Degradation of bisphenol A using electrochemical assistant Fe(II)-activated peroxydisulfate process
* Corresponding author.
?
Degradation of bisphenol A using electrochemical assistant Fe(II)-activated peroxydisulfate process
Chun-wei Yang*
College of Environmental Science and Engineering,Jilin Normal University,Siping 136000,PR China
Received 22 October 2013; accepted 17 December 2014 Available online 15 April 2015
* Corresponding author.
Abstract
Degradation of bisphenol A (BPA)in aqueous solution using sulfate radicals was investigated using the Fe(II)-activated peroxydisulfate (PDS)process,electrochemical process,electrochemical process with 2.5 mmol/L Na2S2O8without Fe(II),and electrochemical assistant Fe(II)-activated PDS process.It was found that the electrochemical assistant Fe(II)-activated PDS process performed best in the degradation of BPA.The variables considered to influence the degradation efficiency of BPA were the initial concentration of Fe2t,the initial concentration of Na2S2O8,and the current density.More than 97% of the BPA removals were achieved within 120 min under the optimum operational condition.The degradation of BPAwas accompanied by the formation of phenol,hydroquinone,and small-molecule compounds such as succinic acid.The electron transfer was the principal step in the oxidation of BPA.
?2015 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
This work was supported by the Natural Science Foundation of Jilin Province (Grant No.20140101215JC),the Key Program in Science and Technologies of Jilin Province (Grant No.20150204049SF),and the Key Laboratory of Industrial Ecology and Environmental Engineering,the Ministry of Education of China (Grant No.KLIEEE-13-07).
E-mail address: chunwei_yang@jlnu.edu.cn (Chun-wei Yang).Peer review under responsibility of Hohai University.
http://dx.doi.org/10.1016/j.wse.2015.04.002
1674-2370/?2015 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords:Sulfate radicals; Peroxydisulfate; Advanced oxidation; BPA
1.Introduction
Bisphenol A (BPA)is a compound widely used in the production of polycarbonate plastics and epoxy resin (Morgan et al.,2014; Mei et al.,2011).It is an endocrine-disrupting chemical (EDC)that affects the endocrine system and produces an adverse effect on aquatic life,animals,and potentially human.There is increasing evidence that EDCs can alter endocrine functions and may disrupt the growth,development,and reproduction of the human body by interfering with the production of the endocrine system.Accordingly,because of the contribution of estrogenic chemicals to the development of hormone-dependent cancers,disorders of the reproductive tract,and other effects,they are often referred to as environmental estrogens (Tyler and Denys,2014; Branco and Lemos,2014).BPA has become a controversial issue because it was detected in different environmental media (aquatic environment,soil,sediment,and air),food,and the human body (Huang et al.,2012; Suzuki et al.,2004).Therefore,the development of a process for BPA degradation has aroused significant interest (Cui et al.,2009; Li et al.,2008).
The electrochemical process is one of the advanced oxidation processes (AOPs)developed in recent years for wastewater treatment (Lei et al.,2013; Li et al.,2005).Effectiveness of electrochemical oxidation for wastewater treatment depends largely upon the properties of the anodes and the organic substances involved in the process (Jin et al.,2014; Chen et al.,2005).Traditional electrodes,such as graphite,are not effective for wastewater treatment (Souza et al.,2014).Recently,there has been a growing interest in using sulfate radicals to treat organic contaminants (Anipsitakis and Dionysiou,2004; Rastogi et al.,2009).Sulfate radicals could be produced from peroxydisulfate (PDS)or peroxymonosulfate via thermal,photochemical,radiolysis,or redox decomposition (Eqs.(1)through (4))(Zhao et al.,2010).
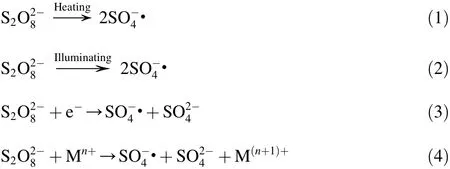
where M denotes the transition metal.
