Transplantation of human telomerase reverse transcriptase gene-transfected Schwann cells for repairing spinal cord injury
Shu-quan Zhang, Min-fei Wu, Jia-bei Liu, Ye Li, Qing-san Zhu, Rui Gu,
1 Department of Orthopedics, Tianjin Nankai Hospital, Tianjin, China
2 Department of Spine Surgery, Orthopedic Hospital, Second Hospital, Clinical Hospital, Jilin University, Changchun, Jilin Province, China
3 Department of Orthopedics, China-Japan Union Hospital, Jilin University, Changchun, Jilin Province, China
Transplantation of human telomerase reverse transcriptase gene-transfected Schwann cells for repairing spinal cord injury
Shu-quan Zhang1, Min-fei Wu2, Jia-bei Liu3, Ye Li3, Qing-san Zhu3, Rui Gu3,*
1 Department of Orthopedics, Tianjin Nankai Hospital, Tianjin, China
2 Department of Spine Surgery, Orthopedic Hospital, Second Hospital, Clinical Hospital, Jilin University, Changchun, Jilin Province, China
3 Department of Orthopedics, China-Japan Union Hospital, Jilin University, Changchun, Jilin Province, China
Transfection of the human telomerase reverse transcriptase (hTERT) gene has been shown to increase cell proliferation and enhance tissue repair. In the present study, hTERT was transfected into rat Schwann cells. A rat model of acute spinal cord injury was established by the modif ed free-falling method. Retrovirus PLXSN was injected at the site of spinal cord injury as a vector to mediate hTERT gene-transfected Schwann cells (1 × 1010/L; 10 μL) or Schwann cells (1 × 1010/L; 10 μL) without hTERT gene transfection. Between 1 and 4 weeks after model establishment, motor function of the lower limb improved in the hTERT-transfected group compared with the group with non-transfected Schwann cells. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling and reverse transcription-polymerase chain reaction results revealed that the number of apoptotic cells, and gene expression of aquaporin 4/9 and matrix metalloproteinase 9/2 decreased at the site of injury in both groups; however, the ef ect improved in the hTERT-transfected group compared with the Schwann cells without hTERT transfection group. Hematoxylin and eosin staining, PKH26 f uorescent labeling, and electrophysiological testing demonstrated that compared with the non-transfected group, spinal cord cavity and motor and sensory evoked potential latencies were reduced, while the number of PKH26-positive cells and the motor and sensory evoked potential amplitude increased at the site of injury in the hTERT-transfected group. These f ndings suggest that transplantation of hTERT gene-transfected Schwann cells repairs the structure and function of the injured spinal cord.
nerve regeneration; spinal cord injury; Schwann cells; transplantation; motor function; telomerase; reverse transcriptase; proliferation; modif cation; cells; neural regeneration
Funding: This study was supported by a grant from the Science and Technology Development Plan Program of Jilin Province of China, No. 2011084.
Zhang SQ, Wu MF, Liu JB, Li Y, Zhu QS, Gu R (2015) Transplantation of human telomerase reverse transcriptase gene-transfected Schwann cells for repairing spinal cord injury. Neural Regen Res 10(12):2040-2047.
Introduction
The regeneration of injured axons after spinal cord injury (SCI) can be promoted by changing the microenvironment of regenerating nerves (Ban et al., 2009; Allodi et al., 2014). Schwann cells are the myelin-forming cells of peripheral nerve f bers and thus, are the main structural and functional cells of these nerves (Chen et al., 2014; Deng et al., 2014; Wu et al., 2015). Schwann cells play a major role after SCI. Numerous studies have shown that Schwann cell transplantation contributes to the functional recovery of the spinal cord (Kang et al., 2004; Dong et al., 2013; Hakim et al., 2015).
Human telomerase reverse transcriptase (hTERT) is a key nutritional factor, which is not expressed in most normal tissues; however, in primary tumor and carcinoma cell lines, hTERT is highly expressed (Marcol et al., 2015; Peterson et al., 2015; Sparling et al., 2015). hTERT has multiple biological ef ects and an important clinical value. After a local application, hTERT is diluted and metabolized rapidly; therefore, it should be administered repeatedly in large dosages (Siddiqui et al., 2015; Thumm et al., 2015; Vasudeva et al., 2015).
pLXSN is a commonly used retrovirus vector (Liu and Wang, 2015), which was used in the current study to transfect rat Schwann cells with the hTERT gene. The aim of the study was to investigate the ef ect of transplanting hTERT gene-modif ed Schwann cells on the electrophysiology of these cells in SCI rats.
Materials and Methods
Experimental animals
Eighty-five healthy, inbred-line female, 1-month-old Sprague-Dawley rats, weighing 200–250 g were purchasedfrom the Animal Laboratory of Chinese Academy of Medical Sciences, China (animal certif cate No. SCXK(Jin)20050076). Experiments were approved by the Animal Ethics Committee of the Department of Orthopedics of Tianjin Nankai Hospital of China.
Culture and identif cation of Schwann cells
The sciatic nerve was aseptically stripped under a microscope from two rats. The tissue was digested with 0.25% trypsin/0.2% collagenase for 40 minutes, and then centrifuged at 300 × g for 5 minutes. The cells were incubated in Dulbecco’s Modif ed Eagle’s medium (DMEM)/F12 medium containing 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) at 37°C in a 5% CO2incubator for 30 minutes. Fibroblasts were then removed by dif erential adherence. Any remaining f broblasts were killed 24 hours later by adding 100 μL cytarabine (10–5mM; Gibco). The fourth passage of Schwann cells was incubated on coverslips for 48 hours, then washed three times with phosphate-buf ered saline (PBS), f xed with 4% paraformaldehyde (pH 7.4) at room temperature (20 minutes), and then washed three times with PBS. These cells were incubated with rat anti-myelin basic protein monoclonal antibody (1:1,000; Amyjet Scientif c Inc., Wuhan, China) in a wet box at 4°C overnight, and then washed three times with PBS followed by incubation with goat anti-rat IgG (1:1,000) at 37°C for 2 hours. The cells were then treated with 4′,6-diamidino-2-phenylindole (DAPI) for 10 minutes and then washed three times with PBS. The samples were mounted with mounting medium. Schwann cells were digested with 0.25% trypsin (Gibco) and the single-cell suspension was obtained. Schwann cells (2 × 107cells/mL) were washed once with serum-free DMEM/F12 medium and resuspended with 0.5 mL of diluent C. All protocols were conducted in accordance with PKH26 dye instructions (Sigma-Aldrich, St. Louis, MO, USA). Immediately after labeling, 1 × 105cells were collected and washed once with PBS, then f xed with 1% paraformaldehyde. Cell proliferation rate was detected using f ow cytometry (BIOS biological, Wuhan, China). Cell characteristics were observed with a f uorescence microscope (Olympus IX71; Olympus, Tokyo, Japan) 24 hours after the culture. Green f uorescence revealed the bodies and processes of normal Schwann cells. For nestin immunof uorescence staining, the most immunoreactive materials were f ne particles and partial immunoreactive materials were coarse particles. Furthermore, some particles were scattered or clustered, while some were f lamentous of dif erent sizes. Nestin was mainly present in the cytoplasm and seldom present in cell processes.
