Optimization of the Callus Induction System of Chenopodium quinoa Willd
Hanyi YU, Yurong JIANG*, Zeyang MAO, Guoquan LU, Guolin CHEN, Qian MAO
1. School of Agriculture and Food Science, Zhejiang A&F University, Lin’an 311300, China;
2. Institute of Hydraulic and Environmental Engineering, Zhejiang University of Water Resources & Electric Power, Hangzhou 310018, China
As an annual Chenopodiaceae herbaceous plant,Chenopodium quinoa Willd. has been planted in Andes Mountains for more than 5000 years. C. quinoa is called the "mother of cereal" and the "real gold of Andes"[1-2]. Protein content is 13% -23% in C. quinoa. There are nine proportionally balanced amino acids essential for human nutrient. C.quinoa is rich in unsaturated fatty acid,flavonoids,vitamin E and other beneficial compounds. It is the only monosomic plant recommended by the Food and Agriculture Organization(FAO),which can meet the demand of all basic material in human body.Known as the "super grain of the future", the "gold nutrition" and the "king of organic grains"[3-4].C.quinoa prefers tropical and subtropical dry and wet climate; the proper growth temperature is 14.0 -18.0 ℃; it is resistant to light frost during vegetative growthphase(-1.0-0 ℃).After seed setting,C.quinoa is resistant to low temperature of -6.0℃, and has very strong resistance capabilities to salinization, drought, frost,pest and disease damage[5]. Introduction trial was carried out in the year 1987 in College of Agriculture and Animal Husbandry of Tibet Autonomous Region[6]. At present, C. quinoa is planted in Xi’an City, Shaxi Province, Qinghai Province, and Sichuan Province in large scale. Researches on C.quinoa are mainly focused on its biological characteristics[6-7], chemical component[8-9], stress resistance and other physiological characteristics[10-11].So far,no reports have been found on the tissue culture of C. quinoa. In this research, suitable explants were screened from nine varieties of C.quinoa. Effects of hormone combination on the callus induction and proliferation of C. quinoa stem were researched, aiming at laying biological basis for the construction of regeneration system of C. quinoa, and for thefinding of new varieties by transgene and somatic hybridization.
Materials and Methods
Materials
Nine varieties of C. quinoa in field planting were selected, including TEMUCO QUINOA TRADI TIONAL,CQ-TEMVCC, Chenopodium quinoa‘Red’, PI596498, Tomico Quinoa,cherry vaniua quinoa, DAVE ( FOURO-SEVEN)Quinoa,Zheli-49 and Zheli-51(Table 1).All the seeds were provided by Zhejiang A&F University.
Cultivation of C. quinoa aseptic seedlings
Plump seeds were heated in water bath pot at 38 ℃for 3 h.After cooling to room temperature, sterilization was carried out by 75% ethanol for 1min, and by 0.2% HgCl2for 5 min.Then, the seeds were washed by sterile water for 4 -5 times. Under aseptic condition, the sterilized seeds were inoculated to MS culture medium without hormone. They were cultured at 24 ℃for 3-4 d in dark, and then cultured under 16 h /8 h photoperiod for 3 d. Thus, aseptic seedlings were obtained.
Treatment of explants
Stem section of aseptic seedlings were cut into 1.0 cm small segments;cotyledons were cut into the size of 0.5 cm × 0.5 cm. They were put in MS +0.5 mg/L 2, 4-D for callus induction,with in all three repetitions. After cultivation at 24 ℃for 28 h under 16 h/8 h photoperiod, the induction rate and growth status of callus from different explants were obtained. The equation for induction rate was as follows:
Induction rate = Explants number that forming callus / Total number of inoculated explants×100%.
Optimization test of callus induction
Segments of aseptic seedlings of three verities were selected, which were TEMUCO QUINOA TRADI TIONAL, Zheli-51 and Zheli-49. Under aseptic condition, they were cut into 1.0 cm segments, and inoculated to MS culture medium + different hormone treatments(Table 2).there were three culture mediums for each variety.
