Cloning, Expression and Activity Analysis of a Novel Fibrinolytic Serine Protease from Arenicola cristata
ZHAO Chunling, and JU Jiyu
?
Cloning, Expression and Activity Analysis of a Novel Fibrinolytic Serine Protease from
ZHAO Chunling1), and JU Jiyu2), *
1),,261053,2),,261053,
The full-length cDNA of a protease gene from a marine annelidwas amplified through rapid amplification of cDNA ends technique and sequenced. The size of the cDNA was 936 bp in length, including an open reading frame encoding a polypeptide of 270 amino acid residues. The deduced amino acid sequnce consisted of pro- and mature sequences. The protease belonged to the serine protease family because it contained the highly conserved sequence GDSGGP. This protease was novel as it showed a low amino acid sequence similarity (<40%) to other serine proteases. The gene encoding the active form ofserine protease was cloned and expressed in. Purified recombinant protease in a supernatant could dissolve an artificial fibrin plate with plasminogen-rich fibrin, whereas the plasminogen-free fibrin showed no clear zone caused by hydrolysis. This result suggested that the recombinant protease showed an indirect fibrinolytic activity of dissolving fibrin, and was probably a plasminogen activator. A rat model with venous thrombosis was established to demonstrate that the recombinant protease could also hydrolyze blood clot. Therefore, this recombinant protease may be used as a thrombolytic agent for thrombosis treatment. To our knowledge, this study is the first of reporting the fibrinolytic serine protease gene in.
;molecular cloning;serine protease;gene expression
1 Introduction
Thrombosis is one of the most frequent diseases that cause death and disability worldwide. Thrombolysis is a predominant and effective method of treating thrombotic diseases. As main clinical thrombolytic agents, fibrinolytic enzymes, such as urokinase and tissue plasminogen activators, have an important function in thrombolysis (Chen., 2013; Evim., 2013). These agents can catalyze the cleavage of fibrin, the main component of blood clots, and then dissolve them. However, once administered, these enzymes are degraded rapidly during blood circulation before they can exert their therapeutic effects. In addition, various side effects, such as immunoreactions and inflammation, appear (Merlini., 2004; Rosenschein., 1991). The search for new fibrinolytic enzymes with higher thrombolytic activity, longer half-life, and lower toxicity poses a significant challenge (Lijnen., 1995; Tang., 2002).
Fibrinolytic proteases are found in plants (Matsubara., 2000), animals (Chudzinski-Tavassi., 1998; Zhang., 1995), and microorganisms (Kotb., 2013; Huang., 2013). Certain serine proteases are endoproteases that may participate in different physiological functions, such as coagulation, cellular and humoral immunity, fibrinolysis, embryonic development, and digestion. Multiple proteases participating in the digestion process are referred to as trypsin (Almonte., 2011).
is a widely distributing polychaete annelid found in the eastern coastland of China. Waxman (1975) isolated the hemoglobin fromand characterized its structure. Parker and Lin (1987) isolated four proteases that activate cyclic AMP phosphodiesterase from lugworm, and reported the characterization and peptidase specificity of protease C. Wang. (2007) reported the anti-proliferation activity of arenicolsterol A, a novel cytotoxic enolic sulfated sterol from, on tumors. However, proteases fromwith fibrinolytic activity have not been cloned.
In the present study, we reported the cloning and sequencing of a novel protease gene namedfrom, as well as its expression in. The fibrinolytic activity of the recombinant AFE was also examined.
2 Materials and Methods
2.1, Bacterial Strains, and Vectors
was collected from BoHai Bay (Yantai, China) in September 2010.JM109 and plasmid pGEM-T (Promega, USA) were used in DNA manipulation.BL21 (DE3) (Invitrogen, USA) and plasmid pET-21a (+) were used for protein expression.was cultured in Luria broth (LB) medium with 100μgmL?1ampicillin.
2.2 Rapid Amplification of cDNA Ends (RACE) of AFE Gene
All primers used for cloning and expressingprotease gene were synthesized by SBS Genetech in Peking, China. The sequences of the primers are presented in Table 1. To obtain the full length of the protease gene, RACE was performed using cDNA amplification kits (Genebiotech, China).
The 3’-end flanking sequence ofcDNA was isolated through 3’-RACE. The gene-specific 3’-RACE primer (3F/3GSP) was designed based on the highly conserved amino acid sequence (GDSGGP) of the serine protease family. Total RNA was extracted fromusing Trizol reagent (Invitrogen, USA). As reverse transcription primer, 3’-RACE adaptor primer (3AP) was used to conduct the first-strand cDNA synthesis. Using cDNA first strand as templates, PCR amplification was performed using 3F (3GSP) as an upstream primer and 3R as a downstream primer. PCR amplification was carried out for 35 cycles. The initial denaturation was conducted at 94℃ for 5min, then 35 cycles of denaturation (94℃, 40s), annealing (60℃, 30s) and extension (72℃, 1min), followed by an extra extension (72℃, 10min).