hTERT transfection and the western immunoblot assay
The fourth passage of Schwann cells was incubated in DMEM containing 10% fetal bovine serum at saturated humidity with 5% CO2and at 37°C. The cells were subcultured once every 3 days. Schwann cells (6 × 104/well) in the logarithmic phase were seeded in a 24-well plate for 3 days. For the PLXSN-hTERT transfection, the medium was discarded and the cells were washed twice with PBS. In accordance with multiple of infection = 105, PLXSN-hTERT diluted with serum-free L-DMEM including 10 mM nicotinamide + 1 mM b-mercaptoethanol + 200 mL/L fetal calf serum was added to the cell culture f ask and incubated at 37°C for 2 hours. Cells were then incubated with fetal bovine serum and L-DMEM medium for 1 week. The cell culture medium was not replenished 3 days before detection. Under the same conditions, the EV group was exposed to the empty virus (EV) transfection.
Three groups were designed in the in vitro experiment: Schwann cells without hTERT transfection (SCs group), EV, and hTERT-transfected group (n = 6 per group). The cell suspension from each group was centrifuged at 800 r/min for 5 minutes. After removal of the culture medium, 400 μL of lysate was added and the total protein was extracted. Protein concentration was determined using the Bradford method (Wang and Zhang, 2015). The proteins were separated on a 5% stacking gel (40 V for 1 hour) and 10% running gel (60 V for 3.5 hours). Proteins were then transferred (via a wet transfer) onto a membrane (14 V for 14 hours). The membrane was blocked in a swing bed at 37°C for 2 hours, then treated with rabbit anti-human f broblast polyclonal antibody (1:800; Hyclone, Logan, UT, USA) at 37°C for 2 hours in a swing bed. After four 5-minute washes with Tris-buf ered saline with Tween-20 (TBST), the membrane was treated with goat anti-rabbit IgG (1:700; Hyclone) at 37°C for 1.5 hours then washed four times (5 minutes) with TBST. Bands were visualized with 3,3′-dimethylbenzidine. The experiment was performed in triplicate. Results were analyzed with Quantity One image software (Bio-Rad, Hercules, CA, USA). Protein expression was determined from the ratio of the target band to the β-actin band.
Cell counting kit-8 (CCK-8) assay
Cells from the exponential growth phase were seeded in 96-well plates at 2 × 103and incubated in DMEM containing 10% fetal bovine serum (200 μL) for 24 hours. Six wells were used for each group. CCK-8 (10 μL; Gibco, Carlsbad, CA, USA) was added to each well followed by incubation for 4 hours. The blank well was considered as the control. Optical density values were measured at 450 nm using a microplate reader. Schwann cells before and after transfection were made into single-cell suspensions. After quantif cation, cells were incubated in a 24-well plate (1.5 × 104/well) in a 5% CO2incubator at 37°C. Three wells were used for each group. From day 2 of the culture, the cells from the three wells of each group were digested daily and at the same time for 5 days to obtain an accurate count. Cell growth curves in each group were then drawn.
Model establishment and maintenance
The remaining 83 Sprague-Dawley rats were acclimatized in the laboratory for 2 weeks. They were intraperitoneally anesthetized with 2.5% ketamine (20 mg/kg) and f xed on the bench in the prone position. After the lower back was shaved, a median incision was made on the back using the T8–9spinous processes as a center to expose the T7–10spinousprocesses and the lamina. The T8–9spinous processes and part of the lamina tissue were removed. This exposed spinal cord tissue was the lesion area. Based on the method by Allen (Xie et al., 2010; Zhang et al., 2010; Chen et al., 2011; Wang and Zhang, 2015), but with some modifications, a 10-g object was vertically dropped from a 2.5-cm height, which impacted directly on the rat spinal cord. Paralysis of the lower limbs was observed after the rat’s tail suf ered from swinging and spasms. These responses conf rmed a successful establishment of the model. The wound was washed with penicillin-saline, and the tissue was sutured layer by layer. Abdominal massage and extrusion were performed in the morning and afternoon on a daily basis to assist in urination of the rats. The 75 successful models were equally and randomly assigned to the following groups: SCI, Schwann cells without hTERT transfection, and hTERT transfection. Six hours after model establishment, 10 μL of culture medium was injected in rats of the SCI group. At the same time point, 10 μL of Schwann cell without hTERT transfection suspension (1 × 1010/L) was slowly injected over 3 minutes at the site of injury (dura mater was retained) to the Schwann cells without hTERT transfection group. The microinjector was maintained in place for 5 minutes. The pinhole was blocked with a biological adhesive to prevent suspension overf ow. The wound was sutured layer by layer. In the hTERT transfection group, 10 μL of hTERT-transfected Schwann cell suspension (1 × 1010/L) was slowly injected, with the subsequent procedures the same as those of the Schwann cells without hTERT transfection group. Rats in each group were used for the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (n = 5), reverse transcription polymerase chain reaction (RT-PCR) (n = 5), hematoxylin and eosin staining (n = 5), chlormethyl-benzamidodialkylcarbocyanine (CM-DiI) count (n = 5), and detection of motor and electrophysiological function. The rats were not sacrif ced. After scoring, they were returned to their respective groups. The remaining f ve rats were kept to prevent data loss.
TUNEL assay
Rats were anesthetized with chloral hydrate 72 hours after transplantation. The chest was then opened and aortic cannulation was conducted through the left ventricle, followed by f xation with 4% paraformaldehyde. Spinal cord tissue (2.0 cm) was collected by using the injury site as the center. The tissue was then f xed with paraformaldehyde. Paraf n sections were dewaxed. TUNEL-positive cells were quantif ed in the samples using the TUNEL assay kit (Roche, Shanghai, China). Cells with brown nuclei were deemed TUNEL positive. Under high power microscopy (× 200; Olympus), ten fields were selected from each section. TUNEL-positive cells in each f eld were quantif ed and the mean value was calculated.