Optimization test of callus proliferation
The early generation of calluses of TEMUCO QUINOA TRADI TIONAL,Zheli-51 and Zheli-49 were cultivated for 12 d,cut into small segments of 0.3 g, and inoculated to culture medium for subculture (Table 3).There were three bottles for each treatment; and four calluses in each bottle. Thus, each treatment had in all 12 calluses.After 20 d,fresh weight of callus was weighed (m2).With the proliferation rate of callus as the index,the optimal culture medium was screened(Table 3).
Proliferation rate of callus= (m2-m1)/m1×100%.

Table 1 Sources of nine varieties C.quinoa
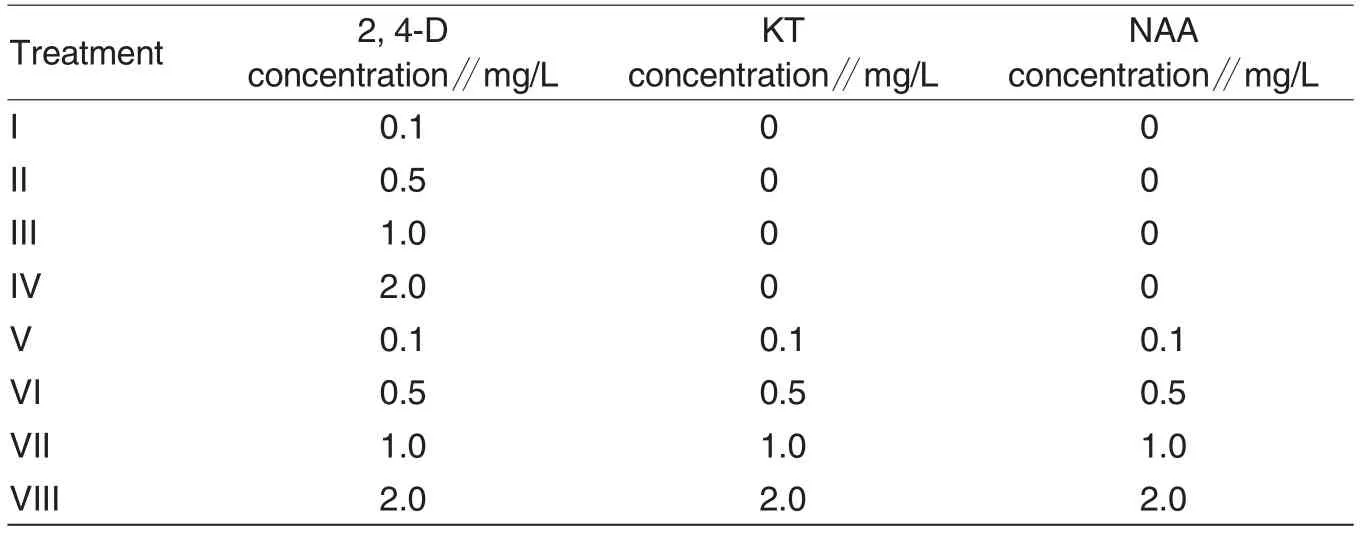
Table 2 Design of callus induction treatment test

Table 3 Test design of callus proliferation treatment
In the equation, m1was the weight of callus in early generation,and m2was the weight after cultivated for 20 d.
Data analysis
Data were analyzed by Excel and SPSS software.
Results and Analysis
Selection of callus explants
The sterilized stem segments and cotyledons were inoculated to culture medium. After 2 d, incision of explants turned to brown. After 4-5 d, explants began to expand.Milk white sticky callus appeared on about 7 d, and explants elongated. On 28 d, explants had no callus induction would no longer produce callus. The induction rates of calluses varied in different vari-eties; the induction rate of callus fro different explants also varied within the same species.Fig.1 illustrated that the callus induction rates of stem segments were all above 78% in the nine varieties of C.quinoa.And the average induction rate was 90%. However, the callus induction rate of cotyledon was only above 58%; and the average induction rate was 80%. Callus rates of stem segments in cherry vaniua quinoa, Tomico Quinoa, Zheli-51,Chenopodium quinoa ‘Red’were significantly higher than those of cotyledon.The callus induction rates of stem segments reached 100% in TEMUCO QUINOA TRADI TIONAL, CQTEMVCC and Chenopodium quinoa‘Red’. Therefore, it could be concluded that callus induction sensitivity of C. quinoa had differences in varieties.Although stem segments and cotyledons could both induce callus, stem segments was more suitable to be used as the explants for callus induction.
