Electroacupuncture promotes the recovery of motor neuron function in the anterior horn of the injured spinal cord
Jian-hui Yang, Jian-guo Lv, Hui Wang, Hui-yong Nie
Rehabilitation Center, First Af liated Hospital of Health Science Center, Xi’an Jiaotong University, Xi’an, Shaanxi Province, China
Electroacupuncture promotes the recovery of motor neuron function in the anterior horn of the injured spinal cord
Jian-hui Yang*, Jian-guo Lv, Hui Wang, Hui-yong Nie
Rehabilitation Center, First Af liated Hospital of Health Science Center, Xi’an Jiaotong University, Xi’an, Shaanxi Province, China
Acupuncture has been shown to lessen the inf ammatory reaction after acute spinal cord injury and reduce secondary injury. However, the mechanism of action remains unclear. In this study, a rat model of spinal cord injury was established by compressing the T8–9segments using a modif ed Nystrom method. Twenty-four hours after injury, Zusanli (ST36), Xuanzhong (GB39), Futu (ST32) and Sanyinjiao (SP6) were stimulated with electroacupuncture. Rats with spinal cord injury alone were used as controls. At 2, 4 and 6 weeks after injury, acetylcholinesterase (AChE) activity at the site of injury, the number of medium and large neurons in the spinal cord anterior horn, glial cell line-derived neurotrophic factor (GDNF) mRNA expression, and Basso, Beattie and Bresnahan locomotor rating scale scores were greater in the electroacupuncture group compared with the control group. These results demonstrate that electroacupuncture increases AChE activity, up-regulates GDNF mRNA expression, and promotes the recovery of motor neuron function in the anterior horn after spinal cord injury.
nerve regeneration; spinal cord injury; electroacupuncture; spine injury; secondary injury; acupoint; motor neurons; acetylcholinesterase; glial cell line-derived neurotrophic factor; inclined board test; Basso, Beattie and Bresnahan locomotor rating scale; functional recovery; neural regeneration
Funding: This study was supported by a grant from the Shaanxi Province Scientif c and Technological Project in China, No. 2014TM4193.
Yang JH, Lv JG, Wang H, Nie HY (2015) Electroacupuncture promotes the recovery of motor neuron function in the anterior horn of the injured spinal cord. Neural Regen Res 10(12):2033-2039.
Introduction
Spinal cord injury (SCI) is the most severe complication of spine injury, and often leads to severe dysfunction below the site of injury. Consequently, promoting the recovery of motor function below the site of injury has been the focus of numerous recent studies (Zhang et al., 2014). Acupuncture is an ef ective method for treating injuries to the central nervous system, as shown by numerous clinical studies. However, the mechanism of action remains poorly understood. Acupuncture has been shown to lessen the inf ammatory reaction after acute SCI and alleviate secondary injury to the spinal cord, thereby improving sensory and motor dysfunction following SCI. Indeed, acupuncture is considered a key therapeutic approach for SCI (Nayak et al., 2001).
After SCI, the recovery of motor function depends on the capacity of the nervous system to repair itself, and on the preservation of motor end plate integrity. Spinal motor nerves have trophic ef ects on target tissue (Bar et al., 1998). Spinal motor neurons contain neurotrophic factors that maintain the morphology of target muscle and the proper functioning of the motor end plate.
Acetylcholine is an important neurotransmitter secreted from spinal motor neurons. Acetylcholinesterase (AChE) is a hydrolase that hydrolyzes the neurotransmitter acetylcholine, and can be used as a marker of cholinergic neurons. Changes in the activity of the enzyme ref ect cellular metabolism and the degree of injury (Nakamura et al., 1996). Changes in the number of Nissl bodies and the number of neurons containing Nissl bodies can also be used to assess the degree of nerve injury (Gulino and Gulisano, 2013). Ramey and Archer (1993) demonstrated that acupuncture can enhance AChE activity in the midbrain reticular formation and increase the number of AChE-positive cells. However, there is no report on AChE activity and Nissl bodies in motor neurons in the spinal cord.
Bregman et al. (1997) showed that the injured central nervous system exhibits plasticity and can regenerate, and that the speed and extent of regeneration are dependent on neurotrophic factors. Glial cell line-derived neurotrophic factor (GDNF) belongs to the transforming growth factor β superfamily, and is the most potent neurotrophic factor for motor neurons (Vianney and Spitsbergen, 2011). GDNF not only improves the microenvironment at the site of injury, it also inhibits apoptosis, contributing to the functional recovery of neurons and neuroglia, and strongly protects against nervous system injury (Cheng et al., 2005; Bakshi et al.,2006; McCullough et al., 2013). Although Ledergerber (1984) found that electroacupuncture promotes the expression of brain-derived neurotrophic factor (BDNF), very few studies have examined the ef ect of acupuncture on GDNF.
In the present study, we examined AChE activity, Nissl bodies, GDNF mRNA expression, and the motor function of the lower extremities in a rat model of SCI after electroacupuncture. This study was undertaken to provide insight into the ef ect and mechanism of action of acupuncture on the recovery of motor neuron function in the anterior horn of the injured spinal cord.