Table 1 The PCR primers used in this study
The 5’ flanking sequence ofcDNA was cloned5’-RACE. The gene-specific 5’-RACE primer (5R1/5GSP1, 5R2/5GSP2) was designed according to the obtained sequence of 3’-RACE fragment. As reverse transcription primer, oligodT (5RP) was used to conduct the first-strand cDNA synthesis. The reverse transcription product was purified, and tailed with dCTP and TdT. The dC-tailed cDNA was amplified through nested PCR using 5F1 (5AP) as the adaptor primer, 5F2 as forward primers, and 5R1 (5GSP1) and 5R2 (5GSP2) as reverse primers. Two rounds of PCR amplifications were performed as follows: denaturation at 94℃ for 5min, followed by 35 cycles of denaturation at 94℃ for 40s, annealing at 60℃ for 30s and extension at 72℃ for 1min, and an extra extension at 72℃ for 10min. All PCR products were subcloned into pGEM-T, transferred intoJM109 and sequenced.
2.3 Analysis of Nucleotide and Amino Acid Sequences
To find nucleotide and amino acid sequences similar toprotease, the NCBI BLAST program (http:// www.ncbi.nlm.nih.gov) was used. The signal peptide was predicted by Signal P 3.0 program (http://www.cbs.dtu. dk/services/Signal P). The alignment of amino acid sequences was obtained using DNAMAN software.
2.4 Nucleotide Sequence Accession Number
The complete cDNA sequence offibrino- lytic enzyme gene has been submitted to GenBank database under accession no. JX974353.
2.5 Phylogenetic Analysis
Multiple alignment ofwas conducted with known sequences using clustal X 1.81 (Thompson., 1997). The sequences aligned included those of other annelid animals, namely,,,,, and. The phylogenetic tree was constructed with neigh- bor-joining method (Saitou and Nei, 1987) and MEGA 4.0 software (Tamura., 2007) with 1000 bootstrap replicates.
2.6 Expression and Purification of a Recombinant
UsingcDNA obtained by the protein reverse transcription primer (PRP/oligodT) as a template, PF and PR (Table 1, restriction enzymeandsites are underlined) were used as PCR amplification for the activation peptide of. The amplified DNA fragment was digested withandand ligated to pET-21a (+), which was then digested withand. The re- constructed plasmid was transferred intoBL21 and subsequently confirmedsequencing.
BL21 containing the recombinant plasmid was cultured on LB agar plate containing ampicillin (100μgmL?1) overnight. A single colony was transferred into 10mL LB broth and allowed to grow at 37℃ with shaking overnight. The expandedwere inoculated in 500mL LB broth containing ampicillin (100μgmL?1) and allowed to grow at 37℃ until an optical density of 1.2 at 600nm was reached. Expression of the protease was then induced by the addition of 1.0mmolL?1of isopropyl- β-D-thiongalactopyranoside (IPTG; INALCO, USA). The cells were incubated at 30℃ for another 4h. Cells were centrifuged at 8000×g and 4℃ for 10min, and then resuspended in lysis buffer (20mmolL?1Tris-Cl pH 7.4, 10mmolL?1imidazole, 0.5molL?1sodium chloride) and disrupted by sonication. The insoluble proteins were separated and resuspended in the lysis buffer. The supernatants and the insoluble protein precipitate were used for SDS-PAGE. The protease was purified from the supernatant by adding 1mL of 50% Ni-NTA slurry (QIAGEN, China) pre-equilibrated in lysis buffer to 4mL of the lysate. After incubation at 4℃ for 1h, the lysate-Ni-NTA mixture was loaded onto a column. Unbound proteins were removed by washing the column with two column volumes of lysis buffer. The column was washed twice with 5mL of 20mmolL?1Tris-Cl (pH 7.4) containing 20 mmolL?1imidazole and 0.5molL?1sodium chloride. The recombinant protein was eluted with four column volumes of elution buffer, and the fractions were analyzed by SDS-PAGE.
2.7Fibrinolytic Activity Assay of the Purified Recombinant Protease
The fibrin plates included plasminogen-free fibrin and plasminogen-rich fibrin. Fibrinolytic activity was measured using the standard fibrin plates through the procedure described previously (Cho., 2004). Twenty microliters of purified recombinant protein solution (1mgmL?1) was spotted onto the fibrin plates and incubated at 37℃ for 16h. The hydrolyzed clear zone was then measured.