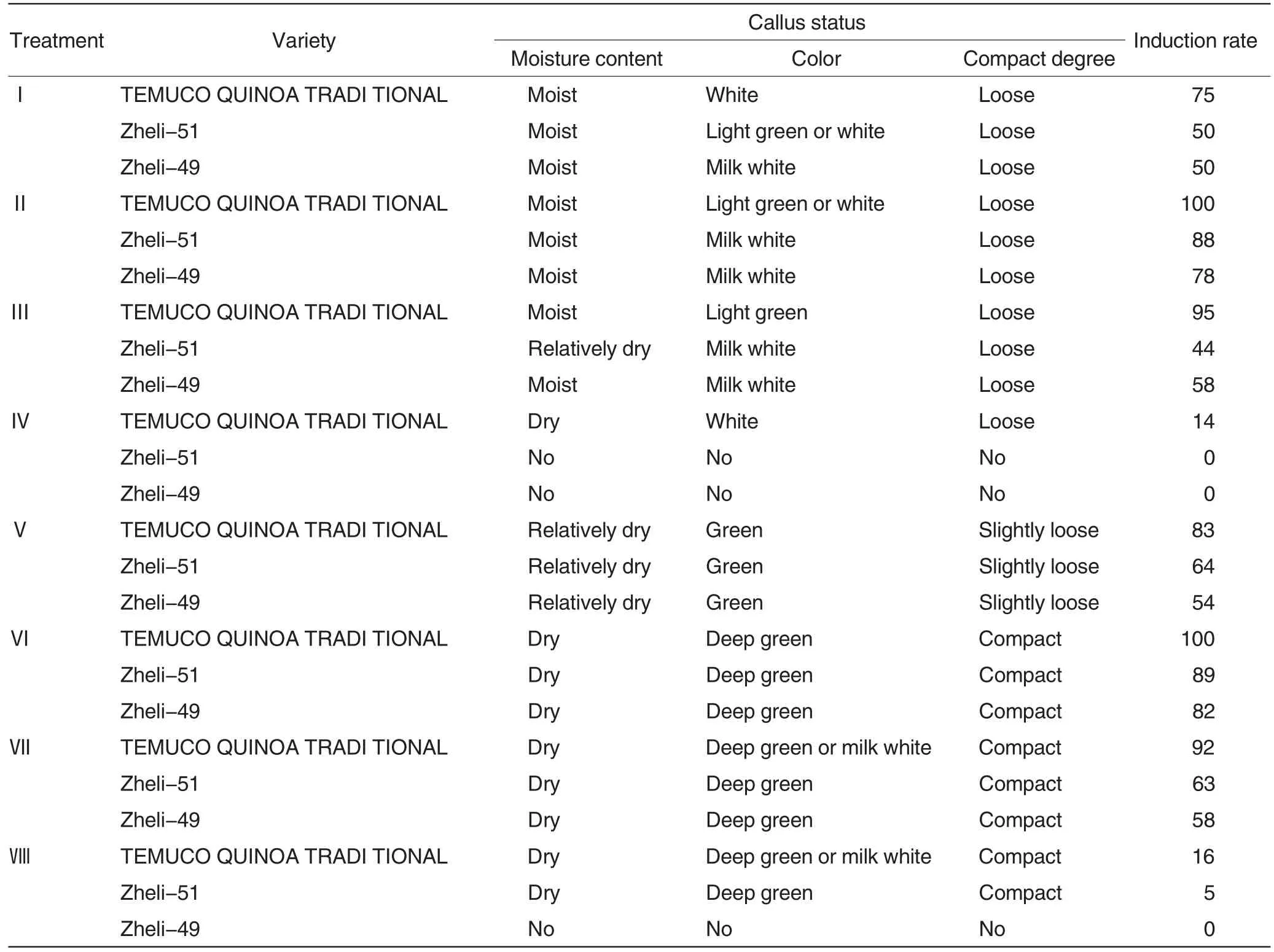
Table 4 Effects of treatment combination on callus induction of different varieties of C.quinoa

Table 5 Proliferation rates of calluses of three varieties of C.quinoa in different treatments
Optimization analysis of callus induction
During the callus induction of C. quinoa, hormone types and concentrations played an important role in callus induction. Table 4 reported that under different combination treat-ments, induction rates of calluses in the same variety had great differences. For instance, callus induction rates of TEMUCO QUINOA TRADI TIONAL in treatments Ⅱ and Ⅵreached 100%, while those in treatments Ⅳand Ⅷwere lower than 20%.Induction rates of TEMUCO QUINOA TRADI TIONAL calluses were scientifically higher than those of Zheli-49 and Zheli-51 in all treatments. As the concentration of 2, 4-D increased, induction rates of three varieties showed a decreasing trend within certain range. Induction rates of Zheli-49 and Zheli-51 decreased to 0 in 2.0 mg/L 2,4-D treatment. Different concentrations of KT and NAA were applied based on 2,4-D; results showed that their change trends of callus induction rates were similar like 2,4-D treatment.The average callus induction rates of three varieties in treatments Ⅱand Ⅵwere between 89% and 90%. Under the former treatment, all the three varieties had humid,milk white and loose calluses. However, the last three treatments produced dry, dark green and compact calluses (Fig.2). Therefore, in the induction and optimization tests of calluses,treatment Ⅱwas the most ideal culture medium according to the induction rate and callus status.