The Fe(II)-activated PDS process has been used in wastewater treatment,especially for in situ chemical oxidation using sulfate radicals (Ahmed et al.,2012; Liang et al.,2004).However,there have existed critical limitations in the application of the Fe(II)-activated PDS process because the reaction requires acid conditions,and the efficiency has been too low.Evidence of the latter is that some of the sulfate radicals produced react with excess Fe2tthereby lowering the concentration of sulfate radicals that are available to degrade contaminants in the system (Eqs.(5)and (6)).

Therefore,for water treatment,it would be interesting to develop environmentally benign methods with environmental and economic considerations.The electrochemical assistant Fe(II)-activated PDS process has been established,offering a new method of solving problems.In the electrolytic cell,pollutants are destroyed by the action of the Fe(II)-activated PDS process in bulk together with anodic oxidation at the anode surface.Simultaneously the electrochemical reduction of Fe3tto Fe2tat the cathode surface (Eq.(7))enhances Eq.(5).Moreover,the oxidation of regenerated Fe2tto Fe3tmay occur at the same time (Eq.(8))on the anode surface in order to maintain the appropriate Fe2tconcentration.
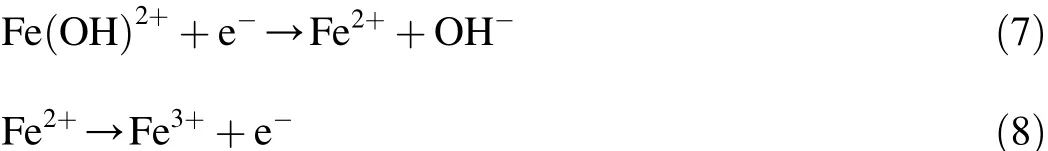
As a result,our research focuses on the destruction of BPA,as an EDC,using the electrochemical assistant Fe(II)-activated PDS process in an effort to propose sulfate radicals-based AOPs as an alternative to the most common and established electro-Fe(II)-activated PDS system for the decomposition of EDCs.In this study,the reaction mechanism and the products of BPA degradation were also evaluated.From the environmentally friendly point of view,the electrochemical assistant Fe(II)-activated PDS process for wastewater treatment provides a promising alternative as a novel technology for elimination of contaminants.
2.Materials and methods
2.1.Chemicals
BPA was obtained from the Shanghai Third Chemical Reagent Factory in China.Sodium sulphate (anhydrous,with a purity of 99%),sodium peroxydisulfate (analytical reagent grade,with a purity of 99%),and ferrous sulfate (analytical reagent grade,with a purity of 99%)were purchased from the Shenyang Chemical Reagent Factory in China.All sample solutions were prepared with deionized water from the ion exchange system.
2.2.Procedures and equipment
Experiments were performed atroom temperature (20±1) C in a 100 mL beaker supplied with a magnetic stirrer under constant potential conditions using a ATTEN APR-6402 potentiostat/galvanostat (Shenzhen,China).Graphite plates (2.5 cm 4.5 cm 0.5 cm)were used as cathode and anode electrodes with the electrode spacing maintained at 1 cm.The aqueous solutions were agitated continuously with a magnetic stirrer atavelocity of75rpm.The initial concentrations of BPA andwere 35 mg/L and 25 mmol/L,respectively.A series of experiments was conducted to investigate the optimal iron catalyst concentration,current density,and sodium peroxydisulfate concentration.The pH of BPA solutions was not adjusted during the reaction.
2.3.Analytical methods
The concentration of BPA was determined using highperformance liquid chromatography (HPLC)incorporating a high-pressure pump (Shimadzu LC-6A)and a UV detector (Waters 481 detector).In the HPLC analysis,a Vydac C18 column (with a size of 1.0 mm 150 mm and a mean particle size of 5 mm)and a mobile phase of methanol/water (with a volume ratio of 3:1)at a steady flow rate of 1 mL/min were used.The injection volume was 2 mL.The retention time of BPA was 4.62 min.Measurements were made in triplicate in each experiment with errors less than 5%.
