Cytokine and Antioxidant Regulation in the Intestine of the Gray Mouse Lemur (Microcebus murinus)During Torpor
Shannon N.TessierBarbara A.KatzenbackFabien Piferi Martine PerretKenneth B.Storey*e
1Institute of Biochemistry&Department of Biology,Carleton University,Ottawa,ON K1S 5B6,Canada
2UMR 7179 Centre National de la Recherche Scientifque,Muse′um National d’Histoire Naturelle,Brunoy 91800,France
3Department of Surgery&Center for Engineering in Medicine,Massachusetts General Hospital&Harvard Medical
School,Charlestown,MA 02129,USA
4Department of Biology,University of Waterloo,Waterloo,ON N2L 3G1,Canada
Cytokine and Antioxidant Regulation in the Intestine of the Gray Mouse Lemur (Microcebus murinus)During Torpor
Shannon N.Tessier1,3,#,a,Barbara A.Katzenback1,4,#,b,Fabien Piferi2,c, Martine Perret2,d,Kenneth B.Storey1,*,e
1Institute of Biochemistry&Department of Biology,Carleton University,Ottawa,ON K1S 5B6,Canada
2UMR 7179 Centre National de la Recherche Scientifque,Muse′um National d’Histoire Naturelle,Brunoy 91800,France
3Department of Surgery&Center for Engineering in Medicine,Massachusetts General Hospital&Harvard Medical
School,Charlestown,MA 02129,USA
4Department of Biology,University of Waterloo,Waterloo,ON N2L 3G1,Canada
Primate torpor;
Cytokines;
Chemokines;
Antioxidant enzymes;
Gut immunology
During food shortages,the gray mouse lemur(Microcebus murinus)of Madagascar experiences daily torpor thereby reducing energy expenditures.The present study aimed to understand the impacts of torpor on the immune system and antioxidant response in the gut of these animals. This interaction may be of critical importance given the trade-off between the energetically costly immune response and the need to defend against pathogen entry during hypometabolism.The protein levels of cytokines and antioxidants were measured in the small intestine(duodenum,jejunum, and ileum)and large intestine of aroused and torpid lemurs.While there was a signifcant decrease of some pro-infammatory cytokines(IL-6 and TNF-α)in the duodenum and jejunum during torpor as compared to aroused animals,there was no change in anti-infammatory cytokines.We observed decreased levels of cytokines(IL-12p70 and M-CSF),and several chemokines(MCP-1 and MIP-2)but an increase in MIP-1α in the jejunum of the torpid animals.In addition,we evaluated antioxidant response by examining the protein levels of antioxidant enzymes and total antioxidant capacity provided by metabolites such as glutathione(and others).Our results indicatedthat levels of antioxidant enzymes did not change between torpor and aroused states,although antioxidant capacity was signifcantly higher in the ileum during torpor.These data suggest a suppression of the immune response,likely as an energy conservation measure,and a limited role of antioxidant defenses in supporting torpor in lemur intestine.
Introduction
A variety of small mammals use torpor to reduce energy expenditures and enhance survival during inactive parts of the day especially when faced with stressful conditions(e.g.,nutrient or water limitation,or low ambient temperatures).By suppressing metabolic rate and allowing core body temperature(Tb)to fall,animals extend the time that they can survive using internal fuelreservesalone.Thegraymouselemur(Microcebusmurinus) uses daily torpor cycles during the dry season as an energy conservation mechanism,which is thought to be related to the extremefuctuationsofthe resourceavailability in the seasonally-aridregionsofMadagascarwherethisspeciesinhabits[1,2].Indeed,as conditions dictate,these lemurs can also extend torpor into multi-day bouts of hibernation,a capability previously-unknown for primate species.Daily torpor lasts 9.3 honaveragebutmaybeaslongas17.5 h[2].Duringtorpor,Tbcanvarysubstantiallyfromrelativelyhighvaluesof28–32°C to recorded lows of 7.8°C,with mean values around 17.3°C (depending in part upon the ambient temperature)[2].Lemurs exhibit low resting metabolic rates and can further reduce their basal metabolic rate to 20%–30%of their euthermic counterpartsbyenteringtorpor,therebyconservingsignifcantamounts of fuel and energy[2–5].The reallocation of energy resources toward essential metabolic processes may create energy defcits in other‘‘non-essential’’physiological processes,such as the activity of immune system.
Mucosal tissues,such as the intestinal mucosa,are primary sites of entry for pathogens.Under normal conditions,the specialized immune cells and molecules that comprise the mucosal immune system defend against pathogen entry and replication, thereby maintaining animal health.However,the immune system is energetically costly to maintain and activate and may be selectively regulated during periods of hypometabolism, thereby compromising mucosal immunity and predisposing animals to disease during this time.For example,modulation of the intestinal immune system during seasonal hibernation has been reported in some small mammals such as ground squirrels[6].This is thought to occur in part due to energetic constraints under hypometabolic conditions,but may also be linked to atrophy of the intestinal tract villi or a shifting microbial environment resulting from prolonged or repeated fasting over the winter months[7].A number of soluble mediators regulate the recruitment,activation,and deactivation of innate immune cells.Chemokines such as monocyte chemoattractant protein-1(MCP-1),macrophage infammatory protein-1 alpha (MIP-1α),and macrophage inhibitory protein-2(MIP-2) regulate the recruitment of immune cells[8–10].In addition, tumor necrosis factor-alpha(TNF-α),macrophage colonystimulating factor (M-CSF),interferon-gamma (IFN-γ), interleukin-12 subunit p40(IL-12p40),IL-12p70,IL-1β,and IL-6 regulate the pro-infammatory processes,whereas IL-10 and transforming growth factor-beta (TGF-β)actas anti-infammatory mediators[8–10].Together,these cytokines provide an overview of the regulation of the immune response.
The balance between pro-infammatory and anti-infammatory cytokines mediates homeostasis in an organism,while the selective regulation and proper functioning of the immune response are important for defense against invading pathogens.