Results analysis of the optimization and proliferation of callus growth
Calluses were inoculated to culture mediums with different hormone treatments for 20 d. callus weight before and after inoculation were weighed,so as to find out the effects of hormone treatments on callus proliferation. Table 5 reported that the proliferation rates had significant differences among different varieties of calluses; the proliferation rates were between 52% and 268% . Proliferation rates of calluses were affected by both hormone combination of culture medium and the variety characteristics.Calluses of TEMUCO QUINOA TRADI TIONAL grew rapidly;proliferation rate was relatively high under different treatments, reaching 158%. Calluses of Zheli-51 grew rapidly slowly; the mean value of proliferation rate in all treatments was only 75%. The callus inducing ability of Zheli-51 was superior to Zheli-49; while the proliferation capacity of Zheli-51 was poorer than Zheli-49. The average proliferation rate of three varieties was 71% in treatment Ⅰ (MS + 0.5 mg/L 2,4 -D);the average proliferation rates enhanced in treatment Ⅱ(MS+0.5 mg/L 2,4-D+0.5 mg/LNAA)and Ⅲ(MS+0.5 mg/L 2,4-D + 0.5 mg/L KT). The proliferative effects of calluses of three varieties of C. quinoa in treatments Ⅳand Ⅴwere the most significant; the average value reached 161% and 134% , respectively. Callus proliferation rate of TEMUCO QUINOA TRADI TIONAL in treatment Ⅳ reached 268.3%, but the proliferation rates in treatment Ⅴdecreased to 180.8% .Therefore, increase of hormone concentration had certain inhibitory effects on its proliferation. And treatment Ⅳ(0.5 mg/L 2,4-D +0.5 mg/L KT + 0.5 mg/L NAA)was relatively suitable.