RT-PCR
Seventy-two hours after transplantation, 50 mg of spinal cord tissue was obtained. In accordance with the manufacturer’s instructions, total RNA was extracted from the spinal cord tissue with Trizol reagent (Gibco). The RNA content was determined by an ultraviolet spectrophotometer. The two-step RT-PCR (Yi et al., 2005) using the TaKaRa kit was performed according to the kit’s instructions. mRNA was reverse transcribed into cDNA, which was then amplif ed by PCR. Primers are listed in Table 1. The amplif ed products underwent electrophoresis and the optical density of the bands was analyzed by Image-ProPlus 6.0 software (Media Cybernetics, Washington, DC, USA). The ratio of integrated optical density of aquaporin 4/9 (AQP4/9) or matrix metalloproteinase 9/2 (MMP9/2) to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was calculated as an index of AQP4/9 and MMP9/2 mRNA expression.
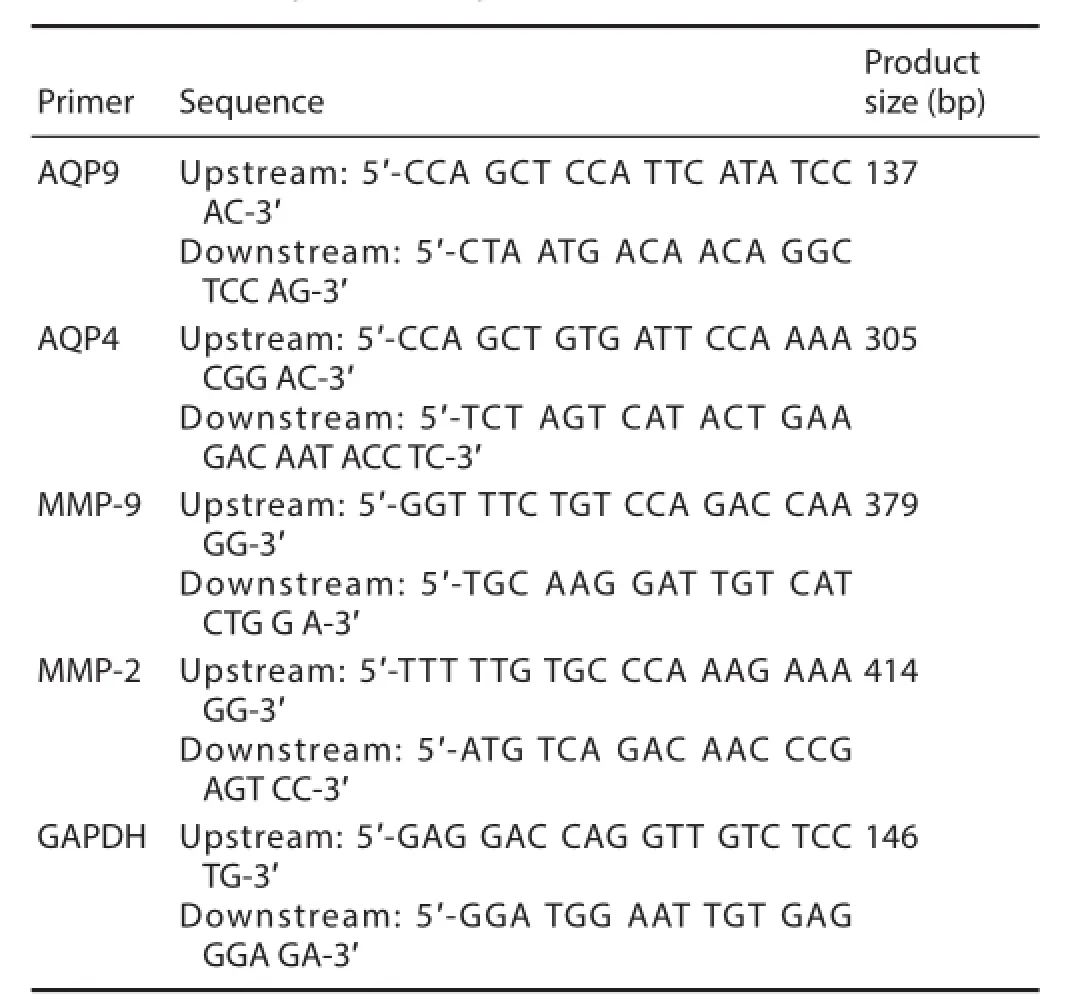
Table 1 Primer sequences and product size
Evaluation of motor function
Motor function was assessed in rats from the three groups before surgery, and then 1 and 3 days and 1, 2, 3, and 4 weeks after transplantation. After scoring, the rats were not sacrif ced but returned to their respective groups. The Basso, Beattie, and Bresnahan (BBB) scale (Barros Filho and Molina, 2008) and the inclined plane test (Wang et al., 2010; Liu et al., 2011) were carried out. The BBB scores ranged from 0 to 21, where 21 = normal and 0 = complete paralysis. The scores were based on the number and range of joint activities, the degree of loading, front and rear limb coordination, and the activities of the front and back claws and tail. In the inclined plane test, rats were placed horizontally on a smooth tiltboard with their heads to the front. The angle was increased every 5°, and the maximum angle at which the rats stayed on the board for 5 seconds was recorded. The evaluation began at 8:00 a.m. The score was obtained by two investigators, and the average value was calculated.
Electrophysiology
Four weeks after transplantation, a Keypoint 4 induced potential instrument (Beijing Weidi Kangtai Medical Instrument Co., Ltd., Beijing, China) was used to measure somatosensory-evoked potentials and motor-evoked potentials, based on a previous method (Chi et al., 2010). The rats were intraperitoneally anesthetized with 10% chloral hydrate and then laid on the horizontal plane. Hind limbs were stimulated with the stimulating electrode. A recording electrode was placed at the intersection of the coronal suture and sagittal suture healing line under the scalp (i.e., the hindlimb cortical sensory area). A reference electrode was placed 0.5 cm posterior to the hindlimb cortical sensory area. A direct current, square wave, and electrical pulses were given until the hind limb suf ered from a slight tic. The conditions were as follows: current intensity: 5–15 mA; pulse width: 0.2 ms; frequency: 3 Hz; superimposed: 50–60 times. Neurophysiological recovery was monitored by recording the somatosensory-evoked potential latency and amplitude. Motor-evoked potentials were detected by the latencies and amplitudes. These were obtained after anesthesia by placing the stimulating electrodes 2 mm anterior to the coronal suture and 2 mm lateral to the sagittal suture under the scalp (i.e. the motor cortex). The electrodes were stimulated as follows: stimulus intensity: 40 mA; pulse width: 0.1 m; frequency: 1 Hz; superimposed: 300–500 times; scanning speed: 5 ms/D; sensitivity: 5 μV/D.
Hematoxylin and eosin staining and f uorescence microscopy
Four weeks after transplantation, five rats from each of the three groups were subjected to hematoxylin and eosin staining. The degree of injury was verif ed by a histological examination. Spinal cord tissue was f xed with 4% paraformaldehyde and sliced into frozen sections. Sections were stained with hematoxylin and eosin, and observed under f uorescence microscopy. Under high power microscopy (× 200), ten f elds were selected from each section. The number of PKH26-positive cells in each f eld was quantif ed and the average value was calculated. The apoptotic rate was then calculated.