3.Results and discussion
3.1.Preliminary studies on treatment method
Preliminary experiments were performed to compare the effect of different combinations of treatment methods,including the Fe(II)-activated PDS process,electrochemical process,electrochemical process with 2.5 mmol/Lwithout Fe(II),and electrochemical assistant Fe(II)-activated PDS process,as shown in Fig.1.The initial concentrations of BPA andwere 35 mg/L and 25 mmol/L,respectively.Theseents were carried out at room temperature (20±1) C and neutral pH.In the Fe(II)-activated PDSprocess the concentrations of Fe(II)and PDS were 0.2 and 2.5 mmol/L,respectively.The current density in the electrochemical process and electrochemical assistant Fe(II)-activated PDS process was maintained at 3.6 mA/cm2.In the electrochemical assistant Fe(II)-activated PDS process the concentrations of Fe(II)and PDS were 0.2 and 2.5 mmol/L,respectively.The reaction duration was kept at 30 min.The Fe(II)-activated PDS process,electrochemical process,electrochemical process with 2.5 mmol/L without Fe(II),and electrochemical assistant Fe(II)-activated PDS process had minimum BPA concentrations with a decreasing order of 30.69,24.17,19.54,and 14.27 mg/L,respectively.It was clearly seen that the best and efficient treatment method for the degradation of BPA was the electrochemical assistant Fe(II)-activated PDS process.
The removal rate of BPA only reaches 12.3% after a 30-min treatment with the Fe(II)-activated PDS process because the Fe(II)-activated PDS process only has high efficiency at a relatively high temperature (50e70 C)and acid condition (with pH values from 2 to 4)(Huang and Huang,2009).Therefore,it is not efficient to treat BPA with the Fe(II)-activated PDS process.The existence of PDS could enhance the efficiency of the electrochemical process because sulfate radicals can be produced through PDS reaction at the cathode electrode surface (Eq.(3)).BPA could also be removed by the anode during the electrochemical reaction.The concentration of BPA decreased to 19.54 mg/L after a 30-min treatment with the electrochemical process with 2.5 mmol/Lwithout Fe(II).By contrast,the concentration of BPA reached 14.27 mg/L with the electrochemical assistant Fe(II)-activated PDS process due to the coupling of electrochemical and Fe(II)-activated PDS processes.Therefore,the electrochemical assistant Fe(II)-activated PDS process is feasible and practical in the treatment of BPA.Further investigations of process factors should be considered.3.2.Influence of initial concentration of Fe2ton BPA removal rate

Fig.1.Variation of BPA concentration with time with different processes.
In order to evaluate the effect of the initial concentration of Fe2ton the degradation of BPA,six discrete values between 0.1 and 1.8 mmol/L were used with the electrochemicalassistant Fe(II)-activated PDS process.Other operating parameters apart from the initial concentration of Fe2twere kept the same (the BPA and concentrations and the current density were 35 mg/L,2.5 mmol/L,and 3.6 mA/cm2,respectively).It was observed that the BPA removal rate increased with the initial concentration of Fe2t,especially below 1.0 mmol/L (Fig.2),and the influence of the initial concentration of Fe2twas less pronounced when it reached 1.0 mmol/L.When the Fe2tconcentration was greater than 1.0 mmol/L the remaining Fe2tcould react with sulfate radicals (Eq.(6)).Finally,the BPA removal rate remained steady even when the initial concentration of Fe2tincreased.Taking into account the practical application of this process,the optimal initial concentration of Fe2tin the following experiments was set as 1.0 mmol/L.
3.3.Influence of initial concentration ofon BPA removal rate
The initial concentration ofhas a significant effect on the degradation of BPA with the electrochemical assistant Fe(II)-activated PDS process.The effect of the initial concentration ofwas essentially investigated and the results are shown in Fig.3.The initial concentration ofvaried from 0.5 to 4.5 mmol/L while other experimental parameters remained constant (the BPA and Fe2tconcentrations were 35 mg/L and 1.0 mmol/L,respectively,and the current density was 3.6 mA/cm2).It was clearly seen that the increase of the initial concentration of from 0.5 to 2.5 mmol/L improved the BPA removal rate from 46.1% to 61.36% after 30 min of treatment,as high concentration can accelerate the generation of sulfate radicals and decomposition of organic pollutants.However,when the initial concentration ofincreased from 2.5 to 4.5 mmol/L,the removal rate of BPA remained almost steady,from 61.36% to 62.45%.The results show that the optimal initial concentration ofwas 2.5 mmol/L for the degradation of BPA using the electrochemical assistant Fe(II)-activated PDS process.