Conclusions and Discussions
Research on tissue culture of C. quinoa was of great significance to the conservation of germplasm resources, the breeding of new varieties and the research of genetic features[12].In this research, nine varieties of C. quinoa could all induce calluses,but there were certain differences in the sensibility of different cultivars under different cultivation conditions.Therefore, when establishing the callus cultivation system of C. quinoa,both common problems and genotype characteristics should be considered[13]. Callus could be induced from the cotyledons and stem segments of C. quinoa. And induction effect of the latter was superior to the former,which was consistent with the research results by An Gui-hua[14], Lu Jie[15], Mo Ying[16],Zhang Bo[17],Xiao Hexia[18]et al.Plant hormone was an important influencing factor regulating the callus induction and differentiation of plant organs[19-20]. 2, 4-D was a commonly used plant growth regulator in tissue culture, which mainly induced the formation of callus,promoted rooting and was widely applied in plant tissue culture[21]. Research results showed that the three varieties of C.quinoa had the maximum induction rate of calluses in 0.5 mg/L 2,4-D treatment.As the concentration of 2, 4-D increases, induction rate of calluses declined. Xing Xiao-ming et al. found out that when 2, 4-D concentration was between 0.10 and 1.00 mg/L, callus induction rate of the leaves of Stranvaesia davidiana Decne increased as the hormone concentration enhanced. High concentration of 2, 4-D showed significant inhibitory effects on callus induction[22]. Dai Liang et al. found out that under 3 mg/L 2, 4-D treatment, callus induction rate of 12 varieties of Poa pratensis reached 86%[24]. Therefore,different concentrations of 2, 4-D all had great impacts on callus induction.Zhu Zhu-ying et al. found out that in treatment of MS+2,4-D,induction rat e of banana leaves was as high as 100%. The induction rate of banana leaves reduced when adding other auxins or cytokinins such as NAA, KT and 6-BA[25]. Research results showed that treatment Ⅵ(MS+0.5 mg/L 2,4-D + 0.5 mg/L KT + 0.5 mg/LNAA) and treatment Ⅱ( MS + 0.5 mg/L 2, 4-D)had close callus induction rates of C. quinoa; but the morphological difference of calluses were relatively great.The latter had loose,glossy and yellowish white calluses. Therefore,culture medium MS + 0.5 mg/L 2, 4-D was the optimal for callus induction,which was different from the previous research results[26-27].This might be because that formation of different plant calluses needed different types of hormones. In the tissue culture system,the proliferation speed of calluses affected the efficiency of tissue culture system, especially in the transgene research[23]. Sun Rui-ming et al. found out that callus of Chimonocalamus delicatus had poorer degree of dependence on 2, 4-D in proliferation stage than in induction stage. But certain concentration should be maintained.KT and NAA at low concentration had good effects on the proliferation of Chimonocalamus delicatus calluses[28].Gao Li-li et al. found out that callus proliferation of indica type rice was not only related to the variety characteristics, but also the growth status and culture medium component of calluses[29]. This research results showed that in treatment Ⅳ (MS + 0.5 mg/L 2, 4-D+0.5 mg/L KT+0.5 mg/L NAA),the callus proliferation rates of three varieties of C.quinoa reached 90.8%-268.3%.The callus proliferation rate of TEMUCO QUINOA TRADI TIONAL was significantly higher than those of Zheli-51 and Zheli-49.
[1]ZHU JH(朱劍宏).Chemical composition and nutrition of quinoa(南美藜的化學(xué)組成和營(yíng)養(yǎng)價(jià)值)[J]. Journal of Chengdu University(成都大學(xué)學(xué)報(bào): 自然科學(xué)版),2002,21(2):24-28.
[2]VEGA-G LVEZ A,MIRANDA M,VERGARA J,et al. Nutrition facts and functional potential of quinoa(Chenopodium quinoa Willd.),an ancient Andean grain:a review[J]. Journal of the Science of Food and Agriculture, 2010, 90 (15):2541-2547.
[3]OSHODI A A,OGUNGBENLE H N,OLADIMEJI M O. Chemical composition,nutritionally valuable minerals and functional properties of benniseed (Sesamun radiatum),pearl millet(Pennisetum typhoides) and quinoa (Chenopodium quinoa) flours[J]. International Journal of Food Sciences & Nutrition, 1999, 50:325-331.
[4]COMAI S,BERTAZZO A,BAILONI L, et al.The content of proteic and nonproteic(free and protein-bound) tryptophan in quinoa and cereal flours [J]. Food Chemistry,2007,100(4):1350-1355.
[5]JACOBSEN S E,MUJICA A,JENSEN C R. The resistance of quinoa(Chenopodium quinoa Willd.) to adverse abiotic factors [J]. Food Reviews International,2003,19(1/2):99-109.
[6]KAMPOT TASHI(貢布扎西),WANGMO(旺姆),ZHANG CX (張崇璽)et al.Biological characteristics of quinoa in Tibet(南美藜在西藏的生物學(xué)特性表現(xiàn))[J].Southwest China Journal of Agricultural Sciences (西南農(nóng)業(yè)學(xué)報(bào)),1994,7 ( 3) :54-62.
[7]MENEGUETTI Q A,BRENZAN M A,BATISTA MR,et al.Biological effects of hydrolyzed quinoa extract from seeds of Chenopodium quinoa Willd.[J]. Journal of Medicinal Food, 2011, 14 (6): 653-657.