Materials and Methods
Animals
A total of 60 adult, healthy, clean, white, male, Sprague-Dawley rats, 8–10 weeks of age, and weighing 250–300 g, were provided by the Laboratory Animal Center, Health Science Center, Xi’an Jiaotong University, China (license No. SYXK-(Shaan)2006-002). The protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health of the United States (1996). All experiments were approved by the Animal Ethics Management Team, Xi’an Jiaotong University, China.
Preparation of the rat models of SCI and group management
In accordance with the modif ed Nystrom method (Black et al., 1988), thoracic segments of the spinal cord of 60 rats were compressed using the posterior approach. Rats were intraperitoneally anesthetized with 2% sodium pentobarbital (30 mg/kg) and f xed on a Jiangwan Type I Stereotaxic Apparatus (Anhui Zhenghua Biological Equipment Co., Ltd., Huaibei, China) in the prone position. The middle of the back was shaved with an electric shaver. A median incision was made on the back. The sacrospinalis muscle was incised along both sides of the spinous process. The T7–10vertebral plate was exposed and carefully removed so as to fully expose the T8–9levels of the spinal cord. Both ends of the spinous process were f xed. The compression device was an 8-cm-long column with a 4-cm-diameter circular plate at the top and a rectangular 2.2 mm × 5.0 mm curved (concave side downward) smooth metal impactor plate at the bottom (Figure 1). The column was inserted into a 4-cm-long smooth plastic pipe, so that the column could move freely up and down. The total weight of the circular plate, column and impactor plate was 15 g. The plastic pipe was f xed to the arm of the stereotactic apparatus, and a 35-g weight was added onto the circular plate. The metal impactor plate compressed the dorsal surface of the T8–9levels of the spinal cord. The total compression weight was 50 g, and the duration of compression was 5 minutes. This resulted in moderate to severe thoracic spinal cord injury. After surgery, the wound was sutured, and the animals were placed in a heating lamp box to keep the body warm. After successful compression, hemorrhage and swelling were seen at the injury site, and the rat twitched its tail. Both lower extremities and torso showed signs of spastic tremor. After regaining consciousness, both lower extremities displayed f accid paralysis. The spinal dura mater was complete at the site of injury. On the following day after injury, the Basso, Beattie and Bresnahan (BBB) score was less than 1.
After regaining consciousness, rats were fed at room temperature in individual cages. Urination was induced once by squeezing or by puncturing the bladder every 2 or 3 days. Rats with spinal cord injury were equally and randomly divided into control and electroacupuncture groups. Rats in the control group did not receive any treatment. Rats in the electroacupuncture group underwent electroacupuncture.
Electroacupuncture
Rats received electroacupuncture 24 hours after injury by puncturing the following acupoints corresponding to the L3–6segments of the spinal cord (Takeshige et al., 1990; Ramer and Bisby, 1998): Zusanli (ST36; 0.5 cm below the front of the capitulum f bulae), Xuanzhong (GB39; 0.2 cm superior to the tip of the lateral malleolus), Futu (ST32; inferior 1/3 of the line between the anterior superior iliac spine and the lateral patella) and Sanyinjiao (SP6; 0.2 cm superior to the tip of the medial malleolus, the rear edge of the medial tibia). Using an HB-EDT-II acupuncture apparatus (Shenzhen Lefukang Science and Technology Co., Ltd., Shenzhen, China), two stainless steel 1-cun needles (Shenzhen Lefukang Science and Technology Co., Ltd.) were pricked into two acupoints as positive and negative electrodes, to a depth of 0.15 cm, with a frequency of 75 cycles/min, and a current of 40–50 μA. Electroacupuncture was performed once a day. The needle was maintained in place for 30 minutes. At 15 minutes, the electrodes were exchanged. One group of acupoints was punctured every day. Two groups of acupoints received electroacupuncture alternately.
Sample collection, preparation of frozen sections and staining
In accordance with previous studies on acupuncture treatment (Takeshige et al., 1990), at 2, 4 and 6 weeks after electroacupuncture, f ve rats were obtained from each group. Under anesthesia, samples were collected and RT-PCR was performed. An additional f ve rats were obtained from each group, anesthetized, perfused with 100 mL physiological saline and 130 mM paraformaldehyde 500 mL through the left ventricle. The spinal cord at the injury site was removed, frozen, and sliced into 15-μm-thick transverse sections. These sections were f xed in 4% paraformaldehyde for 24 hours, permeabilized in xylene, and embedded in wax. Four sections per rat were used.
In accordance with instructions in the hematoxylin-eosin staining kit (Bogoo Biological Technology Co., Ltd., Shanghai, China), sections were treated with xylene, dewaxed, hydrated, stained with hematoxylin for 5 minutes, washed with distilled water for 5 minutes, dif erentiated with a dif erentiation medium for 30 seconds, immersed in distilled water for 10 minutes, stained with eosin for 2 minutes, washed with distilled water, dehydrated with anhydrous alcohol for 5minutes, washed with distilled water for 1 or 2 seconds, permeabilized with xylene, and mounted with neutral resin.