3.4.Influence of current density on BPA removal rate

Fig.2.Effects of initial concentration of Fe2ton BPA removal rate.
Experiments were conducted to determine the effect of current density on the removal of BPA using the electrochemical assistant Fe(II)-activated PDS process while other experimental parameters were kept constant (the BPA,Fe2t,and concentrations were 35 mg/L,1.0 mmol/L,and 2.5 mmol/L,respectively).The values of current density applied were 0.9,1.8,3.6,5.4,7.2,and 9.0 mA/cm2.As shown in Fig.4,the removal rate increased from 31.97% to 61.36% with the increase of current density from 0.9 to 3.6 mA/cm2within 30 min.However,when the current density was greater than 3.6 mA/cm2,the BPA removal rate decreased to 50.31% for current densities from 3.6 to 9.0 mA/cm2.This may be explained by the fact that high current density may lead to a decrease of the concentrations of sulfate radicals and Fe2t,and then influence the removal rate of BPA.Furthermore,the results revealed that there was an optimum current density (3.6 mA/cm2)in treating the BPA solution.
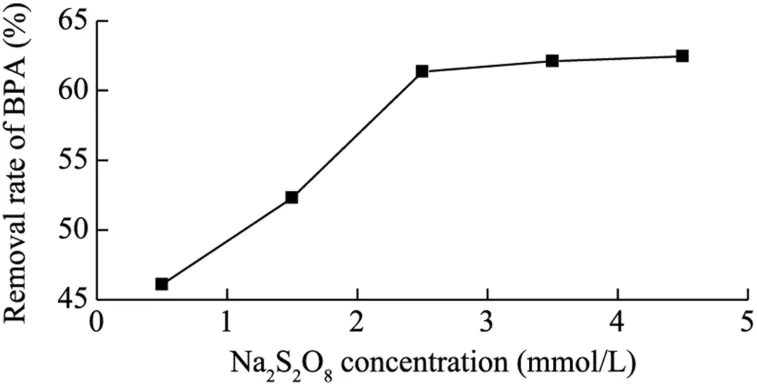
Fig.3.Effects of initial concentration of Na2S2O8on BPA removal rate.
3.5.Kinetics studies of BPA removal
It is necessary to study the kinetics of BPA removal under optimal conditions.The degradation process of BPA was observed when the BPA,Fe2t,and concentrations were 35 mg/L,1.0 mmol/L,and 2.5 mmol/L,respectively,and the current density was 3.6 mA/cm2at ambient temperature and neutral pH.The results are shown in Fig.5.The optimal BPA removal rate reached 97.67% within 120 min of treatment.The electrochemical assistant Fe(II)-activated PDS process is operative in the degradation of BPA.
The kinetics of the BPA removal process followed the firstorder reaction of the BPA concentration.Fig.6 presents the calculation of the pseudo first-order rate constant k,for BPA degradation with the electrochemical assistant Fe(II)-activated PDS process within the 120-min treatment.The first-order kinetics of the removal rate are expressed in Eq.(9).The degradation process of BPA follows Eq.(10).
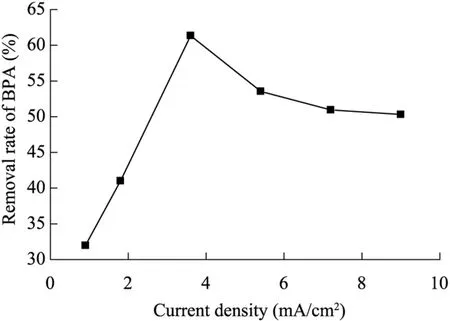
Fig.4.Effects of current density on BPA removal rate.