2.8Fibrinolytic Activity Assay of Purified Recombinant Protease
A venous thrombosis rat model (Kumada., 1980) was used with several modifications to evaluate thefibrinolytic activity of purified recombinant protease. Eight rats were anaesthetized with ketamine and laparotomized to expose the inferior vena cava. Then, a stainless steel wire coil was placed into the lumen of the vena cava with 15mm depth through the renal vein branching. The freeze-dried recombinant protease was dissolved in 50mmolL?1phosphate buffer solution (pH 7.4). The protease (at a dose of 25, 50mg(10mL)?1(kg body wt)?1d?1) was administered orally for 6d. The eight rats in the control group were only treated with 10mL PBS for 6d. One hour after the final administration, the rats were laparotomized under ketamine anesthesia. Immediately after clamping the vena cava, the wire coil with its thrombus was removed carefully. The weight of the thrombus on the wire coil was measured as total protein. Three independent experiments were performed.
2.9 Statistical Analysis
Statistical analysis was performed using SPSS12.0 program. Experimental results were presented as mean ± standard deviation (SD), and evaluated statistically using Student’stest.value less than 0.05 was considered significant.
3 Results
3.1 Cloning and Sequence Analysis ofGene
The full-length cDNA ofgene was obtained through RACE (Table 1 and Fig.1). The primers for reverse transcription were designed based on poly (A) sequence provided by the RACE kit. The specific primers for 3’-RACE and 5’-RACE were designed based on the highly conserved amino acid sequence (GDSGGP) of the serine protease family and the obtained 3’-RACE fragment, respectively. After subcloning the fragments and analyzing the nucleotide sequences of the clones, two kinds of cDNA fragments were obtained and spliced. The full-length cDNA was deposited in GenBank under accession number JX974353.
The cDNA ofwas generated with a length of 936 bp encoding 270 amino acid residues. The complete nucleotide sequence including the poly (A) sequence and the deduced amino acid sequence is shown in Fig.2. In the sequence of 936 nuleotides, an open reading frame from start codon ATG at position 40 to a stop codon at position 850 encoded a polypeptide of 270 amino acid residues. The putative polyadenylation signal AATAAA was found at position 897 to 902.
The sequence ofgene was aligned with other known sequences of protease genes using nucleotide- nucleotide BLAST (blastn) at http://www.ncbi.nlm.nih. gov/Blast. No significant similarity was found betweengene and those retrieved from nucleotide database.
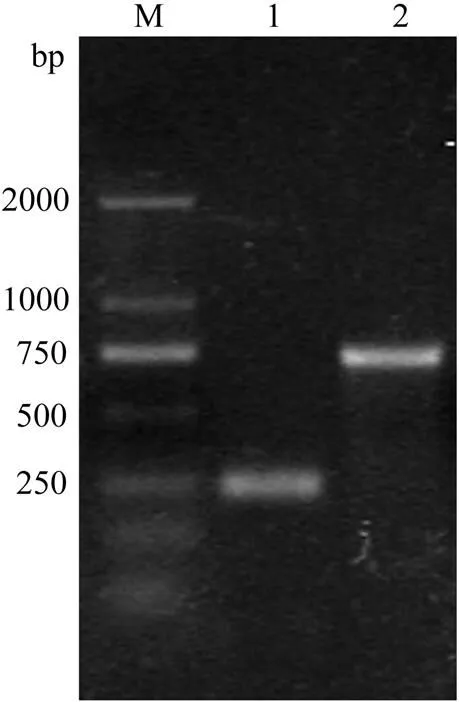
Fig.1 PCR products of Arenicola cristata protease gene cloned through RACE. M, DNA marker; 1, product of 3’-RACE; 2, product of 5’-RACE.
3.2 Amino Acid Sequence Similarity of
The deduced amino acid residues ofgene contained a highly conserved sequence at 223 to 228 amino acid residues (GDSGGP) (Fig.2). Thegene encoded a putative enzyme that consisted of a putative catalytic domain with an active site formed by three residues of His83, Asp129 and Ser225. The primary substrate speci-ficity determinant was situated at Asp217. Based on the analysis of the conserved sequence with other proteases by BLASTp,could be assigned to a trypsin-like serine proteases family. Trypsin-like serine proteases are usually synthesized as preproenzymes that contain an N- terminal signal peptide (11 to 15 amino acid residues) and have a highly conserved N-terminal amino acid sequence (Ile-Val-Gly-Gly) in active form (Wang., 1995). Signal P 3.0 program analysis revealed thatcontained a putative signal peptide of 15 amino acids MKFLLLSALVALASA and had a highly conserved N- terminal amino acid sequence (Ile-Val-Gly-Gly). These showed thatwas translated as a preproenzyme that contained the signal peptide and was changed to the active form through modification after translation.