In accordance with instructions in the Nissl staining kit (Bogoo Biological Technology Co., Ltd.), modified Nissl staining (Thionine-Giemsa method) was performed (Lindroos, 1991). Paraf n sections were dewaxed with xylene, rehydrated through a graded ethanol series, stained with 1% thionine for 5 minutes at room temperature, dif erentiated with anhydrous alcohol and glacial acetic acid, counter-stained with 0.1% eosin, dehydrated with ethanol, permeabilized with xylene, and mounted with resin. In accordance with instructions in the AChE staining kit (Bogoo Biological Technology Co., Ltd.), AChE staining (Karnovsky-Roots method) was performed. Sections were dewaxed, washed with distilled water, incubated in the incubation medium at room temperature for 2–6 hours or at 37°C for 1 or 2 hours, washed with distilled water, dehydrated with anhydrous alcohol, permeabilized in xylene, and mounted with neutral resin.
The sections were observed with a light microscope. Hematoxylin-eosin-stained sections were used to observe nerve tissue swelling, hemorrhage and necrosis, cellular swelling, capsular spaces and vacuolar degeneration. Nissl staining mainly allowed observation of Nissl bodies and the quantif -cation of motor neurons containing Nissl bodies. AChE levels were assessed by quantifying the intensity of AChE staining. Extraction of total RNA and RT-PCR in the rat spinal cord
Using the Trizol one-step method, total RNA from the injured spinal cord was extracted with Trizol solution and an RNA extraction kit (Gibco, New York, NY, USA). A small sample of total RNA was used for UV spectrometry and agarose gel electrophoresis. RNA samples were used for PCR amplif cation with a PCR system (Perkin Elmer, Waltham, MA, USA). GDNF primers were added (Schaar et al., 1994). cDNA was synthesized using a cDNA synthesis kit (Gibco); 2 μL of reaction product served as the template. PCR was performed using the ready-to-use RT-PCR kit (Pharmacia, NJ, USA). PCR conditions were as follows: 94°C for 5 minutes; 35 cycles of 94 °C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute; 72°C for 10 minutes. PCR products were resolved by agarose gel electrophoresis. A gel scanning system (Perkin Elmer) was used for densitometry. β-Actin served as an internal reference. Primers were designed based on a previous study (Nadeau et al., 1995). 100% gray value served as internal control. Semiquantitative analysis was done using an image analysis system (Shanghai Sixing Biological Technology Development Company, Shanghai, China). Results were expressed as the percentage of gray value of the target gene to β-actin.
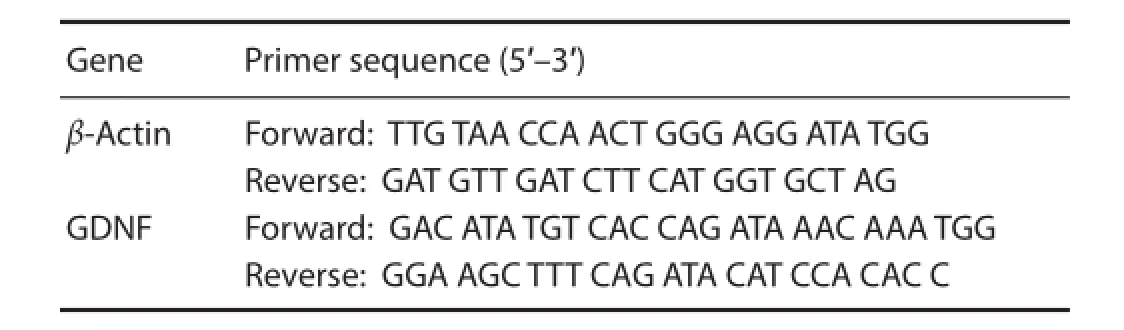
Primer sequences:
Observation of Nissl-stained sections and quantitation of motor neurons in the anterior horn of the injured spinal cord Tissue sections of the injured segment of the thoracic cord were stained using the thionine-giemsa method, and observed with a light microscope. The number of large and mid-sized neurons stained with the modif ed Nissl protocol was calculated in the anterior horn of the spinal cord using a Leica 570 image analysis system (Leica, Wetzlar, Germany). The number of motor neurons in the control and electroacupuncture groups was compared.
Detection of AChE activity in the injured rat spinal cord
AChE activity was assessed using the indirect detection method. The mean gray value of AChE in the same group of sections was calculated based on the background color. At 2, 4 and 6 weeks after SCI, the gray values of four frozen sections for each rat were averaged, and the mean gray values of f ve rats at each time point were determined. The gray value indirectly and inversely ref ects the enzymatic activity in cells. The greater the gray value, the lower the enzymatic activity.
