what drives progressive motor defcits in patients with acute pontine infarction?
Jue-bao Li, Rui-dong Cheng, Liang Zhou, Wan-shun Wen, Gen-ying Zhu, Liang Tian, Xiang-ming Ye
Department of Rehabilitation Medicine, Zhejiang Provincial People’s Hospital, Hangzhou, Zhejiang Province, China
what drives progressive motor defcits in patients with acute pontine infarction?
Jue-bao Li, Rui-dong Cheng, Liang Zhou, Wan-shun Wen, Gen-ying Zhu, Liang Tian, Xiang-ming Ye*
Department of Rehabilitation Medicine, Zhejiang Provincial People’s Hospital, Hangzhou, Zhejiang Province, China
Progressive motor deficits are relatively common in acute pontine infarction and frequently associated with increased functional disability. However, the factors that affect the progression of clinical motor weakness are largely unknown. Previous studies have suggested that pontine infarctions are caused mainly by basilar artery stenosis and penetrating artery disease. Recently, lower pons lesions in patients with acute pontine infarctions have been reported to be related to progressive motor defcits, and ensuing that damage to the corticospinal tracts may be responsible for the worsening of neurological symptoms. Here, we review studies on motor weakness progression in pontine infarction and discuss the mechanisms that may underlie the neurologic worsening.
nerve regeneration; pontine infarction; progressive motor deficits; basilar artery; penetrating artery; corticospinal tract; Wallerian degeneration; review; neural regeneration
Li JB, Cheng RD, Zhou L, Wen WS, Zhu GY, Tian L, Ye XM (2015) What drives progressive motor deficits in patients with acute pontine infarction?. Neural Regen Res 10(3)∶501-504.
Introduction
The brainstem plays an essential role in controlling balance, coordinated movement, hearing, speech, eye movement and swallowing, and patients who have sustained brainstem infarction suffer from not only ataxia and dysphagia, but also paralysis, diplopia and dysarthria (Maeshima et al., 2012). Pontine infarctions are often part of a larger ischemic event involving the brainstem, either in isolation or as part of multilevel ischemia (Moncayo, 2012). Pontine infarctions constitute approximately 7% of all ischemic infarctions and 15% of acute vertebrobasilar ischemic strokes (Silverstein, 1964; Saia and Pantoni, 2009). Unilateral pontine infarctions usually manifest with lacunar syndromes, including pure motor stroke, ataxic hemiparesis or dysarthria clumsy hand (Kim et al., 1995, 2009; Bassetti et al., 1996; Oh et al., 2012). Progressive pure motor hemiparesis is a common feature in acute pontine infarction cases and is frequently associated with increased functional disability (Kunz et al., 2003; Oh et al., 2012). Numerous studies have examined the pathogenesis and etiology of the uniform clinical features; however, the pathological mechanisms underlying the clinical progression of motor deficits in pontine infarctions remain unclear. In this paper, we provide an overview of the current state of knowledge on the mechanisms underlying the development of progressive motor defcits in pontine infarction. Furthermore, we analyze the available data to provide insight into the pathogenesis of the common features and suggest therapeutic targets for combinatorial therapy.
Basilar artery and penetrating artery disease
Patients with unilateral pontine infarctions typically present a pure motor hemiparesis that generally progresses within 72 hours of onset and is accompanied by dysarthria and homolateral ataxia (Kunz et al., 2003). Basilar artery and related branch disease are the most common causes in patients with isolated pontine infarctions and are often correlated with a progressive condition (Fisher and Caplan, 1971; Kaps et al., 1997; Schmahmann et al., 2004; Yamamoto et al., 2011) and a relative frequency of about 40% (Ohara et al., 2010; Yamamoto et al., 2010). Furthermore, these patients have a worse prognosis than patients with lacunar pontine infarctions (Erro et al., 2005; Oh et al., 2012). A large retrospective study showed that basilar artery branch disease is the most common cause of stroke in patients with pontine infarction, and patients with basilar artery branch disease were found to have a worse short-term outcome (Kumral et al., 2002; Vemmos et al., 2005). Basilar artery stenosis was reported to be related to an increase in lesion volume in pontine infarctions (Karepov et al., 2006). High-resolution magnetic resonance imaging (MRI) in patients with pontine infarction identifed atherosclerotic plaques in the basilar artery in more than 70% of cases. The relationships among the occurrence of basilar artery atherosclerotic disease, the increase in pontine lesion volume and clinical outcome have been analyzed by a number of researchers (Kim et al., 2009; Saia and Pantoni, 2009), which revealed that the occurrence of basilar artery atherosclerotic disease is significantly related to an increase in lesion volume in the subacute phase of stroke (Saia and Pantoni, 2009). Pontine infarcts extending to the surface of the pontine base associated with atheromatous plaque occlusions at the penetrating branch of the basilar artery have been described, and ensuing studies have shown that basilar artery branch disease is strongly correlated with atheromatosis of thebasilar arteries and a progressive course (Fisher and Caplan, 1971; Kaps et al., 1997; Kumral et al., 2002; Schmahmann et al., 2004; Kwon et al., 2009; Yamamoto et al., 2011; Ju et al., 2013). Yamamoto et al. (2011) showed that basilar artery atherosclerotic disease is strongly associated with progressive motor deficits and worse functional outcome in both the lenticulostriate artery and anterior pontine artery territories. The presence of basilar artery disease has an impact on lesion size, lesion volume and functional outcome in pontine base infarction patients (Kim et al., 2009). Progressive motor defcits are common in penetrating artery infarctions during the acute stage and sometimes lead to severe disability. Anterior pontine arteries are delicate vessels that branch acutely from the basilar artery and angle in a slightly caudal direction (Yamamoto et al., 2010). A study investigating progressive motor defcits in penetrating artery infarctions suggested that patients with infarctions topographically located within the territories of the anterior pontine arteries constitute about 29.0% of cases of progressive motor defcits (Yamamoto et al., 2010). Basilar artery branch atheromatous disease likely contributes to progressive motor defcits in patients with penetrating artery infarction.