Fig.2Nucleotide and deduced amino acid sequences of cDNA encoding the serine protease from. The nucleotide and amino acids are numbered from the 5’ end of the cDNA and from the N-terminal Met of the native enzyme, respectively. A stop codon is indicated with an asterisk, and a putative polyadenylation signal (AATAAA) is italicized and underlined. The computed signal peptide sequence is double-underlined. The conservative catalytic triad (histidine, aspartic acid and serine) is indicated in italic and boldface. Underlined and boldfaced sequence represents conserved residues of the serine protease family.
Although the sequence around the catalytic site ofshowed significant homology with that of serine protease family and contained the conserved sequence GDSGGP, the amino acid sequence ofhad no significant homology with that of other annelid animals, such as earthworm and(Fig.3). Phylogenetic analysis of the serine proteins from different annelid animals confirmed this result (Fig.4). Comparison of the deduced peptide sequence ofgene with other serine proteases revealed an identity of less than 40%. Compared with serine proteases from annelid creatures,revealed an identity of 40%, 37%, 39% and 36% to(ABW 04905.1),(AAQ13829.1),(ACL 12061.1) and(ADL28819.1), respectively. Therefore, thegene obtained in this study is novel, and the protease may be a new member of the serine protease family.
3.3 Fibrinolytic Activity of Recombinant Protease
The 711 bp cDNA fragment amplified by PCR encoded the active form ofat position Ile34 to Ile270 (Fig.1). The fragment was inserted into the prokaryotic expression vector pET-21a (+). The recombinant protease was expressed with His tag and was analyzed with SDS-PAGE (Fig.5). The expressed protein was mainly in the inclusion body, and could be found in certain soluble proteins. The soluble proteins in the supernatant was purified with His·Band column protein purification system. Electrophoresis of the purified product on SDS-PAGE showed a band with a molecular weight of approximately 26 kDa, which was basically consistent with that calculated from the amino acid sequence. Thus, the mature protein was not modified in.