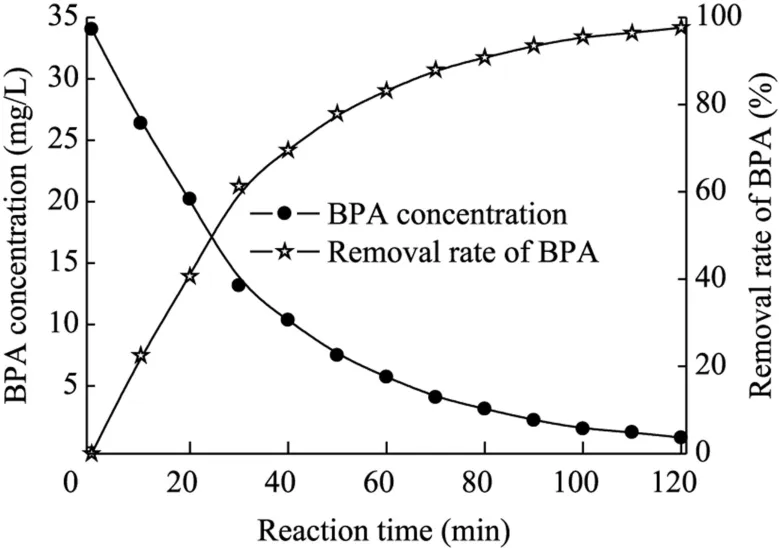
Fig.5.Variations of BPA concentration and removal rate with reaction time.

where C is the concentration of BPA at reaction time t,and C0is the initial concentration of BPA.
3.6.Reaction products from oxidation of BPA
The reaction products from the oxidation of BPA using the electrochemical assistant Fe(II)-activated PDS process were identified by means of HPLC.Fig.7 shows the BPA degradation process in separate reaction time.
The results indicated that after the 120-min reaction the BPA concentration was very low,but hydroquinone,phenol,and succinic acid were found using the standard addition method.The concentration of BPA decreased with the reaction time but the peak height of intermediate products remained steady.Previous reports (Cui et al.,2009; Li et al.,2008)have shown the degradation pathways of BPA.Hydroquinone was one of the key intermediate products through isopropylidene bridge cleavage.Then succinic acid could be discovered through aromatic ring cleavage.The degradation pathway of hydroquinone was determined because hydroquinone and succinic acid were detected throughout the reaction with the electrochemical assistant Fe(II)-activated PDS process.
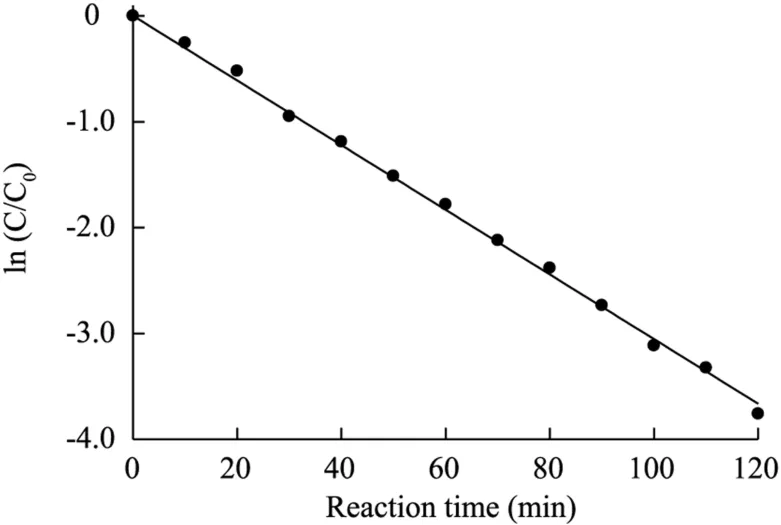
Fig.6.Degradation kinetics of BPA.
Nevertheless,the results also indicated that the concentrations of intermediate products hydroquinone and phenol were very low.Therefore,during the oxidation of BPA,electron transfer was assumed to be the key step of the sulfate radical attack.A sulfate radical mediated attack on BPA leads to the formation of organic radicals via electron transfer from the organic compounds to the sulfate radicals.The degradation of BPA through a hydroxylation reaction of hydroxyl radicals did not prevail in this process.The results regarding reaction intermediates indicated that succinic acid was the primary intermediate from BPA degradation and the electron transfer was the principal step in the oxidation of BPA.
