via the oxidation of Zn in water[23,24].The in situ-formed hydrogen was adsorbed on the surface of Cu/CuO/Zn O through the formation of hydrogen bonds.Then,ethyl lactate was also adsorbed on the surface of Cu/Cu O/Zn O by the combination of Cu/CuO/Zn O with carbonylic C and O atoms[25,26].When the hydrogen reacted with ethyl lactate molecules,an intermediate 2-hydroxypropanal was obtained.Furthermore,both the intermediate 2-hydroxypropanal and the hydrogen formed were adsorbed on the surface of Cu/Cu O/Zn O,H was added to C═Oand an intermediatelinked by anα-bond was formed.Finally,the intermediate acquired one more H from the surface of Cu/CuO/ZnO and the desired 1,2-PDO was obtained.

Fig.10.Proposed pathway for the formation of 1,2-PDO.
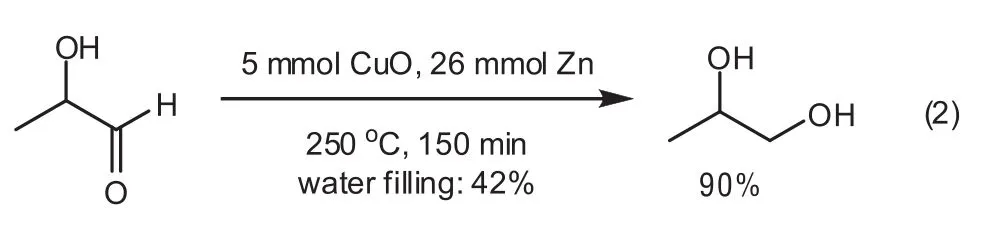
To investigate the possibility of an experimental pathway for the transformation,a validation test for the conversion of intermediate 2-hydroxypropanal to 1,2-PDO was conducted.As a result,intermediate 2-hydroxypropanal was converted completely to 1,2-PDO in 90%yield under optimum conditions[Eq.(2)].This validation process illustrates that the proposed pathway is acceptable.
4.Conclusions
We developed a highly efficient conversion of biomass-derived ethyl lactate to 1,2-PDO over CuOin high temperature water.CuO catalyst exhibits high catalytic activity for the transformation.A high yield of 93.6%was achieved in the presence of 26 mmol Zn and 5 mmol Cu O with 42%water filling at 250°C for 150 min.The use of gaseous hydrogen was in effective and no desired 1,2-PDO was obtained.This study provided a highly efficient process for the conversion of biomass-derived ethyl lactate to 1,2-PDO.Further study will betaken to develop efficient methods for the conversion of biomass-derived compounds to value-added chemicals.
[1]G.X.Xu,C.Shao,Z.L.Xiu,Optimizing control of bio-dissimilation process of glycerol to 1,3-propanediol,Chin.J.Chem.Eng.16(2008)128-134.
[2]R.J.van Putten,J.C.van der Waal,E.de Jong,C.B.Rasrendra,H.J.Heeres,J.G.de Vries,Hydroxymethylfurfural,a versatile platform chemical made from renew able resources,Chem.Rev.133(2013)1499-1597.
[3]Y.Roman-Leshkov,C.J.Barrett,Z.Y.Liu,J.A.Dumesic,Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates,Nature 447(2007)982-985.
[4]C.S.Zhou,X.J.Yu,H.L.Ma,R.H.He,V.Saritporn,Optimization on the conversion of bamboo shoot shell to levulinic acid with environmentally benign acidic ionic liquid and response surface analysis,Chin.J.Chem.Eng.21(2013)544-550.
[5]R.K.Saxena,P.Anand,S.Saran,J.Isar,L.Agarwal,Microbial production and applications of 1,2-propanediol,Indian J.Microbiol.50(2010)2-11.
[6]D.C.Cameron,N.E.Altaras,M.L.Hoffman,A.J.Shaw,Metabolic engineering of propanediol pathways,Biotechnol.Prog.14(1998)116-125.
[7]E.S.Lipinsky,R.G.Sinclair,Biodegradable materials:state of art and future perspectives,Chem.Eng.Prog.82(1986)26-29.
[8]A.Corma,S.Iborra,A.Velty,Chemical routes for the transformation of biomass into chemicals,Chem.Rev.38(2007)2411-2502.
[9]J.H.Clark,The home of cutting-edge research on the development of alternative sustainable technologies,Green Chem.(2006)17-21.