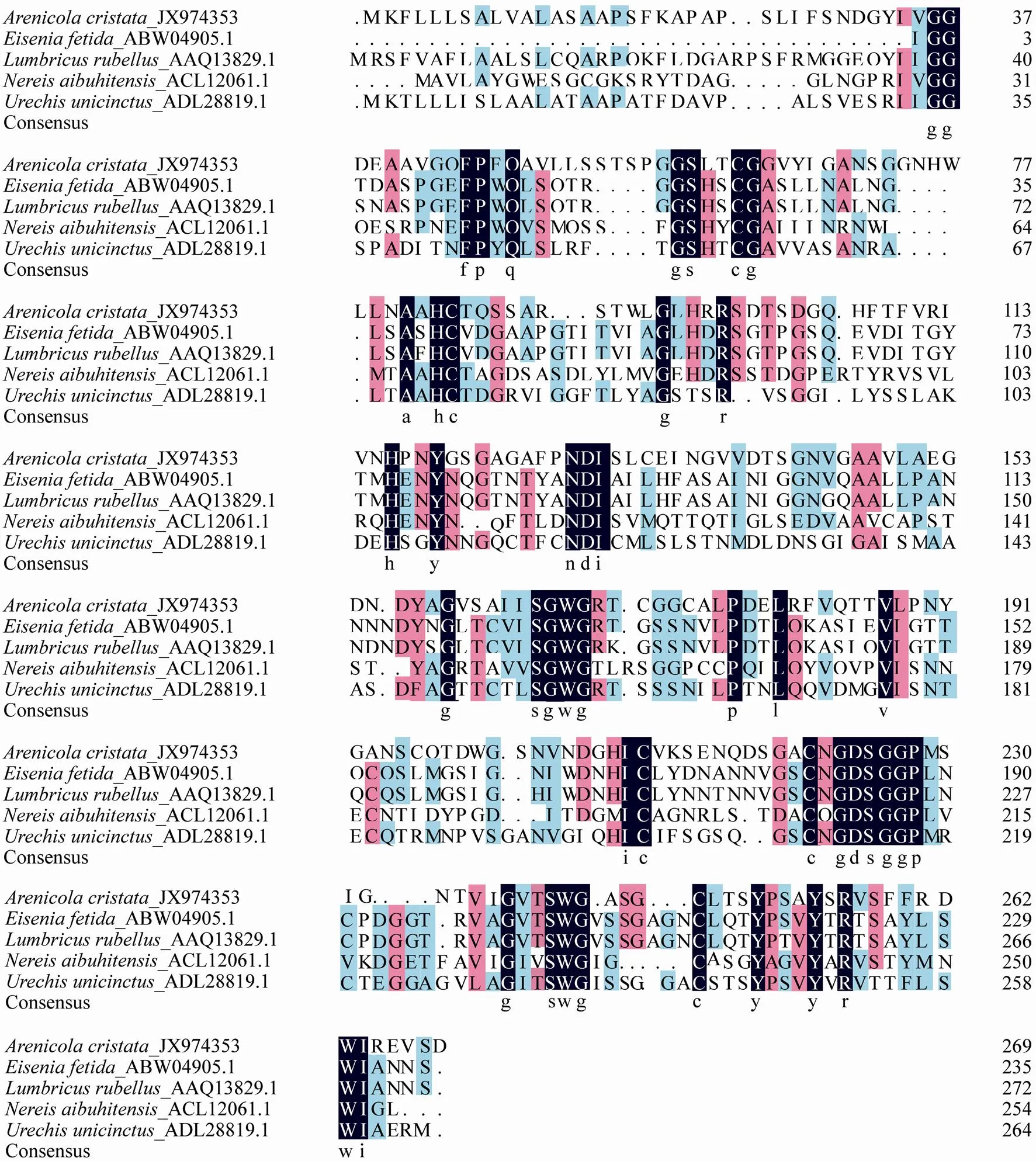
Fig.3Alignment of the deduced amino acid sequence of the protease from Arenicola cristata with other proteases from annelid animals including Eisenia fetida (ABW04905.1), Lumbricus rubellus (AAQ13829.1), Nereis aibuhitensis (ACL12061.1), and Urechis unicinctus (ADL28819.1). The conserved amino acids are shaded in black, and the consensuses are shown.
The fibrinolytic activity of the purified recombinant protease was analyzed using artificial fibrin plates (Fig.6). The fibrin plates included plasminogen-free fibrin and plasminogen-rich fibrin. On the plasminogen-free fibrin, no hydrolyzed clear zones appeared, whereas on the plas- minogen-rich fibrin, the lytic areas were found for the purified recombinant protease and the positive control urokinase. These results indicated that the purified re- combinant protease fromcould hydrolyze the plasminogen-rich fibrin, which showed indirect fibrinolytic activity that can dissolve fibrin. With the same concen- tration, the recombinantshowed higher fibrinolytic activity than urokinase because the diameter of clear zone from recombinantwas approximately two times longer than that of urokinase (Table 2). Infibri- nolytic activity analysis (Table 3), the low concentration of recombinantonly hydrolyzed 23% of the blood clot on stainless wire coil in rats when administrated orally. The thrombus weight was reduced to 59% by higher concentration of recombinantthe control. Therefore, the recombinantshowed significant fibrinolytic activityand, which can be used in the treatment of thrombosis.
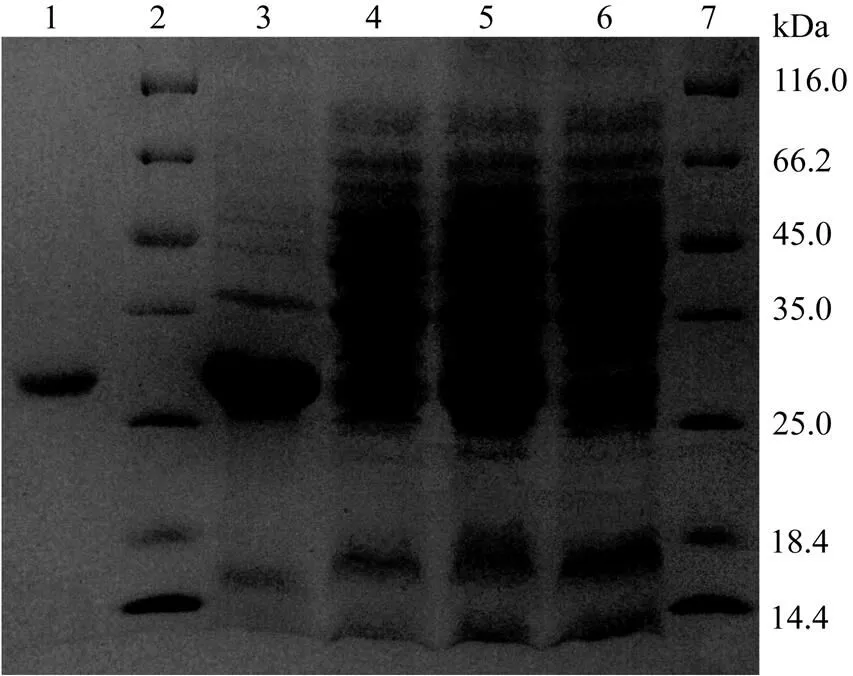
Fig.5SDS-PAGE of the recombinant protease. Lane 1, purified recombinant protein; Lane 2 and 7, protein marker; Lane 3 and 4, precipitate and supernatant from E. coli induced by IPTG; Lane 5, induced E. coli by IPTG; Lane 6, proteins from E. coli.