Oxidative stress pathways are intricately linked to immune function and play critical roles in the general health of the gastrointestinal tract.The immune system uses oxidants as a mechanism of killing pathogens.For example, phagocytes produce both reactive oxygen species(ROS) and reactive nitrogen species(RNS),such as superoxide and nitric oxide,which are highly toxic and damaging to target pathogens[11].While enhanced antioxidant defenses plays a role in deep torpor,antioxidant responses also play crucial roles during a rapid rise in ROS generation associated with the huge increase in oxygen uptake and consumption that powers arousal from torpor[12].Indeed,the involvement of antioxidant enzymes and metabolites appears to be an essential characteristic of most forms of hypometabolism[13],including playing an established cytoprotective role in the intestine of hibernating mammals[14].Important antioxidant enzymes include superoxide dismutase(SOD), present as the Cu/Zn-dependent cytoplasmic form(SOD1) and the Mn-dependent mitochondrial form(SOD2),catalase,peroxiredoxin 2(PRX2),and thioredoxin(TRX1).
The present study examines the regulation of cytokines and antioxidants in the small and large intestine of aroused and torpid gray mouse lemurs.Multiplex and total antioxidant assays were used to determine the relative levels of pro-infammatory cytokines,anti-infammatory cytokines,chemokines,and antioxidantenzymesaswellasantioxidantmetabolites.Thedifferential levels of cytokines in the various intestinal sections of lemurs during torpor suggest regulation of the local immune responseinthegutoflemursduringhypometabolism.However, total protein levels of antioxidant enzymes did not change in response to torpor,although minimal changes in total antioxidant capacity(the sum of low molecular mass antioxidants) were observed in the ileum during torpor.These data suggest either low involvement of antioxidant defenses in the torpor response,or that antioxidant defenses are responsive to other factors not identifed in the present study.
Results
Regulation of pro-infammatory cytokine protein levels in the intestine during torpor
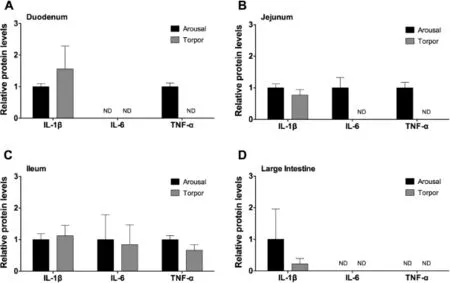
Figure 1 Relative protein levels of pro-infammatory cytokines in the intestine of aroused and torpid lemurs
To assess the regulation of pro-infammatory cytokines in the duodenum,jejunum,ileum,and large intestine,the relative protein levels of IL-1β,IL-6,and TNF-α were examined in torpid and aroused animals.While IL-1β was detected in all intestine samples of torpid and aroused animals,no signifcant differences were observed between torpid and aroused animals (Figure 1).IL-6 protein was detected in the ileum of aroused and torpid lemurs,however,there was no signifcant difference between these two states(P>0.05,Figure 1C).The levels of IL-6 were below the detection limit of the multiplex kit in the duodenum(Figure 1A for both),jejunum(Figure 1B for torpid animals),and large intestine(Figure 1D for both).Similarly,TNF-α was detected in the ileum of aroused and torpid animals,but without signifcant difference(Figure 1C). TNF-α was detected in the duodenum(Figure 1A)and jejunum(Figure 1B)of aroused animals but was below the detectable limit in these tissues of torpid animals.TNF-α could not be detected in the large intestine of aroused or torpid animals (Figure 1D).Taken together,the pro-infammatory cytokines examined in this study were detected in the ileum without signifcant change between the arousal and torpor states,while more IL-6 and TNF-α were present in the jejunum of aroused versus torpid animals.
Regulation of anti-infammatory cytokine protein levels in the intestine during torpor
Production of anti-infammatory cytokines,such as IL-4 and IL-10,are important in dampening and resolving an infammatory response[10].To determine whether an anti-infammatory response occurred in the gastrointestinal tract of torpid lemurs, the relative protein levels of IL-4 and IL-10 were measured.IL-4 and IL-10 were detected in the duodenum,jejunum,ileum, and large intestine for both aroused and torpid animals(Figure 2).However,there were no signifcant differences between aroused and torpid animals(P>0.05).Although,IL-10 protein levels appeared to be lower in the duodenum(Figure 2A) and jejunum(Figure 2B)of torpor animals,the high variability of IL-10 levels in aroused animals precluded the ability to detect signifcant differences.
Regulation of IL-12 and M-CSF cytokine protein levels in the intestine during torpor
The pro-infammatory IL-12p70 cytokine is comprised of two subunits:IL-12p35 and IL-12p40 [9].While the IL-12p70 heterodimer acts as a pro-infammatory signal, the IL-12p40 subunit on its own blocks the proinfammatory action of IL-12p70 by acting as an antagonist.The IL-12p40 homodimer binds to the IL-12 receptor thereby blocking IL-12p70 mediated intracellular signaling [9].IL-12p40 was detectable in the duodenum,jejunum, and ileum(Figure 3A–C),but there was no signifcant difference between aroused and torpid animals.In the large intestine,IL-12p40 was detectable in aroused,but not torpid animals(Figure 3D).Protein levels of IL-12p70 were not signifcantly different between aroused and torpid animals in the duodenum,the ileum,or the large intestine (Figure 3).However,IL-12p70 protein levels were below the detection limitin thejejunum oftorpid animals (Figure 3B).
M-CSF has a multitude of activities and is best known for its role in the development of mature macrophages from early myeloid progenitor cells[8].It also has documented roles in initiating infammatory responses in mature macrophages [8].No signifcant differences,however,were observed in M-CSF levels in the duodenum,ileum,or large intestine(Figure 3A,C,and D)between control and torpid animals.However,while M-CSF levels were detectable in the jejunum of aroused animals,the levels of M-CSF were below the detection limitin thejejunum oftorpid animals (Figure 3B).