Statistical analysis
All data are expressed as the mean ± SD. The dif erence of intergroup data was compared using one-way analysis of variance followed by the least signif cant dif erence test. All data were analyzed using SPSS 15.0 software (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically signif cant.
Results
Identif cation of Schwann cells
Inverted phase-contrast microscopy demonstrated a confluent layer of cells 1 week after culturing. Most of these cells were determined to be Schwann cells, while a few were found to be f broblasts. After purif cation, more than 96% of the total number of cells were Schwann cells. These cells occupied a long, spindle, narrow shape, with small nuclei (Figure 1A). This shape was also conf rmed under f uorescence microscopy (Figure 1B). Immunofluorescence for myelin basic protein revealed green f uorescence of cell bodies and processes of Schwann cells. Furthermore, DAPI immunof uorescence revealed blue-stained nuclei of Schwann cells and f broblasts. The cytoplasm of the f broblasts was not stained and was thus transparent. Therefore, the proportion of Schwann cells could be roughly estimated.
hTERT gene transfection
Western immunoblots confirmed the expression of the hTERT protein in rats 48 hours after hTERT transfection. The expression of this protein was low in the EV group and in the group without hTERT transfection of Schwann cells. These f ndings conf rmed that rats transfected with the hTERT gene into Schwann cells were indeed the hTERT-transfected group (Figure 2).
Ef ect of the transplantation of hTERT gene-transfected
Schwann cells at the site of injury on cell proliferation
The proliferation of Schwann cells was significantly (P <0.05) higher in the hTERT-transfected group compared with the Schwann cells without hTERT transfection group, which was markedly (P < 0.05) higher than the EV group (Figure 3).
Ef ect of the transplantation of hTERT gene-transfected Schwann cells at the site of injury on cell apoptosis
Apoptotic cells were distributed all over the site of injury, including the edge of the injury site. The TUNEL assay revealed that the apoptotic rate was signif cantly (P < 0.05) less in both the Schwann cells without hTERT transfection group and the hTERT-transfected group, with the latter group exhibiting the lowest rate (P < 0.05; Figure 4).
Ef ect of the transplantation of hTERT gene-transfected Schwann cells at the site of injury on mRNA expression of AQP4/9 and MMP9/2
AQP4/9 and MMP9/2 mRNA expression was markedly (P< 0.05) higher in the Schwann cells without hTERT transfection group compared with the hTERT-transfected group 72 hours after injury. The expression of these two genes was signif cantly (P < 0.05) lower in the non-transfected group compared with the SCI group (Figure 5).
Motor function
No signif cant dif erence in motor function was detected in all rats before injury. Two to four weeks after injury, motor function signif cantly (P < 0.05) improved in the Schwann cells without hTERT transfection group and in the hTERT-transfected group compared with the SCI group (Table 2).
Ef ect of the transplantation of hTERT gene-transfected Schwann cells on the number of nerve cells
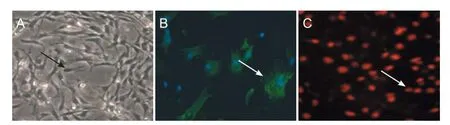
Figure 1 Identif cation of Schwann cells 4 days after the primary culture.

Figure 2 Protein expression of hTERT in Schwann cells 48 hours after transfection (western blot assay).
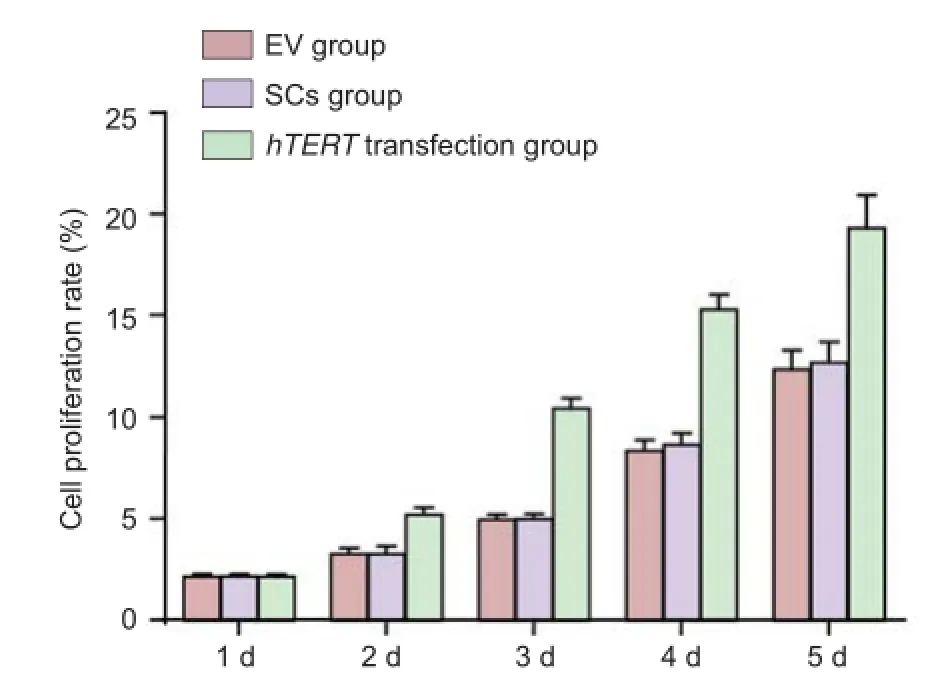
Figure 3 Ef ect of the transplantation of hTERT gene-transfected Schwann cells at the site of injury on cell proliferation.