Evaluation of motor nerve function in the rat hind limb
Motor nerve function was assessed in the rat hind limb at 1–6 weeks after SCI according to a previously published method (Basso et al., 1995; Engesser-cesar et al., 2005). For the BBB locomotor rating scale, motor function was classif ed into 22 grades as follows: 0 = complete paralysis; 21 = normal function. Thirty minutes after scoring, the inclined board test was performed according to a modif cation of the Rivlin method (Rivlin and Tator, 1997). The body axis was perpendicular to the longitudinal axis of the board. The board was elevated 5° each step. The maximum angle at which the rat remained on the board for 5 seconds was recorded.
Statistical analysis
Measurement data are expressed as the mean ± SD, and analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA). Dif erences between groups were compared using one-way analysis of variance and the least signif cant dif erence test. A value of P < 0.05 was considered statistically signif cant.
Results
Ef ects of electroacupuncture on the histomorphology of the injured rat spinal cord
Two weeks after electroacupuncture, in the control group, hematoxylin-eosin staining revealed incomplete gray matter and white matter. At the injury site, swelling, hemorrhage, necrotic foci, cellular swelling, vacuoles, and vacuolar degeneration were visible in the gray matter. Nerve f bers were arranged irregularly (Figure 2A). Apoptosis and inf ammatory inf ltration were observed. These f ndings indicate successful model establishment. In the electroacupuncture group, many neurons had survived, with only light swelling and necrosis. Small karyocytes were observed (Figure 2B).
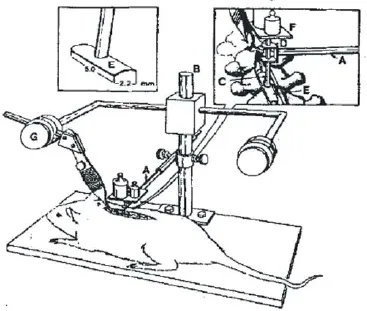
Figure 1 Compression device for producing spinal cord injury through the posterior approach using the Nystrom method.
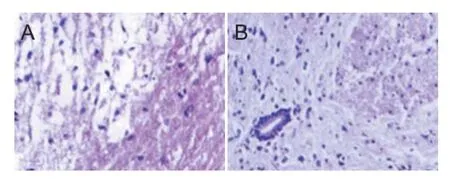
Figure 2 Histomorphological changes in the injured spinal cord 2 weeks after electroacupuncture (hematoxylin-eosin staining, × 200).
Table 1 GDNF mRNA expression (gray value ratio of GDNF to β-actin) in the rat spinal cord after electroacupuncture
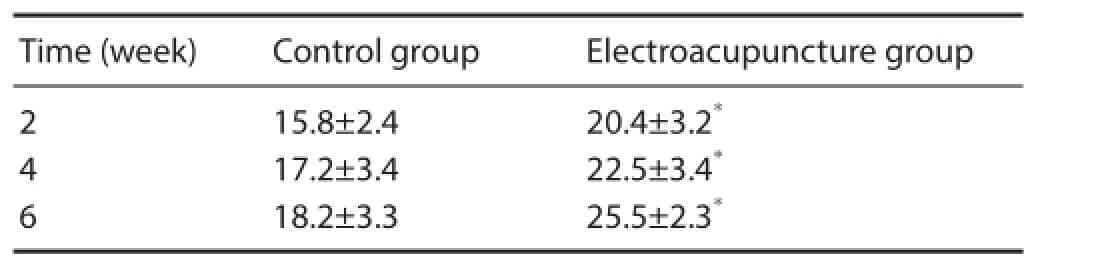
Table 2 Ef ects of electroacupuncture on the number (/200-fold f eld) of Nissl bodies in motor neurons in rats with spinal cord injury
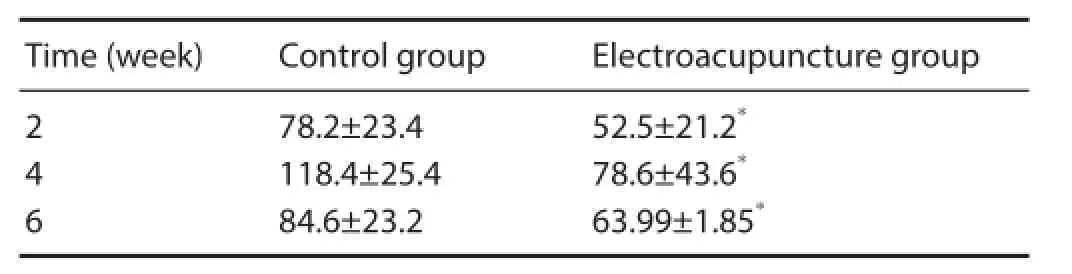
Table 3 Changes in gray values of acetylcholinesterase staining in the rat spinal cord after electroacupuncture
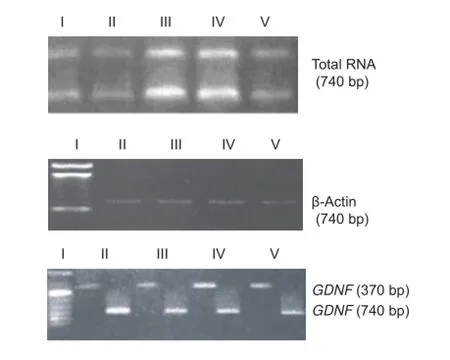
Figure 3 Electrophoretogram of total RNA and GDNF mRNA expression in the injured rat spinal cord.