Regulation of chemokine protein levels in the intestine during torpor
Chemokinesareafamilyofcytokinesthatacttorecruitimmune cells to the site of infection,or alternatively,to control cell migration into tissues during homeostasis.To assess the modulation of chemokines in gray mouse lemurs during torpor,the relative levels of the chemokines MCP-1,MIP-1α,and MIP-2 were examined in the different sections of the intestine in aroused and torpid lemurs.MCP-1 was not detected in all intestine samples expect in jejunum of aroused animals(Figure 4). Conversely,MIP-1α and MIP-2 were detected in all samples for both animal states.MIP-1α protein levels were signifcantly higher in the jejunum of torpid animals compared to those in aroused animals(P=0.029,Figure 4B),however,MIP-1α protein levels did not change signifcantly in duodenum,ileum or large intestine(Figure 4A,C,and D).Similarly,levels of MIP-2 were comparable between the two states in the duodenum,ileum,and large intestine.However,signifcantly lower levels of MIP-2 were found in the jejunum of torpid animals compared to those of aroused animals(P=0.049,Figure 4B).
Regulation of antioxidant defenses in the intestine during torpor
Giventhelinkbetweentheimmuneresponseandoxidativestress (e.g.,respiratoryburst),wemeasuredtherelativelevelsofantioxidantenzymes(Figure5)andmetabolites(Figure6)intheduodenum,jejunum,ileum,and large intestine of aroused and torpid lemurs.First,weexaminedthe protein expressionof antioxidant enzymes including catalase,SOD1,SOD2,TRX1 and PRX2. Our results indicated comparable protein levels for all antioxidant enzymes between aroused and torpid lemurs in all the intestinal subsections examined(Figure 5).We then assayed the total antioxidant capacity using the sum of glutathione, ascorbate(vitamin C),vitamin E,bilirubin,bovine serum albumin(BSA),and uric acid as a readout(each are important compliments to the enzymatic antioxidant response).The total antioxidant metabolite capacity in the ileum increased by 40% during torpor,compared to aroused animals(P<0.05)(Figure 6).However,no signifcant changes were observed in other sections of the intestine between aroused and torpid lemurs.
Discussion
Daily torpor cycles represent large changes in an animal’s physiology over short periods of time.Along with cessation of feeding,torpor is characterized by loweredTband metabolic rate[15]as well as reduced physiological functions including heart beat and breathing rate.Data collected from free-ranging gray mouse lemurs show that daily torpor bouts are several hours in duration with a minimum Tbof~27°C whereas hibernation bouts in this species can last up to 4 weeks with a minimumTbof 11.5°C[16].Previous studies on torpor and the immune system have shown that