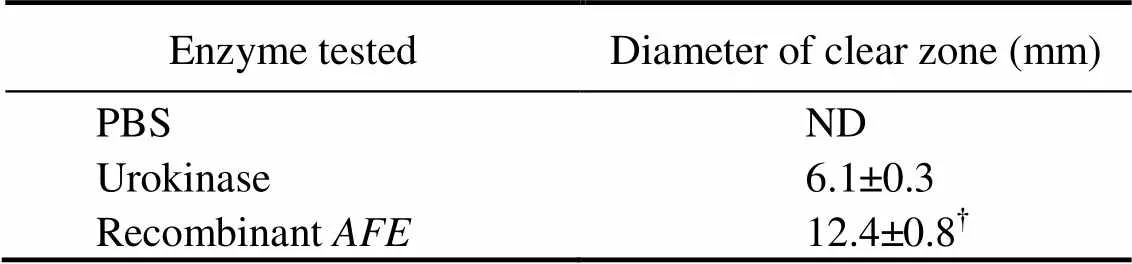
Table 2 In vitro fibrinolytic activity of recombinant AFE on plasminogen-rich fibrin plate
Notes: ND, not detected;?<0.05urokinase. Data are represented as mean ± SD (=5).
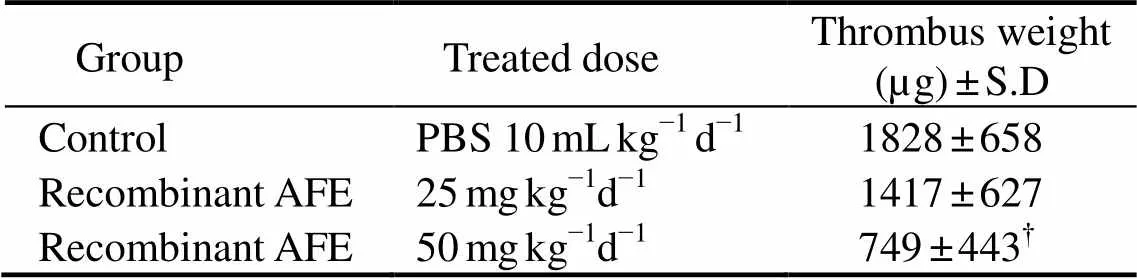
Table 3 Thrombus weight after oral administration of recom- binant AFE on the rat model of venous thrombosis
Note:?<0.05control.
4 Discussion
is classified as an annelid with a shape similar to that of another annelid, which is the earthworm. A number of serine proteases with fibrinolytic activity are reportedly isolated and purified from numerous annelid species. To date, earthworm proteases have been used as orally administered fibrinolytic agents to prevent and treat thrombosis diseases, such as myocardial infarction and cerebral thrombus (Jin., 2000). Earthworm proteases are a mixture of six isoenzymes with molecular weights from 2 kDa to 32 kDa, which can dissolve plasminogen- rich fibrin and plasminogen-free fibrin (Cho., 2004; Han., 2011; Nakajima., 1993;Mihara., 1991; Li., 2003; Hrzenjak., 1998). Currently, the sequences of the isolated isoenzyme genes ofandhave been reported by several investigators (Cho., 2004; Ge., 2005; Sugimoto., 2001; Dong., 2004; Wang., 2011). In addition, serine proteases from other annelid animals, namely,(Zhang., 2007; Wang., 2011) and(Bi., 2013; Bi., 2013), also display fibrinolytic activity. Bothandcontain different isoenzymes. Therefore, as a marine annelid,also possibly contains proteases with fibrinolytic activity and may be a new medical source for the prevention and treatment of thrombosis.
As shown in Figs.1 and 2, we succeeded in cloning and determining the full-length cDNA sequence of the protease genefromThe determined cDNA ofincluded open reading frames that encode a polypeptides of 270 amino acid residues. No significant similarity was found between thegene and those in nucleotide database. Based on sequence analysis, the deduced amino acid residues consisted of pro- and mature sequences.was classified as a novel gene, as determined by the low amino acid sequence homology (less than 40%) with other serine proteases. Theprotease also showed low similarity to the proteases from other annelid animals, such as,,,,and(Figs.3 and 4).