Ef ects of electroacupuncture on GDNF mRNA expression in rats with SCI
In 1% agarose gel electrophoresis, two distinct 28S and 18S bands were visible, with a ratio of approximately 2:1. No obvious degradation was observed (Figure 3A). RNA yield was approximately 2 μg/mg, and ~740-bp DNA fragments were obtained, consistent with the expected β-actin band (Figure 3B). GDNF mRNA expression was high. A 100% standard was used for gray scanning. PCR amplif cation of RNA from the control and electroacupuncture groups (Figure 3C, Table 1) demonstrated that GDNF mRNA expression increased slightly and gradually after SCI, but no significant differences were detected at the various time points (P > 0.05). Compared with the control group, GDNF mRNA expression increased signif cantly over time in the electroacupuncture group (P < 0.01). Significant differences in GDNF mRNA expression were detectable in the electroacupuncture group at the various time points (P < 0.05). These results suggest that electroacupuncture ef ectively increases GDNF mRNAexpression in rats with SCI.
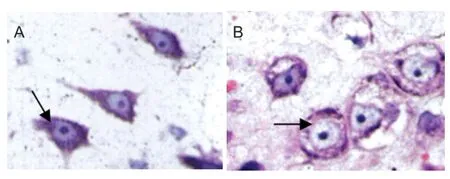
Figure 4 Ef ects of electroacupuncture on the morphology of Nissl bodies in motor neurons in rats with spinal cord injury (thionine-giemsa staining, light microscope, × 1,000).
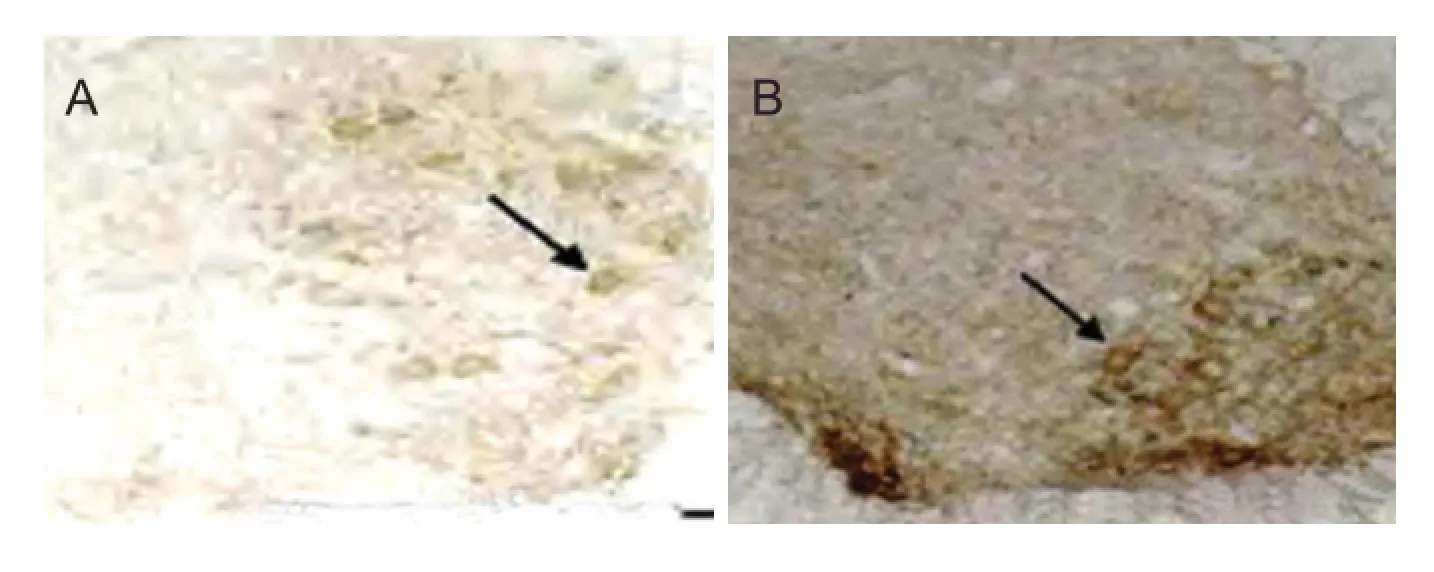
Figure 5 Ef ects of electroacupuncture on AChE activity in the injured rat spinal cord (Karnovsky-Roots method, light microscope, × 100).