[8]ABUGOCH J L. Quinoa (Chenopodium quinoa Willd.): composition, chemistry,nutritional,and functional properties[J].Advances in Food and Nutrition Research,2009,58:1-31.
[9]OGUNGBENLE H N. Nutritional evaluation and functional properties of quinoa(Chenopodium quinoa)flour[J].Interna
tional Journal of Food Sciences and Nutrition,2003,54:153-158.
[10]RUIZ-CARRASCO K,ANTOGNONI F,COULIBALY A K, et al. Variation in salinity tolerance of four lowland genotypes of quinoa (Chenopodium quinoa Willd.) as assessed by growth,physiological traits,and sodium transporter gene expression [J]. Plant Physiology and Biochemistry,2011,49(11):1333-1341.
[11]HARIADI Y, MARANDON K, TIAN Y,et al. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.)plants grown at various salinity levels[J]. Journal of Experimental Botany,2011,62(1):185-193.
[12]JACOBSEN S E.The worldwide potential for quinoa (Chenopodium quinoa Willd.)potential[J]. Food Rev Int,2003,19:167-177.
[13]LAURAIN D, TR MOUILLAUXGUILLER J, CH NIEUX J C. Embryogenesis from microspores of Ginkgo biloba L.,a medicinal woody species[J]. Plant Cell Reports,1993,12(9):501-505.
[14]AN GH (安桂花),XU MZ (許明子),LI MS(李美善),et al.Effects of plant hormones on the callus induction of test tube seedlings of Rhodiola sachalinensis(植物激素對(duì)高山紅景天試管苗愈傷組織誘導(dǎo)的影響)[J].Journal of Agricultural Science Yanbian University(延邊大學(xué)農(nóng)學(xué)學(xué)報(bào)), 2007, 29(3):153-156.
[15]LU J (蘆婕),ZHANG XL (張曉麗),LIU W (劉雯),et al.Effects of explant and plant growth regulator on callus induction of Dioscorea zingiberensis(外植體和植物生長(zhǎng)調(diào)節(jié)劑對(duì)盾葉薯蕷愈傷組織誘導(dǎo)的影響)[J]. Hubei Agricultural Sciences (湖北農(nóng)業(yè)科學(xué)), 2013, 52(15):3693-3696.
[16]MO Y (莫英),LAN LQ (蘭利瓊),QING RW(卿人韋),et al.Research on seed germination condition of Dioscorea zingiberensis and induction explants callus (盾葉薯蕷種子萌發(fā)條件及誘導(dǎo)外植體愈傷的研究)[J]. Journal of Sichuan University (四川大學(xué)學(xué)報(bào): 自然科學(xué)版),2004,41(4):837-841.
[17]ZHANG B(張勃),QIN Y(秦彧),WANG LM (王黎明),et al.Callus induction of different explants of Medicago sativa cv "Derby"(紫花苜蓿品種“德寶”不同外植體愈傷組織誘導(dǎo)研究)[J]. Journal of Gansu Agricultural University (甘肅農(nóng)業(yè)大學(xué)學(xué)報(bào)),2012,47(4):100-104.
[18]XIAO HX (肖荷霞), WANG Y (王瑛),GAO F(高峰),et al.Effects of explants and hormones on the callus induction and differentiation of SANDITI Medicago sativa L.( 外植體及激素對(duì)SANDITI 紫花苜蓿愈傷組織誘導(dǎo)和分化的影響)[J]. Journal of Hebei Agricultural Sciences (河北農(nóng)業(yè)大學(xué)學(xué)報(bào)), 2003,26(4):47-52.
[19]WANG LY(王麗艷),XING RY(荊瑞勇),GUO YX (郭永霞), et al. Optimum combination of hormone concentration during callus subculture of soybean(大豆愈傷組織繼代培養(yǎng)中激素濃度組合的優(yōu)化)[J]. Chinese Journal of Oil Crop Sciences (中國(guó)油料作物學(xué)報(bào)),2013,35(4):446-450.
[20]WANG YY(王玉英),GAO XY(高新一).Plant tissue culture technology handbook(植物組織培養(yǎng)技術(shù)手冊(cè))[M].Beijing(北京):Golden Shield Press (金盾出版社),2006.