The gene encoding the mature form ofserine protease was expressed into produce a soluble protease and inclusion body (Fig.5). The purified recombinant protease in the supernatant could only hydrolyze the plasminogen-rich fibrin, which showed an indirect fibrinolytic activity in dissolving fibrin and a higher activity than urokinase (Fig.6, Table 2). The treatment result in a rat model with venous thrombosis demonstrated that the recombinant protease could hydrolyze blood clots(Table 3). Currently used thrombolytic agents, such as t-PA, u-PA, urokinase, and SK, promote rapid dissolution of thrombi by activating the human body’s natural fibrinolytic system and generating plasmin from plasminogen (Collen., 1991). Therefore, the activity analysis of the recombinant protease suggested that the protease can act as a plasminogen activator and can be used for thrombotic disease treatments. The possible roles ofin thrombolysis therapy and its physicochemical characteristics need further studies. More fibrinolytic proteases fromare expected to be found, and related research is underway in our laboratory.
In summary, a novel serine protease with fibrinolytic activity was cloned fromand expressed in.This protease may act as a plasminogen activatorfor thrombotic diseases treatments.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 30901779) and the Natural Science Foundation of Shandong Province, China (No. ZR2009CM019).
Almonte, A. G., and Sweatt, J. D., 2011. Serine proteases, serine protease inhibitors, and protease-activated receptors: roles in synaptic function and behavior., 1407: 107- 122.
Bi, Q., Han, B., Feng, Y., Jiang, Z., Yang, Y., and Liu, W., 2013. Antithrombotic effects of a newly purified fibrinolytic protease from., 132 (2): e135-144.
Bi, Q., Chu, J., Feng, Y., Jiang, Z., Han, B., and Liu, W., 2013. Purification and characterization of a new serine protease with fibrinolytic activity from the marine invertebrate,., 170 (3): 525-540.
Chen, G., Liu, Y., Xie, Y., Li, J., Liu, H., Sun, L., Peng, Y., and Liu, F., 2013. High dose urokinase against massive pulmonary embolism in nephrotic syndrome., 24 (4): 439-443.
Cho, I. H., Choi, E. S., Lim, H. G., and Lee, H. H., 2004. Purification and characterization of six fibrinolytic serine-prote- ases from earthworm., 37 (2): 199-205.
Chudzinski-Tavassi, A. M., Kelen, E. M., de Paula Rosa, A. P., Loyau, S., Sampaio, C. A., Bon, C., and Angles-Cano, E., 1998. Fibrino(geno)lytic properties of purified hementerin, a metalloproteinase from the leech., 80 (1): 155-160.
Collen, D., and Lijnen, H. R., 1991. Basic and clinical aspects thrombolysis., 78 (12): 3114-3124.
Dong, G. Q., Yuan, X. L., Shan, Y. J., Zhao, Z. H., Chen, J. P., and Cong, Y. W., 2004. Molecular cloning and characterization of cDNA encoding fibrinolytic enzyme-3 from earthworm., 36 (4): 303-308.
Evim, M. S., Bostan, ?., Baytan, B., Semizel, E., and Günes, A. M., 2013. Thrombolysis with recombinant tissue plasminogen activator in 7 children., 19 (5): 574-577.
Ge, T., Sun, Z. J., Fu, S. H., and Liang, G. D., 2005. Cloning of thrombolytic enzyme (lumbrokinase) from earthworm and its expression in the yeast., 42 (1): 20-28.
Han, T. T., Ta, T. D., Nguyen, D. T., Van Den Broek, L. A., and Duong, G. T., 2011. Purification and characterization of novel fibrinolytic proteases as potential antithrombotic agents from earthworm., 1 (1): 26-36.
Hrzenjak, T., Popovic, M., Bozic, T., Grdisa, M., Kobrehel, D., and Tiska-Rudman, L., 1998. Fibrinolytic and anticoagulative activities from the earthworm., 119 (4): 825-832.
Huang, S., Pan, S., Chen, G., Huang, S., Zhang, Z., Li, Y., and Liang, Z., 2013. Biochemical characteristics of a fibrinolytic enzyme purified from a marine bacterium,HQS-3., 62C: 124-130.
Jin, L., Jin, H., Zhang, G., and Xu, G., 2000. Changes in coagulation and tissue plasminogen activator after the treatment of cerebral infarction with lumbrokinase., 23 (2-4): 213-218.
Kotb, E., 2013. Activity assessment of microbial fibrinolytic enzymes., 97 (15): 6647-6665.
Lijnen, H. R., and Collen, D., 1995. Fibrinolytic agents: Mechanisms of activity and Pharmacology., 74 (1): 387-390.
Li, L., Zhao, J., and He, R. Q., 2003. Isolation and some characterizations of a glycosylated fibrinolytic enzyme of earthworm,., 10 (2): 183-190.
Matsubara. K., Hori, K., Matsuura, Y., and Miyazama, K., 2000. Purification and characterization of a fibrinolytic enzyme and identification of fibrinogen clotting in a marine green alga,., 125 (1): 137-143.