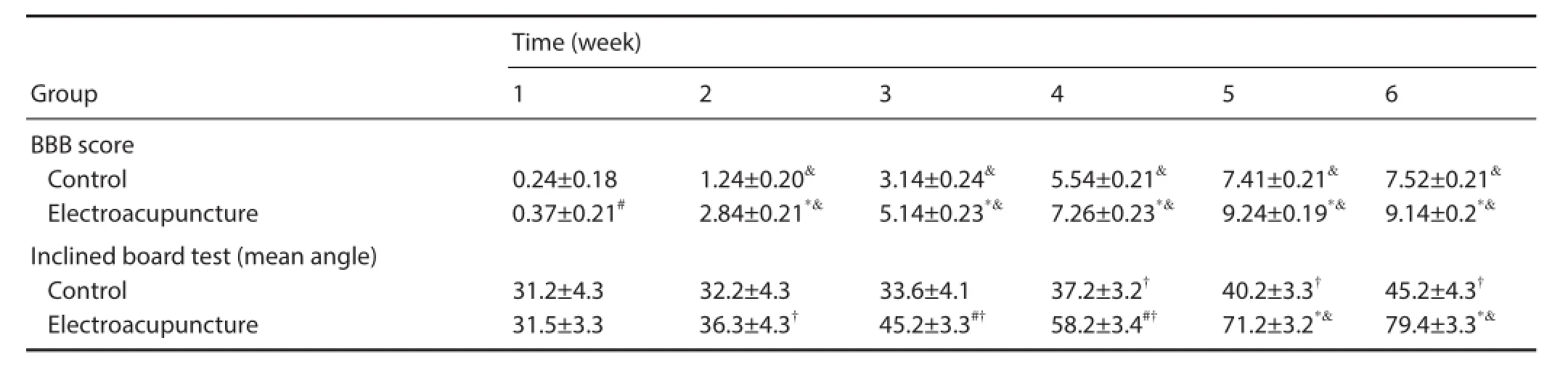
Table 4 Ef ects of electroacupuncture on neurological function in rats with spinal cord injury
Ef ects of electroacupuncture on the morphology of neurons and the number of motor neurons in the anterior horn of rats with SCI
Using the thionine-giemsa method, the background was light pink in each group. Nuclei were darkly stained blue. Nissl bodies exhibited royal blue f uorescence. The Nissl bodies were massive around the nuclei, and were much smaller proximal to the edges (Figure 4). At 4 weeks after electroacupuncture, Nissl bodies were abundant and large in the electroacupuncture group (Figure 4A). In the control group, a small number of Nissl bodies were visible and were lightly stained. Cellular edema and vacuolar degeneration were seen, and Nissl bodies disappeared (Figure 4B). Nissl body staining revealed that at 2, 4 and 6 weeks after electroacupuncture, the number of motor neurons in the anterior horn of the spinal cord was signif cantly higher in the electroacupuncture group compared with the control group (P < 0.01). Signif cant dif erences in the number of motor neurons were found in the electroacupuncture group at the dif erent time points (P < 0.05; Table 2). These results suggest that electroacupuncture promotes the survival of motor neurons in the anterior horn of the spinal cord.
Ef ects of electroacupuncture on AChE activity in rats with SCI
At 4 weeks after electroacupuncture, AChE activity diminished, and staining was weak in motor neurons of the anterior horn of the spinal cord (Figure 5). Compared with the control group (Figure 5A), AChE staining at the injury site was slightly darker in the electroacupuncture group (Figure 5B). Mean gray values for the various groups are given in Table 3. At 2, 4 and 6 weeks after electroacupuncture, the gray value was signif cantly less in the electroacupuncture group compared with the control group (P < 0.01), suggesting that electroacupuncture increased AChE activity in motor neurons in the anterior horn in the early stage of SCI. In the control group, the gray value was highest at 4 weeks compared with the other time points (P < 0.01). These resultsdemonstrate that AChE activity was lowest at 4 weeks after electroacupuncture, and gradually increased by 6 weeks.
Ef ects of electroacupuncture on the hindlimb motor function of rats with SCI
At 1 week after electroacupuncture, the BBB score was signif cantly greater in the electroacupuncture group compared with the control group (P < 0.05). Compared with 1 week after treatment, the BBB score was signif cantly higher in both groups at 2 weeks (P < 0.01), but the increase was greater in the electroacupuncture group than in the control group (P <0.01; Table 4).
Inclined board test results demonstrated that the critical angle increased with time after injury in both groups. No signif cant dif erence in the critical angle was observed at 2 weeks after treatment (P > 0.05), but the increase in the critical angle was greater in the electroacupuncture group than in the control group at 3 weeks. Signif cant dif erences in the critical angle were detected between the electroacupuncture group and the control group at 3–4 weeks (P < 0.05) and at 5–6 weeks (P < 0.01). There were signif cant dif erences in the critical angle between 1 and 2 weeks in the electroacupuncture group (P < 0.05), and the dif erence was extremely signif cant at 5 weeks (P < 0.01). Signif cant dif erences in the critical angle were also seen in the control group between 1 and 4 weeks (P < 0.05; Table 4).