immune function can be compromised or depressed during torpor while being restored or,in some cases,heightened during arousal[6].Studies on little brown bats have demonstrated the susceptibility of this species to white nose disease during hibernation,suggesting a depressed or unresponsive systemic immune response [17–23].A similar immune suppression response has been seen in the 13-lined ground squirrel,which can enter torpor for a period of 6–40 days[6,24–27].Hibernating squirrels displayed lower numbers of circulating leukocytes and proliferative splenic T-cells and were unable to respond to injection with lipopolysaccharide(LPS)[25–27].Indeed,one proposal for the function of interbout arousals in hibernators is that theyserve to initiate or restore optimal immune system function for the purposes of dealing with potential pathogenic threats that may be encountered during hibernation[26].Given that immune system function and oxidative stress pathways are intrinsically linked(e.g.,phagocytic leukocytes produce superoxide()[11]),we endeavored to characterize their responsein the intestine by comparing aroused and torpid gray mouse lemurs.
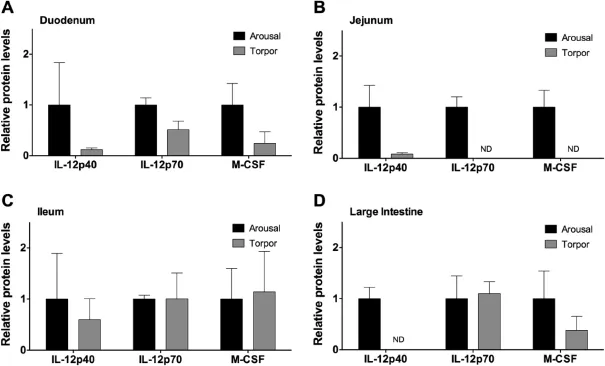
Figure 3 Relative protein levels of IL-12 subunits and M-CSF in the intestine of aroused and torpid lemurs
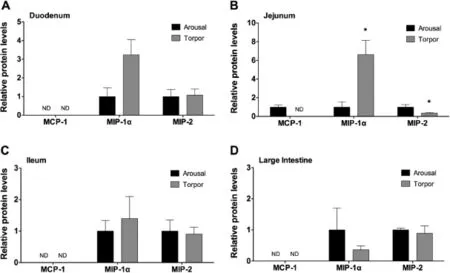
Figure 4 Relative protein levels of chemokines in the intestine of aroused and torpid lemurs
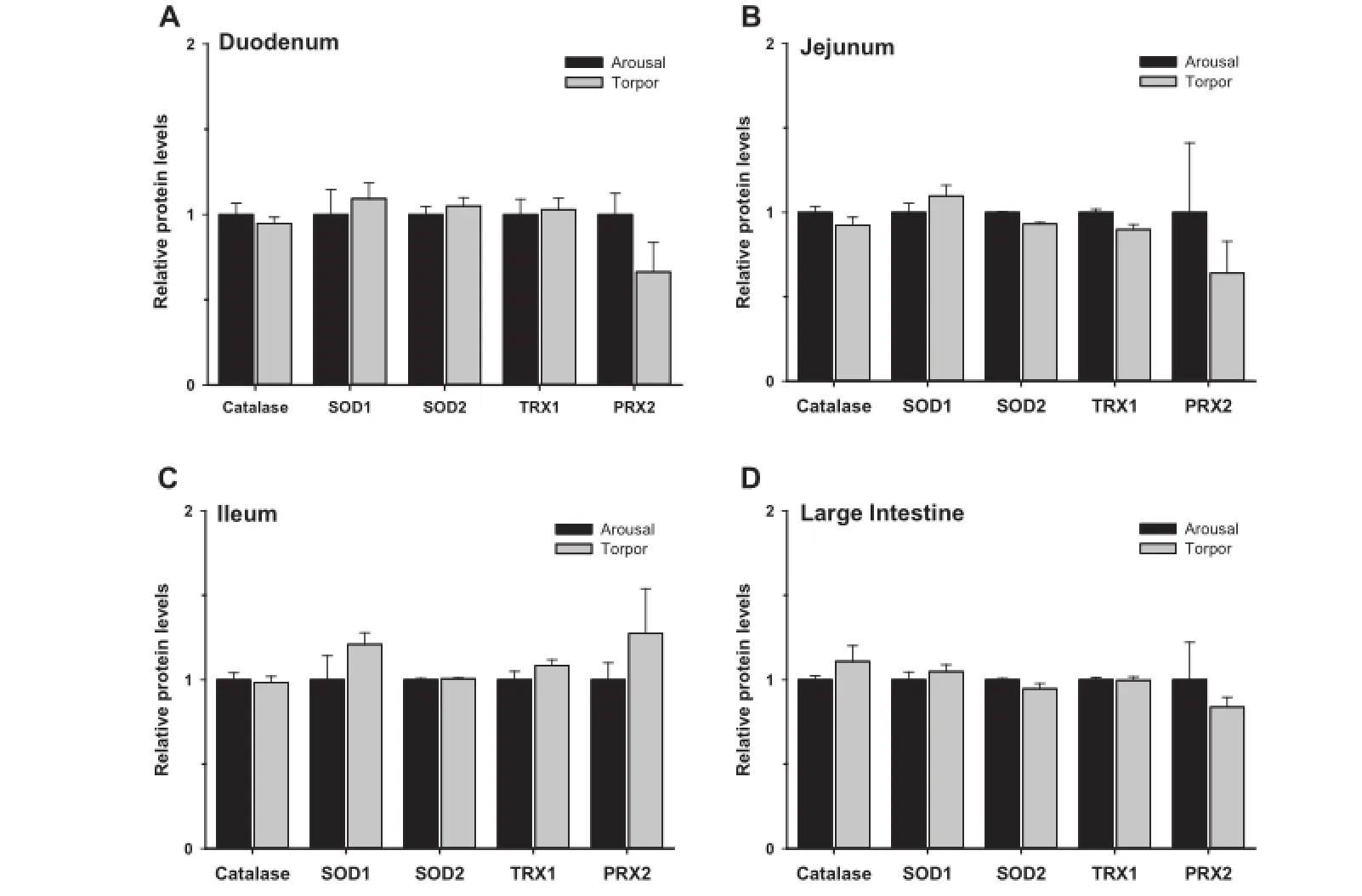
Figure 5 Relative protein levels of antioxidant enzymes in the intestine of aroused and torpid lemurs
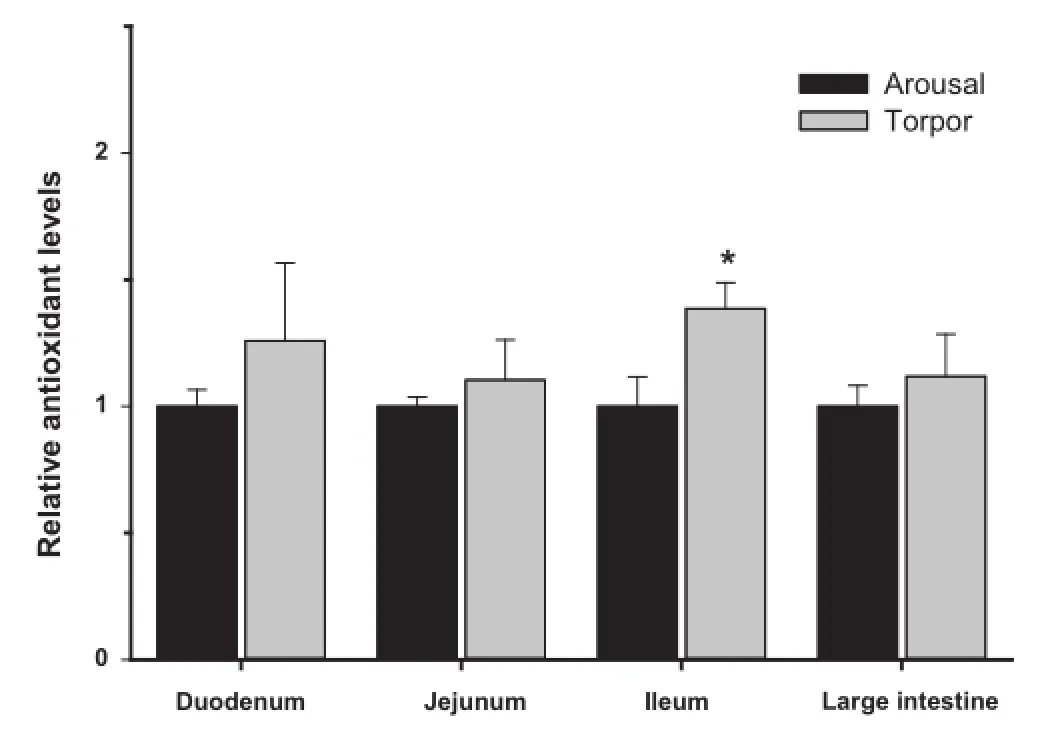
Figure 6 Relative antioxidant capacity in the intestine of aroused and torpid lemurs
The intestinal mucosal system is in constant contact with the externalenvironmentandisexposedtoahighantigenicburden, composed of both commensal and pathogenic microfora [28–33].As such,the intestinal mucosa is equipped with a tailored immune system comprised of specialized mucosalassociatedstructures,suchasPeyer’spatches,mesenteric lymph nodes,and diffuse immune cells[28–33].Commensal microbial fora aid in nutrient acquisition and provide metabolic competition with pathogenic microbes.Commensal microbes also help with the development and stimulation of the intestinal immune system,thereby enhancing the capability of the system to fend off pathogenic bacteria[29].Meanwhile,the general tolerance of the intestinal immune system,in part dictated by regulatory T-cells and the secretion of anti-infammatory cytokines such as IL-4,IL-10,and TGF-β permits the survival and growth of intestinal microfauna[28,29,32].Thus,a balance exists between the intestinal immune system and the microbial fauna that exist and persist in the gastrointestinal tract.