Merlini, P. A., Cugno, M., Rossi, M. L., Agricola, P., Repetto, A., Fetiveau, R., Diotallevi, P., Canosi, U., Mannucci, P. M., and Ardissino, D., 2004. Activation of the contact system and inflammation after thrombolytic therapy in patients with acute myocardial infarction., 93 (7): 822-825.
Mihara, H., Sumi, H., Yoneta, T., Mizumoto, H., Ikeda, R., Seiki, M., and Maruyama, M., 1991. A novel fibrinolytic enzyme extracted from the earthworm,., 41 (3): 461-472.
Nakajima, N., Mihara, H., and Sumi, H., 1993. Characterization of potent fibrinolytic enzymes in earthworm., 57 (10): 1726-1730.
Parker, G. R., and Lin, Y. M., 1987. Isolation from lugworm () of four proteases that activate cyclic AMP phosphodiesterase., 88 (1): 349- 357.
Rosenschein, U., Lenz, R., Radnay, J., Ben Tovim, T., and Rozenszajn, L. A., 1991. Streptokinase immunogenicity in thrombolytic therapy for acute myocardial infarction., 27 (10): 541-545.
Saitou, N., and Nei, M., 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees., 4: 406-425.
Sugimoto, M., and Nakajima, N., 2001. Molecular cloning, sequencing, and expression of cDNA encoding serine protease with fibrinolytic activity from earthworm., 65 (7): 1575-1580.
Tamura, K., Dudley, J., Nei, M., and Kumar, S., 2007. MEGA 4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0., 24: 1596- 1599.
Tang, Y., Liang, D., Jiang, T., Zhang, J., Gui, L., and Chang, W., 2002. Crystal structure of earthworm fibrinolytic enzyme component A: Revealing the structural determinants of its dual fibrinolytic activity., 321 (1): 57-68.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G., 1997. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools., 25 (24): 4876-4882.
Wang, K., Gan, L., Lee, I., and Hood, L., 1995. Isolation and characterization of the chicken trypsinogen gene family., 307 (Pt 2): 471-479.
Wang, L., Chen, B., Shen, X. R., Zhou, Y. Y., Jiang, D. W., Li, J., and Kong, J. L., 2007. Growth inhibition and induction of early apoptosis by arenicolsterol A, a novel cytotoxic enolic sulphated sterol from the marine annelid,., 9 (6-8): 753- 761.
Wang, S. H., Li, Q., Deng, Z. H., Ji, X., Jiang, X., Ge, X., Bo, Q. Q., Cui, J. Y., Zhang, L. Z., Liu, J. K., and Hong, M., 2011.(Iznka) fibrinolytic enzyme reduced cerebral infarction, cerebral edema and increased antioxidation in rat models of focal cerebral ischemia., 489 (1): 16-19.
Wang, X., Chang, L., and Sun, Z., 2011. Differential expression of genes in the earthwormfollowing exposure toO157:H7., 35 (5): 525-529.
Waxman, L., 1975. The structure of annelid and mollusc hemoglobins., 250 (10): 3790- 3795.
Zhang, Y., Cui, J., Zhang, R., Wang, Y., and Hong, M., 2007. A novel fibrinolytic serine protease from the polychaete(Neanthes)(Sars): Purification and characterization., 89 (1): 93-103.
Zhang, Y., Wisner, A., Xiong, Y., and Bon, C., 1995. A novel plasminogen activator from snake venom. Purification, characterization and molecular cloning., 270 (17): 10246-10255.
(Edited by Qiu Yantao)
10.1007/s11802-015-2488-1
(November 2, 2013; revised January 19, 2014; accepted July 10, 2014)
. Tel: 0086-536-8462053 E-mail: juimmu@163.com
ISSN 1672-5182, 2015 14 (3): 533-540
? Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
 Journal of Ocean University of China2015年3期
Journal of Ocean University of China2015年3期
- Journal of Ocean University of China的其它文章
- Research on China’s Aquaculture Efficiency Evaluation and Influencing Factors with Undesirable Outputs
- Sustainability Evaluation of Different Systems for Sea Cucumber (Apostichopus japonicus) Farming Based on Emergy Theory
- Tolerance, Oxygen Consumption and Ammonia Excretion of Ophiopholis sarsii vadicola in Different Temperatures and Salinities
- Effect of Shrimp (Litopenaeus vannamei) Farming Waste on the Growth, Digestion, Ammonium-Nitrogen Excretion of Sea Cucumber (Stichopus monotuberculatus)
- Larval and Juvenile Growth Performance of Manila Clam Hybrids of Two Full-Sib Families
- Proline with or without Hydroxyproline Influences Collagen Concentration and Regulates Prolyl 4-Hydroxylase α (I) Gene Expression in Juvenile Turbot (Scophthalmus maximus L.)