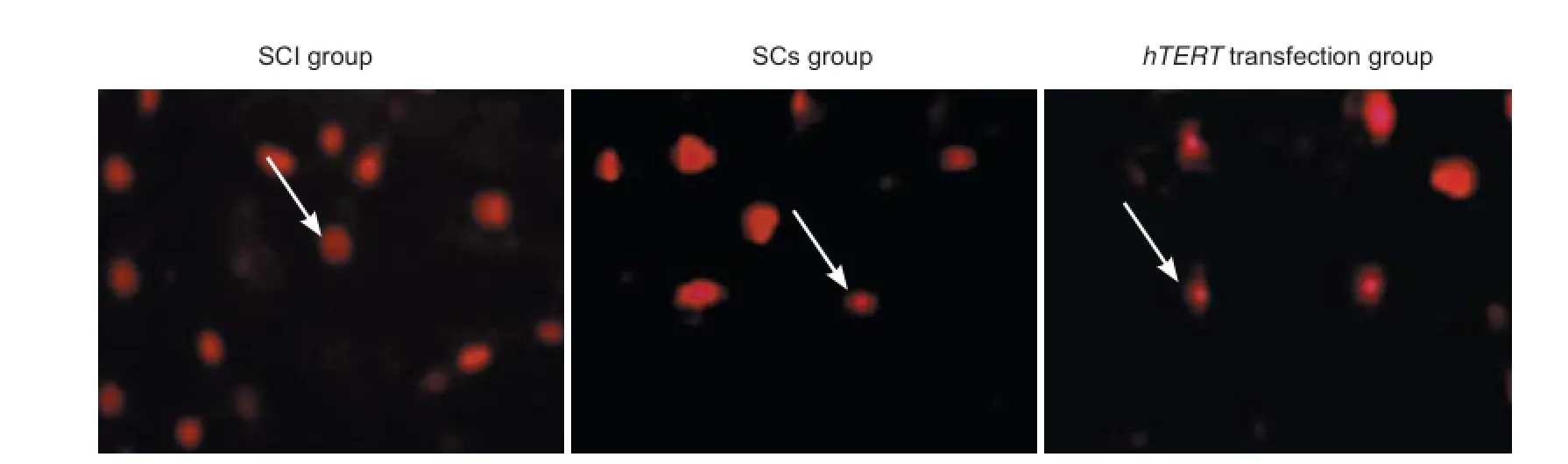
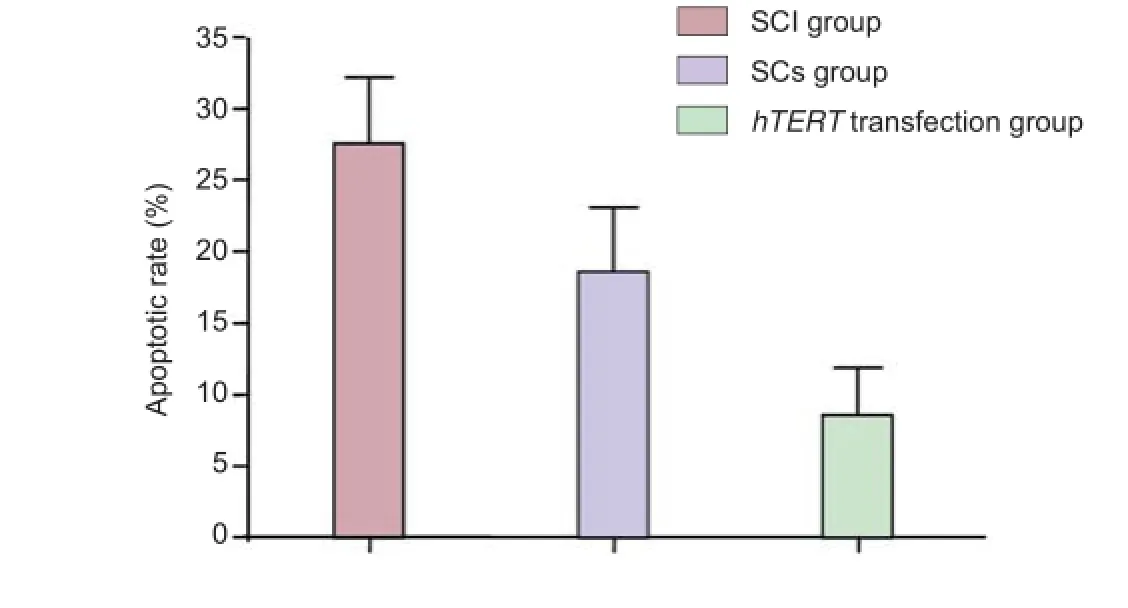
Figure 4 Ef ect of the transplantation of hTERT-transfected Schwann cells at the site of injury on cell apoptosis (TUNEL assay, f uorescence microscope).
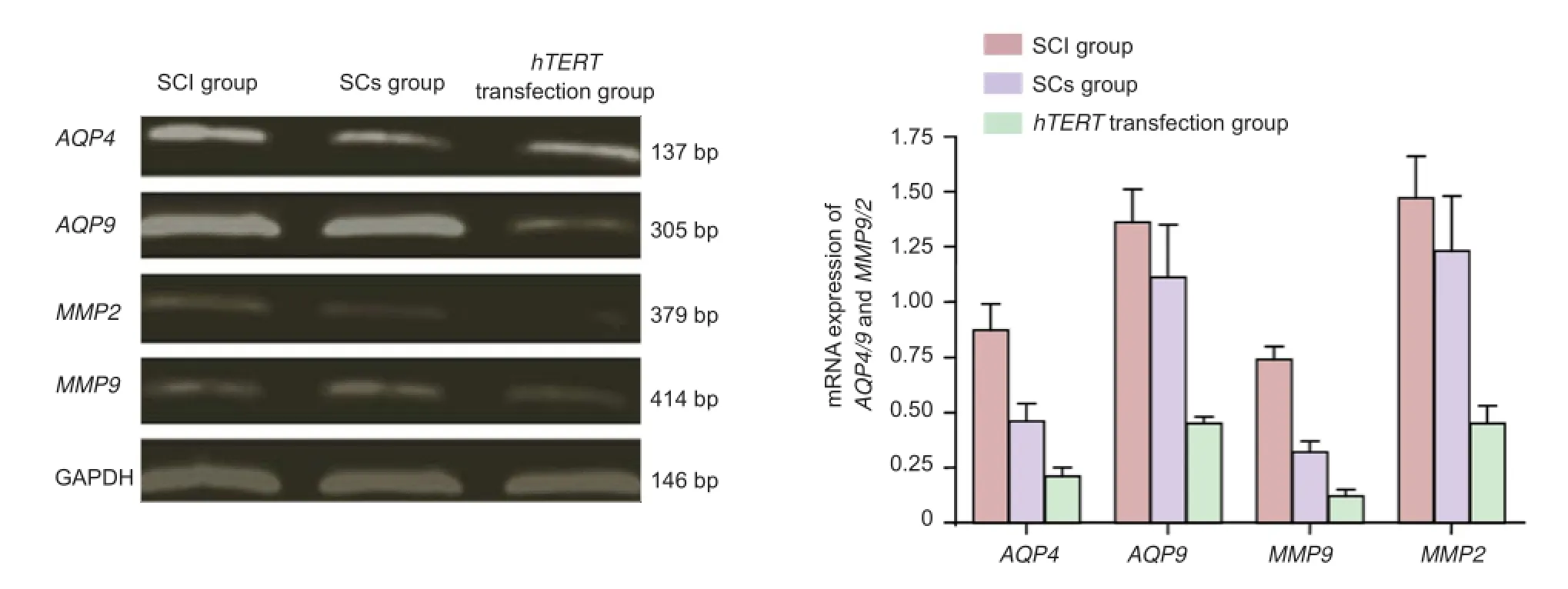
Figure 5 Ef ect of the transplantation of hTERT-transfected Schwann cells at the site of injury on mRNA expression of AQP4/9 and MMP9/2.