Studies examining the effects of torpor and/or seasonal hibernation on the mucosal immune system are limited.Work with 13-lined ground squirrels demonstrated the regulation of intestinalleukocytenumbersand maturation stage,as well as cytokine levels during hibernation[6].In the present study,examination of pro-infammatorycytokines,antiinfammatory cytokines,and chemokines in the duodenum, jejunum,ileum,and large intestine in aroused and torpid animals revealed that the most obvious modulation of cytokine and chemokine levels was in the jejunum.In the jejunum of torpid lemurs,protein levels of the pro-infammatory cytokines IL-6,TNF-α,IL-12p70,andM-CSFweregreatlyreduced,often tobelowthedetectable limitofthe multiplexcytokinekit.However,protein levels of anti-infammatory cytokines such as IL-4 andIL-10intorpidanimalswerenotsignifcantlydifferentfrom those in aroused lemurs.These results are suggestive of a suppression of the mucosal immune response during torpor and are similar to fndings of immune suppression during torpor inhibernators,such as bats and ground squirrels,from cold climates[25].In the large intestine,higher levels of IL-12p40 were observedinarousedlemurscomparedtotorpidlemurs,suggesting that IL-12p40 may be produced as a mechanism to limit infammatory signals mediated by the IL-12 receptor during arousal in this region of the intestine.However,the generalized reductioninlevelsofpro-infammatorycytokinesmaybedueto a decrease in the energy budget of the animal during torpor or may,in part,be due to the interruption of feeding and the loweredantigenicburdenonthegutduringthisperiod[34].Inmammalian species that do not undergo periods of torpor or hibernation,the circadian clock has been shown to govern the systemic immune system as reviewed in[35].During periods of rest in these mammals,the immune system is also at rest and is activated immediately before the mammals re-enter their active state[35].Indeed,pathogen challenge experiments have demonstrated that non-hibernating mammals are most susceptibleto infection atthe endoftheir reststate,prior tothe activation of the immune response[35].Daily bouts of torpor in the gray mouse lemur may very well involve this same circadianclock controlled immune system response.However,further studies are warranted to determine if daily torpor exaggerates theeffectsobservedasaresultofcircadianrhythms.Regardless, the decrease in pro-infammatory cytokines in the intestinal mucosa of lemurs during torpor may signal an increased risk of infection in the same way as hibernating species that may be underprepared to respond to pathogens during their prolonged torpor bouts[21,22,26,27].
Chemokines play an important role in the immune response since their production is often triggered by a danger signal and acts to recruit varying types of cells to the site of insult.In this study,we examined the protein levels of MCP-1,MIP-1α,and MIP-2.Like the response of the pro-infammatory cytokines, protein levelsofthechemokines,MCP-1and MIP-2, decreased in the jejunum of torpid lemurs.However,the protein level of MIP-1α was~5-fold higher in the jejunum of torpid lemurs and also tended to be higher in the duodenum of torpid lemurs.MIP-1α is chemoattractive to a variety of lymphocytes and leukocytes,but is particularly potent to neutrophils[36].The increased levels of MIP-1α in the jejunum during torpor may suggest the recruitment of lymphocytes and neutrophils to the intestine tissues.This is in agreement with the decrease in numbers of circulating lymphocytes and neutrophils concurrent with an infux of leukocytes into the gut,which has been observed during periods of torpor in a number of hibernating mammals[6,37]and as reviewed in [22].Thus,MIP-1α may mediate an infux of neutrophils and lymphocytes into the gut of torpid animals.However,further studies are needed to validate the recruitment of neutrophils into the gut tissues of the gray mouse lemur.
Cycles of torpor and arousal mimic ischemia–reperfusion. During torpor,heart rate and organ perfusion rates are reduced butriseagainveryrapidlywhennon-shiveringthermogenesisby brown adipose tissue is activated to restoreTbto a euthermic state.Enhanced cytoprotective strategies(e.g.,antioxidant response and unfolded protein response,)are known to play animportantrolein mammalianhibernators[12,38],suggesting that they may also be important during daily torpor.However, the protein levels of antioxidant enzymes presently measured in the duodenum,jejunum,ileum,and large intestine of aroused versus torpid lemurs were not signifcantly different.Furthermore,the antioxidant capacity represented by low molecular mass metabolites only increased in the ileum during torpor(by 40%).These data may suggest that the intestinal antioxidant defenses are only minimally required during the relatively shallowtorporexperiencedbygraymouselemurs.Indeed,studiesof arousal in hibernators indicate that intestinal blood fow is disproportionately low compared to other organs and that the gut isoneofthelast organstohavenormal bloodfowrestored[14]. Alternatively,antioxidant enzymes of other animals have recently been shown to be regulated by post-translational modifcations[39],opening up the possibility that other regulatory mechanisms(apart from changes in protein expression)might modulate antioxidant enzyme responses during primate torpor. Additionally,it is possible that other cytoprotective strategies not presently measured could also play an important role in the gut during primate torpor.