[10]Z.Han,L.Rong,J.Wu,L.Zhang,Z.Wang,K.Ding,Catalytic hydrogenation of cyclic carbonates:a practical approach from CO2and epoxides to methanol and diols,Ange wandte 51(2012)13041-13045.
[11]G.Fan,Y.Zhang,Y.Zhou,R.Li,H.Chen,X.Li,Synthesis of ZnO and ZnO2nanoparticles by the oil-in-water microemulsion reaction method,Chem.Lett.37(2008)132-134.
[12]G.Luo,S.Yan,M.Qiao,J.Zhuang,K.Fan,Effect of tin on Ru-B/γ-Al2O3catalyst for the hydrogenation of ethyl lactate to 1,2-propanediol,Appl.Catal.275(2004)95-102.
[13]L.Huang,Y.Zhu,H.Zheng,M.Du,Y.Li,Vapor-phase hydrogenolysis of biomassderived lactate to 1,2-propanediol over supported metal catalysts,Appl.Catal.349(2008)204-211.
[14]J.Feng,W.Xiong,Y.Jia,J.Wang,D.Liu,R.Qin,Hydrogenation of ethyl lactate over ruthenium catalysts in an additive-free catalytic system,React.Kinet.Mech.(2008)89-97.
[15]N.Akiya,P.E.Savage,Roles of water for chemical reactions in high-temperature water,Chem.Rev.102(2002)2725-2750.
[16]M.Watanabe,T.Sato,H.Inomata,R.L.Smith,K.Arai,A.Kruse,E.Dinjus,Chemical reactions of C1compounds in near-critical and supercritical water,Chem.Rev.104(2004)5803-5822.
[17]R.W.Shaw,Y.B.Brill,A.A.Clifford,C.A.Eckert,E.U.Franck,Dissipative hydrodynamics of rigid spherical particles,Chem.Eng.23(2010)23-26.
[18]W.L.Marshall,E.U.Frank,Ion product of water substance,0-1000°C,1-10,000 bars New International Formulation and its background,J.Phys.Chem.Ref.Data(1981)295-304.
[19]L.L.Xu,Z.B.Huo,J.Fu,F.M.Jin,Highly efficient conversion of biomass-derived glycolide to ethylene glycol over CuO in water,Chem.Commun.50(2014)6009-6012.
[20]F.Jin,A.Kishita,T.Moriya,H.Enomoto,Kinetics of oxidation of food wastes with H2O2in supercritical water,Supercrit.Fluids 19(2001)251-262.
[21]Y.Li,G.Xu,C.Liu,B.Eliasson,B.Xue,Gram-scale synthesis of zinc oxide nanorods in basic ethanol solutions,Energy Fuel 15(2001)299-302.
[22]D.Larcher,R.Patrice,Preparation of metallic pow ders and alloys in polyol media:a thermodynamic approach,J.Solid State Chem.154(2000)405-411.
[23]F.L.P.Resende,P.E.Savage,Effect on metal on superficial water gasification of cellulose and lignin,Ind.Eng.Chem.Res.49(2010)2694-2700.
[24]D.C.Elliott,Catalytic hydrothermal gasification of biomass,Biofuels Bioprod.Biore fin.2(2008)254-265.
[25]Z.P.Yan,L.Lin,S.Liu,Synthesis of γ-valerolactone by hydrogenation of biomass derived levulinic acid over Ru/C catalyst,Energy Fuel 23(2009)3853-3858.
[26]A.P.G.Kieboom,F.van Rantwiik,Hydrogenation and Hydrogenolysis in Synthetic Organic Chemistry,Science Press,Beijing,China,1981.295-300.
 Chinese Journal of Chemical Engineering2016年1期
Chinese Journal of Chemical Engineering2016年1期
- Chinese Journal of Chemical Engineering的其它文章
- Economic analysis in product design—A case study of a TCM dietary supplement
- Effect of ionic liquids on stability of O/W miniemulsion for application of low emission coating products☆
- Characterization and adsorption behaviors of a novel synthesized mesoporous silica coated carbon composite☆
- Experiment and simulation of foaming injection molding of polypropylene/nano-calcium carbonate composites by supercritical carbon dioxide☆
- Salt-free reactive dyeing of betaine-modified cationic cotton fabrics with enhanced dye fixation☆
- A computational analysis of the impact of mass transport and shear on three-dimensional stem cell cultures in perfused micro-bioreactors