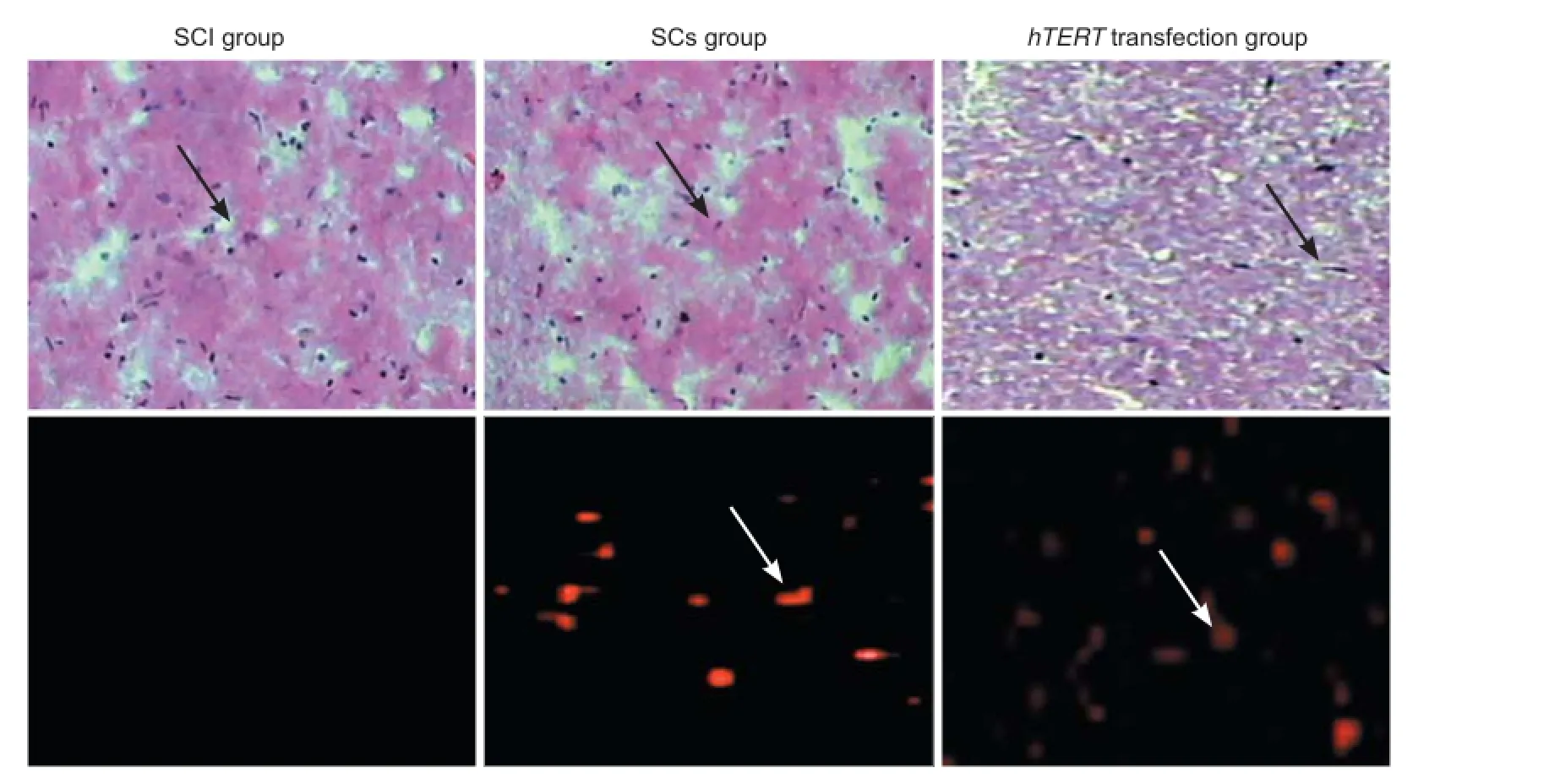
Figure 6 Morphology of nerve cells (arrows) in the rat spinal cord after transplantation with hTERT-transfected Schwann cells.
Four weeks after injury, hematoxylin and eosin staining results revealed that the spinal cord tissue was disrupted; i.e. scarring, a clear cavity, and structural disorder were visible at the injury site (Figure 6). These typical morphological changes to nerve cells were seen in the transplanted region of Schwann cells without hTERT transfection group. The cavity was smaller in this group compared with the SCI group, but larger compared with the hTERT-transfected group. Typical nerve cell-like morphological changes were visible, and the cavity disappeared in the hTERT-transfected group. Scattered distribution of PKH26 (red f uorescence) was visible both in the group without Schwann cell hTERT transfection and in the hTERT-transfected group. Significant (P < 0.01) differences were found between the groups under high power: SCI group: 0; Schwann cells without hTERT transfection group: 18.64 ± 4.57; hTERT-transfected group: 29.86 ± 7.26.
Changes in electrophysiological indices after transplantation
After injury, electrophysiological recordings revealed that evoked potential waveforms completely disappeared in all rats. Four weeks after transplantation, motor and sensory evoked potential latencies were signif cantly (P < 0.05) greater in the hTERT-transfected group compared with the Schwann cells without hTERT transfection group, which was markedly (P < 0.05) higher than the SCI group. Motor and sensory evoked potential amplitudes were signif cantly (P <0.05) higher in the hTERT-transfected group compared withthe Schwann cells without hTERT transfection group, which was markedly (P < 0.05) higher than the SCI group (Table 3).