The data herein suggests that the intestinal immune response is suppressed during torpor relative to the aroused state,or conversely,that the immune response is activated in aroused animals relative to the torpor state.As such,gray mouse lemurs may be more vulnerable to pathogen attack via the gut during periods of hypometabolism if the immune system has a reduced ability to control gut pathogens during torpor,potentially allowing for pathogen outgrowth and invasion.However,the antioxidant defense system appears to play a minimal role in the intestine during primate torpor and arousal,although other cytoprotective mechanisms may be important.Finally,future studies may include deeper transcriptomic and/or proteomic analysis on daily torpor to identify more candidate genes,pathways or networks,which may mediate the transitions between daily torpor and arousal.
Materials and methods
Animals
Adult female gray mouse lemurs(2–3 years of age,n=8;mean body mass 106.3±15.5 g)used in this study were born in the authorized breedingcolony atthe National Museum ofNatural History(Brunoy,France).Animal holding,experimentation and sampling were conducted by Dr.Martine Perret and the MECADEV team (Mecanismes Adaptatifs et Evolution, Department of Ecology and Management of Biodiversity)as described previously[40,41].Briefy,mouse lemurs were separated into two experimental groups(n=4 aroused andn=4 torpid).Alllemurswereindividuallyhousedincagesinaclimate chamber,maintained under short day conditions,and torpor–arousal state was assessed by monitoringTband locomotor activity.Animals used for the torpor group were given a calorie-restricted diet for 5 days(60%of the control diet; 86×10ˉ3J/day versus a normal diet of 144×10ˉ3J/day)to enhancethedepthofdailytorpor,whereascontrolanimalswere fedad libitum.Aroused animals were sacrifced at the end of a daily torpor bout(Tb35–36°C),while torpid lemurs were sacrifced during a torpor bout(whenTbwas at its minimum,30–33°C).Animals were euthanized by approved protocols used by the French team(decapitation)and the tissue samples were rapidly excised and immediately frozen in liquid nitrogen.Frozen samples were packed in dry ice and air freighted to Carleton University(Ottawa,Canada)where they were stored atˉ80°C until use.The highest standards in ethics and transparency that are applied in Europe were used for all experiments,especiallythe Directive 86/609/EEC on the protection of animals used for experimental and other scientifc purposes.
Protein homogenates
Protein extracts of frozen duodenum,jejunum,ileum,and large intestine(~50 mg)were prepared as per the manufacturers’instructions provided with the Milliplex kits(EMD Millipore, Billerica,MA,USA).Briefy,tissue samples were Dounce homogenized 1:4 w:v in ice-cold lysis buffer(Millipore;catalog No.43-045)with the phosphatase inhibitors(1 mM Na3VO4and 10 mM ?-glycerophosphate)and protease inhibitors (BioShop,Canada,catalog No.PIC001).After incubation on ice with occasional agitation for 30 min,samples were centrifuged at 12,000×gfor 20 min at 4°C and the supernatants were collected as total soluble protein lysates.Soluble protein concentrations were determined using the Bradford assay(catalogNo.500-0005)andthenaliquotsoflysateswerestandardized to either 6 or 10 μg/μl for the antioxidant and cytokine panels, respectively.Separate aliquots were used for the antioxidant metabolite assay and were not standardized.Proteins extracts were stored atˉ80°C until further use.
Cytokine multiplex analysis
Acustomizedmousecytokine/chemokineMilliplexMapkitwas ordered from Millipore for analyzing cytokines/chemokines, including IL-1β,IL-4,IL-6,IL-10,IL-12p40,IL-12p70,MCP-1,MIP-1α,MIP-2,and TNF-α.Aliquots of standardized protein lysates(25 μl,10 μg/μl)were added to individual wells of thesupplied96-wellplate.Thenegativecontrolwas25 μloflysis buffer(backgroundcontrol).Anadditional25 μlofassaybuffer was then added to experimental and negative control wells. Mousecytokine/chemokinequalitycontrol 1(QC1)andquality control 2(QC2)(catalog No.MXM6070)were used as positive controls;each vial of lyophilized reagent was reconstituted with 250 μl deionized water,vortexed and allowed to sit for 5 min before 25 μl aliquots were added to the positive control wells as per manufacturer’s instructions.All wells then received a further 25 μl of lysis buffer followed by 25 μl of mixed beads.After overnight incubation at 4°C with continuous orbital shaking, theplatewaswashedtwotimeswithwashbuffer.Afterwashing, 25 μl of detection antibodies were then added to each well and incubated for 1 h at room temperature with orbital shaking. Subsequently,25 μl of streptavidin–phycoerythrin solution was added to each well and incubated for an additional 30 min at room temperature with orbital shaking.Wells were washed two times with 1×wash buffer,re-suspended in 150 μl of sheath buffer,and read on a Luminex?200 machine with Milliplex Analyst software(Millipore,Billerica,MA).
Oxidative stress multiplex analysis
The oxidative stress magnetic bead panel(catalog No. H0XSTMAG-18K,Millipore)wasusedfortheanalysisofcatalase,PRX2,SOD1,SOD2,andTRX1.Tissue extractswereprepared as above,standardized to 6 μg/μl and then aliquots were combined with Milliplex MAP Assay Buffer 1(catalog No. 43-045)to yield a fnal working concentration of 0.75 μg/μl, from which 25 μl(18.75 μg total protein)was added to individual wells.The negative control consisted of 25 μl of assay buffer (background control).For the positive control,25 μl of the provided HepG2 Cell Lysate(unstimulated),prepared as per manufacturer’s instructions,was added to wells.Aliquots of 25 μl of mixed beads were then added to all wells.The plate was incubated for 2 h at room temperature with continuous orbital shaking.Following incubation,the plate was washed three times with 1×wash buffer.After washing,50 μl of detection antibodies were added to each well and incubated for 1 h at room temperature with orbital shaking.Wells were once again washed three times with 1×wash buffer.Subsequently,50 μl of streptavidin–phycoerythrin solution was added and the plate was incubated for an additional 30 min at room temperature with orbital shaking.Wells were washed three times with 1× wash buffer,and then magnetic beads were re-suspended in 100 μl of sheath buffer and read on a Luminex?200 machine with Milliplex Analyst software(Millipore,Billerica,MA).