[21]CHEN Y(陳豫),HU W(胡偉),HE L(何磊). Effects of different concentrations of hormone on callus induction of carrot(不同濃度激素對(duì)胡蘿卜愈傷組織誘導(dǎo)的影響)[J]. Jiangsu Agricultural Sciences(江蘇農(nóng)業(yè)科學(xué)),2013,41(2):54-56.
[22]XING XM (邢小明),LIN XZ (林夏珍),WANG XY(王旭艷)et al.Callus induction of leaves of Stranvaesia davidiana(波葉紅果樹(shù)葉片愈傷組織誘導(dǎo)研究)[J]. Northern Horticulture (北方園藝),2013(12):108-110.
[23]DAI L(代亮),LIU YX(柳玉霞),LIU J(劉潔), et al. Callus induction system of 12 varieties of Poa pratensis (12 個(gè)草地早熟禾品種愈傷組織誘導(dǎo)體系的研究)[J].Journal of Yunnan University(云南大學(xué)學(xué)報(bào): 自然科學(xué)版), 2012, 34(6):722-730.
[24]XU MZ (許明子),JU HG (具紅光),LIU XH(劉憲虎),et al.Cultivar variation of callus increment and plant regeneration rate of paddy (水稻愈傷組織生長(zhǎng)量和植株再分化率的品種間差異)[J].Journal of Jilin Agricultural Sciences(吉林農(nóng)業(yè)科學(xué)),2000,25(2):29-2,49.
[25]ZHU ZY(朱祝英),ZHENG YL(鄭錦玲),YANG YM (楊玉梅), et al. Effects of plant growth regulator on callus induction of banana leaves in vitro (植物生長(zhǎng)調(diào)節(jié)劑對(duì)離體香蕉葉片愈傷組織誘導(dǎo)的影響)[J]. Guangdong Agricultural Sciences (廣東農(nóng)業(yè)科學(xué)), 2013, 40(11):29-32.
[26]ZHOU JM(周金梅),GONG JL(宮敬利),CHEN L (陳磊),et al.Callus induction technology of cooperation 918 tomato(合作918 番茄愈傷組織誘導(dǎo)技術(shù)研究)[J]. Journal of Jilin Agricultural Sciences(吉林農(nóng)業(yè)科學(xué)), 2013, 38(2):78-80.
[27]ZHANG CJ(張朝軍),FAN SL(范術(shù)麗),WU ZX (武芝霞),et al.Establishment of the petioles tissue culture system of cotton field plants (棉花大田植株葉柄組織培養(yǎng)體系的建立)[J].Acta Botanica Boreali-Occidentalia Sinica(西北植物學(xué)報(bào)),2011,31(6):1257-1263.
[28]SUN RM (孫瑞明),WANG J (王娟),CHEN F (陳芳),et al.Callus induction research of Chimonocalamus delicates (香竹愈傷組織誘導(dǎo)研究)[J].Journal of Fujian College of Forestry(福建林學(xué)院學(xué)報(bào)),2013,33(1):48-51.
[29]GAO LL (高麗麗),CHEN YL (陳遠(yuǎn)玲),JIAN YY (簡(jiǎn)玉瑜). Research on enhancing the induction rate and proliferation rate of callus of indica type rice(提高秈稻愈傷組織誘導(dǎo)率和增殖率的研究)[J]. Guangdong Agricultural Sciences(廣東農(nóng)業(yè)科學(xué)),2005(4):28-30.
 Agricultural Science & Technology2015年10期
Agricultural Science & Technology2015年10期
- Agricultural Science & Technology的其它文章
- Research Advances in Gene Regulation and Genetic Improvement of Fish Feeding
- Instrucions for Authors
- Cambridge Scientific Abstracts (CSA)
- Overview of Pharmaceutical Research on the Poria with Hostwood of Traditional Chinese Medicine
- Molecular Marker Assisted Selection for Fusarium Wilt Resistance Breeding in Watermelon(Citrullus lanatus)
- Study on Relative Soil and Water Conservation Benefits of Ridge Tillage in Different Terrain Conditions in the Black Soil Area of Northeast China