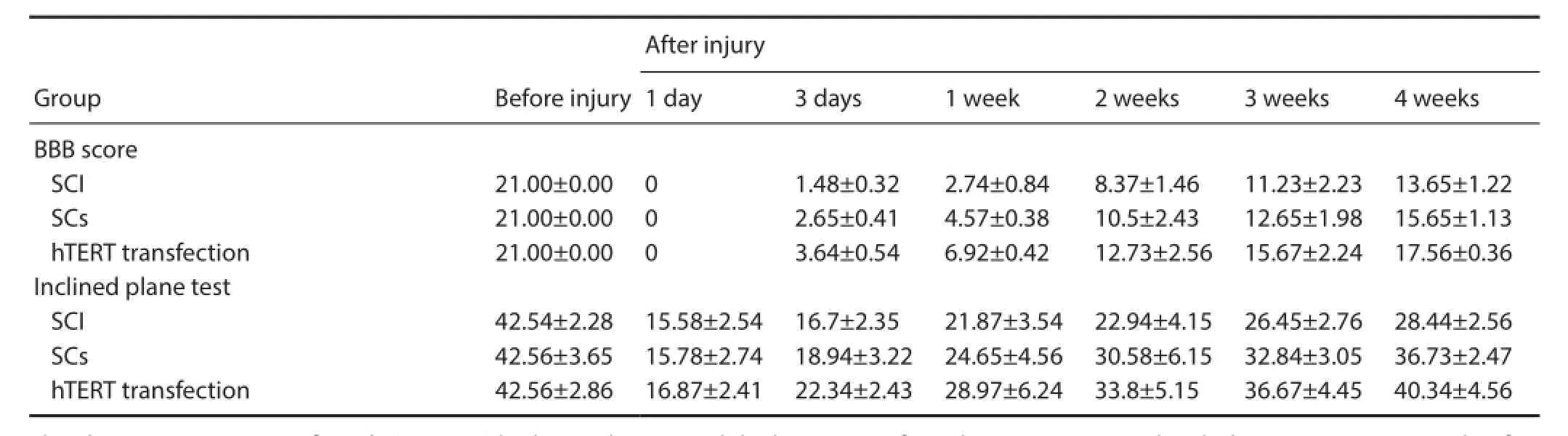
Table 2 Ef ect of the transplantation of hTERT-transfected Schwann cells in SCI rats on motor function
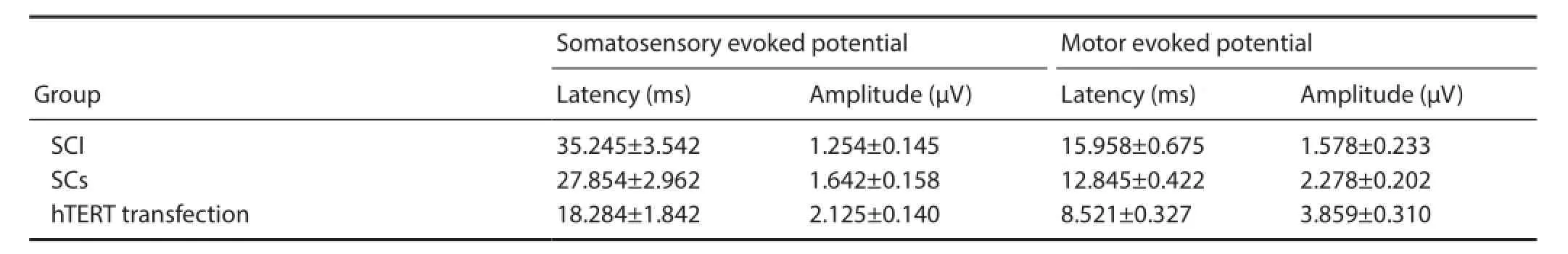
Table 3 Ef ects of hTERT-transfected Schwann cell transplantation on electrophysiological function in SCI rats
Discussion
This study explored the ef ects of the transplantation of hTERT gene-transfected Schwann cells on electrophysiological changes in SCI rats, the results of which were consistent with previous studies (Jin et al., 2013; Wu et al., 2014). Our results showed that gene products were detectable in Schwann cells–mainly in the nuclei–48 hours after transfection. Western immunoblots revealed that rat Schwann cells transfected with the hTERTT gene expressed hTERT. Seventy-two hours after model establishment, results from RT-PCR showed that gene expression of AQP4/9 and MMP9/2 reduced in the hTERT-transfected group compared the SCI group. Lower limb motor function improved in the hTERT-transfected group compared with the group without the transplantation of hTERT-transfected Schwann cells, which was more improved than the SCI group. Hematoxylin and eosin staining in the SCI group revealed spinal cord disruption; the formation of cavities and loss of axons. A few axon-like structures and a small cavity were observed at the injury site in the Schwann cells without hTERT transfection group. In the hTERT-transfected group, numerous axon-like structures were seen, but no cavity was found. The TUNEL assay revealed fewer apoptotic cells in the hTERT-transfected group, suggesting that the transplantation of hTERT gene-transfected Schwann cells may diminish apoptosis of nerve cells at the injury site. The number of PKH-26-positive cells was highest in the hTERT-transfected group followed by the Schwann cells without hTERT transfection group, and was the lowest in the SCI group. Sensory and motor evoked potential latencies were as follows (from lowest to highest): hTERT transfected group < the Schwann cells without hTERT transfection group < the SCI group. Motor and sensory evoked potential amplitudes were as follows (from highest to lowest): hTERT transfection group >the Schwann cells without hTERT transfection group > the SCI group.
In conclusion, compared with the transplantation of Schwann cells without hTERT transfection in SCI rats, the gene and protein expression of AQP4/9 and MMP9/2 was lower, fewer apoptotic cells and improved tissue repair were observed, and the number of PKH-26-positive cells at the injury site was increased in the hTERT-transfected group. There was also an improvement in the recovery of lower limb motor function. Our results suggest that transplantation of hTERT gene-transfected Schwann cells promotes the protein expression of hTERT in these cells. Compared with the transplantation of Schwann cells without hTERT transfection in SCI rats, the transplantation of hTERT gene-transfected Schwann cells provided superior results whether itwas histological or functional. Therefore, this treatment may be a novel strategy for optimizing stem cell transplantation in the treatment of nervous system injury.
Acknowledgments: We are very grateful to Dr. Bao-bin Liu from General Hospital of Tianjin Medical University of China for presenting hTERT PLXSN expression vector.
Author contributions: SQZ and JBL participated in study concept and design. MFW analyzed data. SQZ wrote the paper and was in charge of paper authorization. RG performed statistical analysis. JBL obtained funding. YL and QSZ provided technical or material support and served as a principle investigator.
All authors approved the f nal version of the paper.
Conf icts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Allodi I, Mecollari V, Eggers R (2014) Schwann cells transduced with a lentiviral vector encoding Fgf-2 promote motor neuron regeneration following sciatic nerve injury. Glia 62:1736-1746.
Ban DX, Kong XH, Feng SQ (2009) Intraspinal cord graft of autologous activated Schwann cells ef ciently promotes axonal regeneration and functional recovery after rat’s spinal cord injury. Brain Res 1256:149-161.
Barros Filho TE, Molina AE. (2008) Analysis of the sensitivity and reproducibility of the Basso, Beattie, Bresnahan (BBB) scale in Wistar rats. Clinics (Sao Paulo) 63:103-108.
Chen G, Wan H, Yang F (2011) Observation of spinal cord injury after intramedullary PLGA scaf old cells transplanted tissue. Zhonghua Shenjing Waike Zazhi 25:83-86.