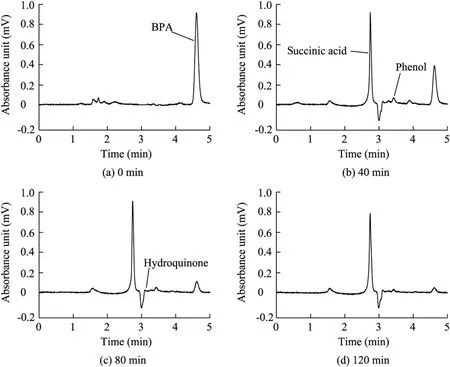
Fig.7.HPLC chromatograms of BPA in water at different reaction time.
4.Conclusions
The electrochemical assistant Fe(II)-activated PDS process was proven to be feasible and effective in the treatment of BPA solution.The influence of some operational parameters on the degradation of BPA was discussed.This promising and ecofriendly oxidation system is likely a useful contribution to the field of AOPs.The main conclusions are as follows:
(1)The removal rate of BPAwas strongly dependent on the initial concentration of Fe2t,the initial concentration ofand the current density.
(2)Investigation of the mechanism of the degradation of BPA with the electrochemical assistant Fe(II)-activated PDS process suggested that this process obeyed the first-order kinetics.
(3)The formation of intermediates,such as hydroquinone and succinic acid,showed that electron transfer was the foremost step in the oxidation of BPA.
References
Ahmed,M.M.,Barbati,S.,Doumenq,P.,Chiron,S.,2012.Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination.Chem.Eng.J.197,440e447.http://dx.doi.org/10.1016/j.cej.2012.05.040.
Anipsitakis,G.P.,Dionysiou,D.D.,2004.Radical generation by the interaction of transition metals with common oxidants.Environ.Sci.Technol.38(13),3705e3712.http://dx.doi.org/10.1021/es035121o.
Branco,A.T.,Lemos,B.,2014.Interaction between bisphenol A and dietary sugar affects global gene transcription in Drosophila melanogaster.Genomics Data 2,308e311.http://dx.doi.org/10.1016/j.gdata.2014.09.005.
Chen,X.M.,Gao,F.R.,Chen,G.H.,2005.Comparison of Ti/BDD and Ti/electrodes for pollutant oxidation.J.Appl.Electrochem.35(2),185e191.http://dx.doi.org/10.1007/s10800-004-6068-0.
Cui,Y.H.,Li,X.Y.,Chen,G.H.,2009.Electrochemical degradation of bisphenol A on different anodes.Water Res.43(7),1968e1976.http://dx.doi.org/10.1016/j.watres.2009.01.026.
Huang,Y.F.,Huang,Y.H.,2009.Identification of produced powerful radicals involved in the mineralization of bisphenol A using a novel)two-stage oxidation process.J.Hazard.Mater.162(2e3),1211e1216.http://dx.doi.org/10.1016/j.jhazmat.2008.06.008.
Huang,Y.Q.,Wong,C.K.C.,Zheng,J.S.,Bouwman,H.,Barra,R.,Wahlstr€om,B.,Neretin,L.,Wonh,M.H.,2012.Bisphenol A (BPA)in China: a review of sources,environmental levels,and potential human health impacts.Environ.Int.42,91e99.http://dx.doi.org/10.1016/j.envint.2011.04.010.
Jin,P.P.,Chang,R.,Liu,D.Q.,Zhao,K.,Zhang,L.X.,Ouyang,Y.J.,2014.Phenol degradation in an electrochemical system with TiO2/activated carbon fiber as electrode.J.Environ.Chem.Eng.2(2),1040e1047.http://dx.doi.org/10.1016/j.jece.2014.03.023.