Discussion
Electroacupuncture has been shown to produce an increase in the number AChE-positive cells and AChE activity in the midbrain reticular formation, suggesting that electroacupuncture enhances AChE activity in neurons (Ramey and Archer, 1993). In the present study, we found that electroacupuncture at Zusanli (ST36) increased contraction of the contralateral gastrocnemius muscle, suggesting that electroacupuncture enhances cholinergic activity in the nerve. We presume that electroacupuncture-induced sensory impulses in deep tissues are transmitted af erently and activate motor neurons at the level of the spinal cord, which results in increased cholinergic activity, resulting in muscle contraction.
Zusanli (ST36), Xuanzhong (GB39), Futu (ST32) and Sanyinjiao (SP6) are two groups of acupoints in the sciatic nerve projection area dominated by the L3–6spinal segments (Ramer and Bisby, 1998). These acupoints have been shown to be associated with spinal cord plasticity (Takeshige et al., 1990). The present study demonstrates that electroacupuncture exerts noticeable protective ef ects on neurons after SCI. AChE activity was substantially higher in the electroacupuncture group at the various time points compared with the control group, which shows that electroacupuncture can alleviate the reduction in AChE activity in motor neurons of the anterior horn in the early stage of SCI. The number of Nissl bodies and the number of large and mid-sized neurons stained by Nissl staining were greater in the electroacupuncture group than in the control group and increased over time. These f ndings suggest that electroacupuncture promotes the survival of motor neurons in the anterior horn of the spinal cord within 3 months after SCI, and that it enhances protein synthesis. Indeed, it was previously shown that acupuncture promotes the formation of Nissl bodies (Gulino and Gulisano, 2013), protein synthesis, and recovery following nerve injury.
The BBB score and the critical angle of the inclined board were higher in the electroacupuncture group at the various time points compared with the control group, which demonstrates that electroacupuncture contributes to the recovery of hind limb motor function. Furthermore, electroacupuncture simultaneously increased GDNF mRNA expression in the spinal cord. GDNF is a major trophic factor for nerve cells, and its expression can be increased by electroacupuncture after SCI. BBB scores showed an improvement in motor function, which suggests that GDNF may protect nerve cells and promote nerve repair and regeneration. Collectively, our f ndings provide insight into the mechanisms underlying the therapeutic ef cacy of electroacupuncture for SCI.
Electroacupuncture has been shown to increase BDNF expression and to enhance AChE activity in nerve cells in the midbrain reticular formation (Ledergerber, 1984; Ramey and Archer, 1993). However, our present study is the f rst to demonstrate that electroacupuncture promotes GDNF mRNA expression in motor neurons of the anterior horn after SCI. This enhancement of GDNF mRNA expression may have compensated for the reduction in GDNF expression resulting from the death of a large number of cells, ef ectively preventing neuronal apoptosis, and increasing cell viability. Indeed, increased GDNF expression has a neuroprotective ef ect and promotes recovery of motor neuron function (Yuan et al., 2007; Koeberle and Bahr, 2008; Naoi and Maruyama, 2009), consistent with the present study. Previous studies have also shown that GDNF mitigates the reduction in AChE activity in motor neurons after SCI (Vianney et al., 2014) and promotes functional recovery (Xu et al., 2013).
Taken together, our f ndings suggest that increased GDNF mRNA expression and increased AChE activity play important roles in the recovery of motor function. A limitation of our study is that AChE activity was not compared between the various time points. Future studies will address this issue.
Acknowledgments: We are very grateful to the staf who provided the experimental site-Central Laboratory, Health Science Center, Xi’an Jiaotong University in China and the staf from Experimental Rat Center for providing rats. We thank Lian-he Zhang from Department of Public Health, Health Science Center, Xi’an Jiaotong University in China for data processing. Author contributions: JHY provided data, ensured the integrity of the data, participated in study concept and design, data analysis, wrote the paper, was in charge of manuscript authorization and statistical analysis, and obtained the funding. JGL provided technical and data support. HW performed the experiments. HYN served as a principle investigator. All authors approved the f nal version of the paper.
Conf icts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Bakshi A, Shimizu S, Keck CA, Cho S, Sean CB, David G, Morales D, Arenas E, Snyder EY, Watsn DT, Mclntosh TK (2006) Neural progenitor cells engineered to secrete GDNF show enhanced survival neuronal dif erentiation and improve cognitive function following traumatic brain injury. Eur J Neurosci 23:2119.
Bar KJ, Saldanha GJ, Rennedy AT, Facer P, Birch R, Caristedt T, Anand P (1998) GDNF and its receptor component Ret in injured humen nerves and dorsal root ganglia. Neuroreport 9:43-47.
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for openf eld testing in rats. J Neurotrauma 12:1.
Black P, Markowitz RS, Damjanov I, Finkelstein SD, Kushner H, Gillespie T, Feldman M (1988) Model of spinal cord injury: Part 3 dynamic load tech-nique. Neurosurgery 22:51-54.
Bregman BS, McAtee M, Dai HN, kuhn PL (1997) Neurophic factors increase axonal growth after spinal coral injury and transplantation in the adult rat. Exp Neurol 148:475-494.