Chen HH, Yang PS, Zhang JX (2015) Pleural ef usion cell lung cancer shedding f ow cytometry and preliminary clinical application. Guoji Jianyan Yixue Zazhi 38:106-110.
Chen L, Huang H, Xi H (2014) A prospective randomized double-blind clinical trial using a combination of olfactory ensheathing cells and Schwann cells for the treatment of chronic complete spinal cord injuries. Cell Transplant 23:35-44.
Chi GF, Kim MR, Kim DW (2010) Schwann cells differentiated from spheroid-forming cells of rat subcutaneous fat tissue myelinate axons in the spinal cord injury. Exp Neurol 222:304-317.
Deng LX, Walker C, Xu XM (2014) Schwann cell transplantation and descending propriospinal regeneration after spinal cord injury. Brain Res 26:1260-1268.
Hakim JS, Esmaeili Rad M, Grahn PJ (2015) Positively charged oligo [poly (ethylene glycol) fumarate] scaf old implantation results in a permissive lesion environment after spinal cord injury in rat. Tissue Eng Part A 2015:17.
Jin B, Wang W, Liu ZY (2013) exogenous hTERT gene transfection on the aged rats liver ischemia-reperfusion injury protective ef ect. Shandong Daxue Xuebao 51:13-16.
Kang SK, Putnam L, Dufour J (2004) Expression of telomerase extends the lifespan and enhances osteogenic dif erentiation of adipose tissue derived stromal cells. Stem Cells 22:l356-1372.
Liu LL, Deng WM (2015) Biological characteristics of Schwann cells of human telomerase reverse transcriptase transfected rat. Zhongguo Zuzhi Gongcheng Yanjiu 14:2250-2254.
Liu YY, Chun QX, Zhang DZ (2011) hTERT antisense oligodeoxynucleotide on chordoma cell cycle and proliferation. Modern Oncol 19:1922-1924.
Marcol W, lusarczyk W, Larysz-Brysz M (2015) Grafted activated schwann cells support survival of injured rat spinal cord white matter. World Neurosurg 21:1878-1887.
Peterson SL, Nguyen HX, Mendez OA (2015) Complement protein C1q modulates neurite outgrowth in vitro and spinal cord axon regeneration in vivo. J Neurosci 35:4332-4349.
Siddiqui AM, Khazaei M, Fehlings MG (2015) Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog Brain Res 218:45-54.
Sparling JS, Bretzner F, Biernaskie J (2015) Schwann cells generated from neonatal skin-derived precursors or neonatal peripheral nerve improve functional recovery after acute transplantation into the partially injured cervical spinal cord of the rat. J Neurosci 35:6714-6730.
Thumm M, Simons M (2015) Myelinophagy: schwann cells dinein. J Cell Biol 210:9-10.
Vasudeva VS, Abd-El-Barr MM, Chi JH (2015) implantation of neonatal skin-derived precursor schwann cells improves outcomes after incomplete cervical spinal cord injury in rats. Neurosurgery 77:15-17.
Wakao S, Matsuse D, Dezawa M (2015) Mesenchymal stem cells as a source of Schwann cells: their anticipated use in peripheral nerve regeneration. Cells Tissues Organs 200:31-41.
Wang D, Zhang JJ (2015) Ef ects of hypothermia combined with neural stem cell transplantation on recovery of neurological function in rats with spinal cord injury. Mol Med Rep 11:1759-1766.
Wang D, Fan YD, Zhang JJ (2013) Transplantation of Nogo-66 receptor gene-silenced cells of in a poly (D,L-lactic-co-glycolic acid) scaf old for the treatment of spinal cord injury. Neural Regen Res 8:677-685.
Wang QY, Liu WG, Wang ZY (2010) FTY720 On RhoA expression in rats after acute spinal cord injury in rats. Zhongguo Guke Linchuang yu Jichu Yanjiu Zazhi 4:292-296.
Weaver FM, Burns SP, Evans CT (2009) Provider perspectives on soldiers with new spinal cord injuries returning from iraq and afghanistan. Arch Phys Med Rehabil 90:517-521.
Wu SH, Huang SH, Cheng KI (2015) Third-degree hindpaw burn injury induced apoptosis of lumbar spinal cord ventral horn motor neurons and sciatic nerve and muscle atrophy in rats. Biomed Res Int 2015:372819.
Wu SH, Huang SH, Lo YC (2015) Autologous adipose-derived stem cells attenuate muscular atrophy and protect spinal cord ventral horn motor neurons in an animal model of burn injury. Cytotherapy 17:1066-1075.
Xie QS, Xu XL, Wei XJ (2010) spinal cord tissue engineering scaf olds: a polylactic glycolic acid optimum pore size f lter water. Zhongguo Zuzhi Gongcheng Yanjiu 14:393-396.
Xue F, Wu EJ, Zhang PX (2015) Biodegradable chitin conduit tubulation combined with bone marrow mesenchymal stem cell transplantation for treatment of spinal cord injury by reducing glial scar and cavity formation. Neural Regen Res 10:104-111.
Yi CL, Chen AM, Bai XJ, Song XZ, Xu WG (2005) The ef ect of bcl-xL gene transfection in vivo on the expression of Caspase-3 and neuroprotective function following spinal cord injury. Zhonghua Shiyan Waike Zazhi 22:984-986.
Zeng X, Qiu XC, Ma YH (2015) Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials 53:184-201.
Zhang SD, Yu HS, Li JH (2010) polylactic acid stent graft evoked potentials of spinal cord hemisection hind legs and exercise in rats. Zhongguo Zuzhong Zazhi 5:286-290.
Zhang SQ, Wu MF, Piao Z (2015) Edaravone combined with Schwann cell transplantation may repair spinal cord injury in rats. Neural Regen Res 10:230-236.
Zhi WL, Li SB, Cheng GW (2014) hTERT gene transfection on human neuronal viability. Gannan Yixueyuan Xuebao 34:170-173.
Copyedited by Mark F, Raye W, Wang J, Qiu Y, Li CH, Song LP, Zhao M
*Correspondence to: Rui Gu, M.D., fandiliucl@126.com.
orcid: 0000-0003-4406-1553 (Rui Gu)
10.4103/1673-5374.172324 http://www.nrronline.org/
Accepted: 2015-11-12
- 中國神經再生研究(英文版)的其它文章
- Neuroplasticity in post-stroke gait recovery and noninvasive brain stimulation
- Structural and functional connectivity in traumatic brain injury
- Neglected corticospinal tract injury for 10 months in a stroke patient
- Polyurethane/poly(vinyl alcohol) hydrogel coating improves the cytocompatibility of neural electrodes
- Electroacupuncture promotes the recovery of motor neuron function in the anterior horn of the injured spinal cord
- A novel method for evaluating brain function and microstructural changes in Parkinson’s disease