Lei,Y.M.,Liu,H.,Shen,Z.M.,Wang,W.,2013.Development of a trickle bed reactor of electro-Fenton process for wastewater treatment.J.Hazard.Mater.261,570e576.http://dx.doi.org/10.1016/j.jhazmat.2013.08.010.
Li,C.,Li,X.Z.,Graham,N.,Gao,N.Y.,2008.The aqueous degradation of bisphenol A and steroid estrogens by ferrate.Water Res.42(1e2),109e120.http://dx.doi.org/10.1016/j.watres.2007.07.023.
Li,X.Y.,Cui,Y.H.,Feng,Y.J.,Xie,Z.M.,Gu,J.D.,2005.Reaction pathways and mechanisms of the electrochemical degradation of phenol on different electrodes.Water Res.39(10),1972e1981.http://dx.doi.org/10.1016/j.watres.2005.02.021.
Liang,C.,Bruell,C.J.,Marley,M.C.,Sperry,K.L.,2004.Persulfate oxidation for in situ remediation of TCE,II: Activated by chelated ferrous ion.Chemosphere 55(9),1225e1233.http://dx.doi.org/10.1016/j.chemosphere.2004.01.030.
Mei,S.R.,Wu,D.,Jiang,M.,Lu,B.,Lim,J.,Zhou,Y.K.,Lee,Y.,2011.Determination of trace bisphenol A in complex samples using selective molecularly imprinted solid-phase extraction coupled with capillary electrophoresis.Microchem.J.98(1),150e155.http://dx.doi.org/10.1016/j.microc.2011.01.003.
Morgan,A.M.,El-Ballal,S.E.,El-Bialy,B.E.,El-Borai,N.B.,2014.Studies on the potential protective effect of cinnamon against bisphenol A- and octylphenol-induced oxidative stress in male albino rats.Toxicol.Reports 1,92e101.http://dx.doi.org/10.1016/j.toxrep.2014.04.003.
Rastogi,A.,Al-Abed,S.R.,Dionysiou,D.D.,2009.Sulfate radical-based ferrous-peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems.Appl.Catal.B: Environ.85(3e4),171e179.http://dx.doi.org/10.1016/j.apcatb.2008.07.010.
Souza,F.L.,Teodoro,T.Q.,Vasconcelos,V.M.,Lima Gomes,P.C.,Ferreira,N.G.,Baldan,M.R.,Haiduke,R.L.,Lanza,M.R.,2014.Electrochemical oxidation of imazapyr with BDD electrode in titanium substrate.Chemosphere117,596e603.http://dx.doi.org/10.1016/j.chemosphere.2014.09.051.
Suzuki,T.,Nakagawa,Y.,Takano,I.,Yaguchi,K.,Yasuda,K.,2004.Environmental fate of bisphenol A and its biological metabolites in river water and their xeno-estrogenic activity.Environ.Sci.Technol.38(8),2389e2396.http://dx.doi.org/10.1021/es030576z.
Tyler,P.,Denys,D.,2014.Presence and bioavailability of bisphenol A in the uterus of rats and mice following single and repeated dietary administration at low doses.Reprod.Toxicol.49,145e154.http://dx.doi.org/10.1016/j.reprotox.2014.08.005.
Zhao,J.Y.,Zhang,Y.B.,Quan,X.,Chen,S.,2010.Enhanced oxidation of 4-chlorophenol using sulfate radicals generated from zero-valent iron and peroxydisulfate at ambient temperature.Sep.Purif.Technol.71(3),302e307.http://dx.doi.org/10.1016/j.seppur.2009.12.010.
 Water Science and Engineering2015年2期
Water Science and Engineering2015年2期
- Water Science and Engineering的其它文章
- Water requirement pattern for tobacco and its response to water deficit in Guizhou Province
- Hydraulic metal structure health diagnosis based on data mining technology
- Effects of leachate infiltration and desiccation cracks on hydraulic conductivity of compacted clay
- Receptivity of plane Poiseuille flow to local micro-vibration disturbance on wall
- Distribution and release of 2,4,5-trichlorobiphenyl in ice
- Effects of benthic algae on release of soluble reactive phosphorus from sediments: a radioisotope tracing study