Cheng H, Huang SS, Lin SM (2005) The neuroprotective ef ect of glial cell line-derived neurotrophic facror in f brin in glue against chronic focal cerebral ischemia in conscious rats. Brain Res 1033:28.
Engesser-cesar C, Anderson AJ, Basso DM (2005) Voluntary wheel running improves recovery from a Moderate spinal cord injury. J Neurotrauma 22:157.
Gulino R, Gulisano M (2013) Noggin and Sonic hedge hog are involved in compensatory changes within the motoneuron-depleted mouse spinal cord. J Neurosci 332:102-109.
Koeberle PD, Bahr M (2008) The upregulation of GLAST-1 is an indirect antiapoptotic mechanism of GDNF and neurturin in the adult CNS. Cell Death Dif er 15:471.
Ledergerber CP (1984) Spinal cord injuries treated by TENS and transcutaneous dectriacupuncture. Am J Acupuncture 12:149-152.
Lindroos OF (1991) Short Nissl staining for in cubated cryostat sections of the brain. Biotech Histochem 66:208-209.
McCullough MJ, Gyorkos AM, Spitsbergen TM (2013) Shopt-term exercise increase GDNF protein levels in the spinal cord of young and old rats. Neuroscience 240:258-268.
Nadeau KC, Azuma H, Tilney NL (1995) Sequential cytokine dynamics in chronic rejection of rat renal allografts: roles for cytokines RANTES and MCP-1. Proc Natl Acad Sci U S A 92:8729-8733.
Nakamura M, Fujimura Y, Yato Y, Watanabe M, Yabe Y (1996) Changes in choline acetyltransferase activity and distribution following incomplete cervical spinal cord injury in the rat. J Neuroscience 75:481-494.
Naoi M, Maruyama W (2009) Functional mechanism of neuroprotection by inhibitors of type B monoamine oxidase in Parkinson’s disease. Expert Rev Neurother 9:1233.
Nayak S, Matheis RJ, Agostinelli S, Shif eft SC (2001) The use of complementary and altemative therapies for chronic pain following spinal cord injury: a pilot survey. J Spinal Cord Med 24:54-62.
Ramer MS, Bisby MA (1998) Dif erences in sympathetic innervation of mouse DRG following proximal or distal nerve lesions. Exp Neurol 152:197-207.
Ramey JW, Archer DF (1993) Peritoneal f uid its relevance to the development of endometriosis. Fertil Steril 60:1-14.
Rivlin AS, Tator CH (1997) Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg 47:577-581.
Schaar DG, Sieber BA, Sherwood AC, Dean D, Mendoza G, Ramakrishnan L, Dreyfus CF, Black IB (1994) Multiple astrocyte transcripts encode nigral trophic factors in rat and human. Exp Neurol 130:287-193.
Takeshige C, Luo CP, Hishida F, Igarashi O (1990) Differentiation of acupuncture and nonacupuncture points by dif erence of associated opioids in the spinal cord in production of analysia by acupuncture and nonacupuncture point stimulation and relations between sodium and those opioids. Acupunct Electrother Res 15:193.
Vianney JM, Miller DA, Spitsbergen TM (2014) Ef ects of acetylcholine andb electrical stimulation on glial cell line-derived neurotrophic factor production in skeletal muscle cells. Brain Res 1588:47-54.
Vianney JM, Spitsbergen TM (2011) Cholinergic neurons regulates secretion of glial cell line-derived neurotrophic factor by skeletal muscle cells in culture. Brain Res 1390:1-9.
Xu P, Rosen KM, Hedstrom K, Rey O, Guha S, Hart C, Corfas G (2013) Nerve injury induces glial cell line-derived neurotrophic factor(gdnf) expression in Schwann cells through purinergic signaling and the pkc-pkd pathway. Glia 61:1029-1040.
Yuan QL, Yang CX, Xu P, Gao XQ, Chen P, Sun ZL, Chen QY (2007) Neuroprotective ef ects of ginsenoside Rb1 on transient cerebral ischemia in rats. Brain Res 1167:1.
Zhang N, Fang M, Chen H, Gou F, Ding M (2014) Evaluation of spinal cord injury animal models. Neural Regen Res 9:2008-2012.
Copyedited by Patel B, Raye W, Wang J, Qiu Y, Li CH, Song LP, Zhao M
*Correspondence to: Jian-hui Yang, hiuyang@sina.cn.
orcid: 0000-0001-5470-890X (Jian-hui Yang)
10.4103/1673-5374.172323 http://www.nrronline.org/
Accepted: 2015-05-20
- 中國神經(jīng)再生研究(英文版)的其它文章
- Neuroplasticity in post-stroke gait recovery and noninvasive brain stimulation
- Structural and functional connectivity in traumatic brain injury
- Neglected corticospinal tract injury for 10 months in a stroke patient
- Polyurethane/poly(vinyl alcohol) hydrogel coating improves the cytocompatibility of neural electrodes
- Transplantation of human telomerase reverse transcriptase gene-transfected Schwann cells for repairing spinal cord injury
- A novel method for evaluating brain function and microstructural changes in Parkinson’s disease