Antioxidant capacity assay
Total antioxidant capacity of lemur tissues was evaluated using the antioxidant assay kit from Cayman Chemical Company (catalog No.709001,Ann Arbor,MI,USA),which measures cumulativeantioxidantcapacityprovidedbyglutathione,ascorbate(vitamin C),vitamin E,bilirubin,BSA,and uric acid.The assayreliesontheabilityofantioxidantsinthesampletoinhibit metmyoglobin-catalyzed oxidation of 2,2′-Azino-di-[3-ethyl benzthiazoline sulfonate](ABTS).The assay was prepared as per manufacturer’s instructions.Briefy,the standard curve was prepared by combining 10 μl of a Trolox preparation (increasing amounts of reconstituted Trolox diluted with antioxidant assay buffer),10 μl of metmyoglobin(lyophilized powder reconstituted in antioxidant assay buffer),and 150 μl of Chromogen(containing ABTS reconstituted in HPLC-grade water)per well.Tissue lysates were prepared as described above.Samplewellswerepreparedbyadding10 μlofa100-fold dilution of each sample(diluted in antioxidant assay buffer), 10 μl of metmyoglobin,and 150 μl of chromogen.The reactions wereinitiatedbyadding40 μlof441 μMhydrogenperoxidefollowedbyincubatingonashakerfor5 minatroomtemperature. Absorbance was read at 750 nm,using a microplate reader,and quantifed as Trolox equivalents(mM/mg wet mass).
Statistical analysis
All numerical data are expressed as means±SEM(n=4). Statistical analysis was performed using SigmaPlot statistical package(v.12)software.All statistical testing used a twotailed Student’st-test.Differences were considered signifcant atP<0.05.
Authors’contributions
All authors contributed to the conception and design of the project and to the editing of the manuscript.MP and FP carried out the animal experiments and SNT and BAK conducted biochemical assays.Data analysis and writing of the manuscript were carried out by SNT,BAK,and KBS.All authors read and approved the fnal manuscript.
Competing interests
The authors have declared no competing interests.
Acknowledgments
We thank Janet M.Storey for editorial review of the manuscript and L.Haro and P.Guesnet for technical and material assistance in the preparation of the lemur tissue samples.This work was supported by a Discovery grant from the Natural Sciences and Engineering Research Council(NSERC)of Canada(Grant No.6793)and a grant from the Heart and Stroke Foundation of Canada(Grant No.G-14-0005874)to KBS.KBS holds the Canada Research Chair in Molecular Physiology;SNT held a NSERC postgraduate scholarship and BAK held a NSERC postdoctoral fellowship.
[1]Wright PC.Lemur traits and Madagascar ecology:coping with an island environment.Am J Phys Anthropol 1999(Suppl.29):31–72.
[2]Schmid J.Daily torpor in free-ranging gray mouse lemurs.Int J Primatol 2001;22:1021–31.
[3]Geiser F.Metabolic rate and body temperature reduction during hibernation and daily torpor.Annu Rev Physiol 2004;66:239–74.
[4]Schmid J.Oxygen consumption and torpor in mouse lemurs (Microcebus murinusandMicrocebus myoxinus):preliminary results of a study in western Madagascar.In:Geiser F,Hulbert AJ,Nicol SC,editors.Adaptations to the cold.The 10th Hibernation Symposium.Armidale,Australia:University of New England Press;1996.p.47–54.
[5]Schmid J.Daily torpor in the gray mouse lemur(Microcebus murinus)in Madagascar:energetic consequences and biological signifcance.Oecologia 2000;123:175–83.
[6]Kurtz CC,Carey HV.Seasonal changes in the intestinal immune system of hibernating ground squirrels.Dev Comp Immunol 2007;31:415–28.
[7]Carey HV,Walters WA,Knight R.Seasonal restructuring of the ground squirrel gut microbiota over the annual hibernation cycle. Am J Physiol Regul Integr Comp Physiol 2013;304:R33–42.
[8]Chitu V,Stanley ER.Colony-stimulating factor-1 in immunity and infammation.Curr Opin Immunol 2006;18:39–48.
[9]Gee K,Guzzo C,Mat NFC,Ma W,Kumar A.The IL-12 family of cytokines in infection,infammation and autoimmune disorders.Infamm Allergy Drug Targets 2009;8:40–52.
[10]Turner MD,Nedjai B,Hurst T,Pennington DJ.Cytokines and chemokines:at the crossroads of cell signalling and infammatory disease.Biochim Biophys Acta 2014;1843:2563–82.
[11]Robinson JM.Phagocytic leukocytes and reactive oxygen species. Histochem Cell Biol 2009;131:465–9.
[12]Storey KB,Storey JM.Metabolic rate depression:the biochemistry of mammalian hibernation.Adv Clin Chem 2010;52:77–108.
[13]Storey KB,Storey JM.Tribute to P.L.Lutz:putting life on‘pause’–molecular regulation of hypometabolism.J Exp Biol 2007;210:1700–14.
[14]Carey HV,Mangino MJ,Southard JH.Changes in gut function during hibernation:implications for bowel transplantation and surgery.Gut 2001;49:459–61.
[15]Storey KB.Out cold:biochemical regulation of mammalian hibernation–a mini-review.Gerontology 2010;56:220–30.
[16]Schmid J,Ganzhorn JU.Optional strategies for reduced metabolism in gray mouse lemurs.Naturwissenschaften 2009;96:737–41.
[17]Barlow A,Ford S,Green R,Morris C,Reaney S.Investigations into suspected white-nose syndrome in two bat species in Somerset.Vet Rec 2009;165:481–2.
[18]Blehert DS,Hicks AC,Behr M,Meteyer CU,Berlowski-Zier BM, Buckles EL,et al.Bat white nose syndrome:an emerging fungal pathogen?Science 2009;323:227.
[19]Buchen L.Disease epidemic killing only US bats.Nature 2010;463:144–5.
[20]Puechmaille SJ,Verdeyroux P,Fuller H,Gouilh MA,Bekaert M, Teeling EC.White nose syndrome fungus(Geomyces destructans) in bat,France.Emerg Infect Dis 2010;16:290–3.
[21]Zimmerman R.Biologists struggle to solve bat deaths.Science 2009;325:393.
[22]Bouma HR,Carey HV,Kroese FG.Hibernation:the immune system at rest?J Leukoc Biol 2010;88:619–24.
[23]Wibbelt G,Kurth A,Hellmann D,Weishaar M,Barlow A,Veith M,et al.White-nose syndrome fungus(Geomyces destructans)in bats,Europe.Emerg Infect Dis 2010;16:1237–43.
[24]McCarron RM,Siekmann DG,Yu EZ,Frerichs K,Hallenbeck JM.Hibernation,a state of natural tolerance to profound reduction in organ blood fow and oxygen delivery capacity.In: Storey KB,editor.Molecular mechanisms of metabolic arrest:life in limbo.Oxford:BIOS Scientifc Publishers;2001.p.23–42.
[25]Novoselova EG,Kolaeva SG,Maker VR,Agaphonova TA. Production of tumor necrosis factor in cells of hibernating ground squirrelsCitellusundulatusduring annualcycle.Life Sci 2000;67:1073–80.
[26]Prendergast BJ,Freeman DA,Zucker I,Nelson RJ.Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels.Am J Physiol Regul Integr Comp Physiol 2001;273:R1861–9.
[27]Spurrier WA,Dawe AR.Several blood and circulatory changes in the hibernation of the 13-lined ground squirrel,Citellus tridecemlineatus.Comp Biochem Physiol A 1973;44:267–82.
[28]Bernard D.Human intestinal dendritic cells as controllers of mucosal immunity.Rev Esp Enferm Dig 2013;105:279–90.
[29]Chang SY,Ko HJ,Kweon MN.Mucosal dendritic cells shape mucosal immunity.Exp Mol Med 2014;46:e84.
[30]Mayer L.Mucosal immunity.Pediatrics 2003;111:1595–600.
[31]Montalvillo E,Garrote JA,Bernardo D,Arranz E.Innate lymphoid cells and natural killer T cells in the gastrointestinal tract immune system.Rev Esp Enferm Dig 2014;106:334–45.
[32]Zeissig S,Blumberg RS.Commensal microbial regulation of natural killer T cells at the frontiers of the mucosal immune system.FEBS Lett 2014;588:4188–94.
[33]Caricilli AM,Castoldi A,Camara NOS.Intestinal barrier:a gentlemen’s agreement between microbiota and immunity.World J Gastrointest Pathophysiol 2014;5:18–32.
[34]Walrand S,Chambon-Savanovitch C,Felgines C,Chassagne J, Raul F,Normand B,et al.Aging:a barrier to renutrition? Nutritional and immunologic evidence in rats.Am J Clin Nutr 2000;72:816–24.
[35]Curtis AM,Bellet MM,Sassone-Corsi P,O’Neill LAJ.Circadian clock proteins and immunity.Immunity 2014;40:178–86.
[36]AjueborMN,KunkelSL,Hogaboam CM.Theroleof CCL3/macrophage infammatory protein-1α in experimental colitis.Eur J Pharmacol 2004;497:343–9.
[37]Inkovaara P,Suomalainen P.Studies on the physiology of the hibernating hedgehog.18.On the leukocyte counts in the hedgehog’s intestine and lungs.Ann Acad Sci Fenn Biol 1973;200:1–21.
[38]RoubleAN,TessierSN,StoreyKB.Characterization of adipocyte stress response pathways during hibernation in thirteen-lined ground squirrels.Mol Cell Biochem 2014;393:271–82.
[39]Dawson NJ,Katzenback BA,Storey KB.Free-radical frst responders:the characterization of CuZnSOD and MnSOD regulation during freezing of the freeze-tolerant North American wood frog,Rana sylvatica.Biochim Biophys Acta 2014;1850:97–106.
[40]Biggar KK,Wu CW,Tessier SN,Zhang J,Pifferi F,Perret M, Storey KB.Primate torpor:regulation of stress-activated protein kinases during daily torpor in the gray mouse lemur,Microcebus murinus.Genomics Proteomics Bioinformatics 2015;13:81–90.
[41]Giroud S,Blanc S,Aujard F,Bertrand F,Gilbert C,Perret M. Chronic food shortage and seasonal modulation of daily torpor and locomotor activity in the grey mouse lemur(Microcebus murinus).Am J Physiol 2008;294:R1958–E1967.
Received 13 February 2015;accepted 24 March 2015
Available online 17 June 2015
Handled by Jun Yu
*Corresponding author.
E-mail:kenneth_storey@carleton.ca(Storey KB).
#Equal contribution.
aORCID:0000-0003-2373-232x.
bORCID:0000-0001-6171-5040.
cORCID:0000-0001-9316-1935.
dORCID:0000-0002-3801-0453.
eORCID:0000-0002-7363-1853.
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences and Genetics Society of China.
http://dx.doi.org/10.1016/j.gpb.2015.03.005
1672-0229?2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Beijing Institute of Genomics,Chinese Academy of Sciences and Genetics Society of China.
This is an open access article under the CC BY license(http://creativecommons.org/licenses/by/4.0/).
 Genomics,Proteomics & Bioinformatics2015年2期
Genomics,Proteomics & Bioinformatics2015年2期
- Genomics,Proteomics & Bioinformatics的其它文章
- Genomics,Proteomics&Bioinformatics
- Special Issue on‘‘Biomarkers for Diseases’’
- Special Issue on‘‘Computational Cardiology’’
- Special Issue on‘‘Metagenomics of Marine Environments’’
- Acknowledgments to Reviewers 2014
- Induction of Antioxidant and Heat Shock Protein Responses During Torpor in the Gray Mouse Lemur,Microcebus murinus