Topographic location of pontine infarctions
In ischemic stroke, there is a gradual progression in neurological impairment leading to increased mortality and functional disability. Clinical deterioration was found to be more frequent in large vessel disease and in the vertebrobasilar arterial territory in a large retrospective analysis. These studies also suggest that the mechanisms involved in the development of ischemic damage are very complex, and that different mechanisms could be responsible for clinical deterioration in different etiologic subtypes and lesion locations (Yamamoto et al., 1998; Saia and Pantoni, 2009). However, only a few studies have focused on the topography of lesions in brainstem infarctions. In a study on isolated pontine infarction, neurologic worsening seemed to be more prevalent in patients with lesions extending to the basal surface of the pons, compared with patients with deep pontine lesions. Moreover, in the same study, clinical deterioration was positively correlated with large vessel disease and branch atheromatous disease (Watson and Colebatch, 2002; Saia and Pantoni, 2009). Oh et al. (2012) examined factors impacting the progression of motor weakness in pontine infarction cases during the acute phase, such as the presence of basilar artery stenosis and the location of the infarction. The authors found that lower pons lesions may contribute to progressive motor defcits in patients with acute pontine infarction. The infarct in the lower pons may affect the extent of ischemic degeneration in the corticospinal tract, leading to progressive motor defcits. Infarct topography is, therefore, a potential prognostic factor for progressive motor defcits. The location of the infarction was found to be a predictor of motor progression in subcortical infarct patients (Konishi et al., 2005; Kim et al., 2008; Oh et al., 2012). Subcortical infarctions are known to have similar causes as pontine infarctions. In the present study, our analysis revealed that lower pontine infarctions were signifcantly associated with progressive motor deficits in patients with acute pontine infarctions. In pure motor pontine infarcts, the topography of the infarct lesion has been reported to be related to prognosis; lesions causing severe hemiparesis are generally large and involve the ventral surface of the paramedian caudal or middle pons (Kim et al., 1995; Kataoka et al., 1997; Oh et al., 2012).
Corticospinal tract and wallerian degeneration in the basis pontis
The classical mechanisms underlying pontine infarction cannot explain the majority of neurologic worsening (Saia and Pantoni, 2009). A previous study reported that basilar artery stenosis is only related to the subacute increase in lesion volume in pontine infarctions and not to neurological progression. Progression of motor defcits is unlikely to be caused by hemodynamic compromise related to basilar artery stenosis (Kim et al., 2009; Oh et al., 2012). The corticospinal tract is located in the center of the pontine basis, which is surrounded by transpontine fibers (Jang, 2011). The corticospinal tracts are situated in the dorsolateral part of the pontine base at the level of the upper pons (Kim and Pope, 2005; Ino et al., 2007; Yu et al., 2009; Jang, 2011). Therefore, infarcts in the lower pontine region may cause more damage to the corticospinal tracts than upper pontine region infarcts because of proximity (Oh et al., 2012).
Degeneration of distal axons and their myelin sheaths after proximal axonal or cell body injury is referred to as Wallerian degeneration, which occurs in both the peripheral and central nervous systems (Qin et al., 2012). In the early stage, axonal swelling and fragmentation with disruption of the myelin sheaths occur, followed by degradation of the myelin sheath and infltration by macrophages and microglia. Wallerian degeneration of the fiber tract in the middle cerebellar peduncle after pontine infarction has been studied (Grassel et al., 2010; Qin et al., 2012). These studies suggest that Wallerian degeneration in the middle cerebellar peduncle may hinder neurological recovery following a focal pontine infarct. The degeneration of axons and their myelin sheaths after proximal axonal or cell body injury in fiber tracts distal to a focal cerebral infarct has been demonstrated in animal experiments and in postmortem studies, as well as by MRI and diffusion tensor imaging (Kobayashi et al., 2005; Gresle et al., 2006; Matsusue et al., 2007; Sylaja et al., 2007; Liang et al., 2009). In a Japanese-language study on autopsy of pontine lesions in the elderly, necrosis of the basis pontis, characterized by loss of myelin and axons, without reactive astrocytes or infammatory cells, was found (Inagaki et al., 1996). Wallerian degeneration of the corticospinal and pyramidal tracts after motor pathway ischemic stroke can be characterized by diffusion tensor imaging. A prospective study on Wallerian degeneration of the corticospinal tract after paramedian pons infarction showed that Wallerian degeneration can be detected after the onset of symptoms (Forster et al., 2010; Grassel et al., 2010).
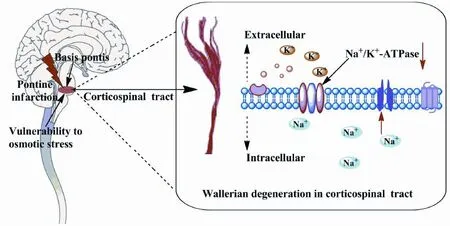
Figure 1 Schematic: The basis pontis may have a relatively higher vulnerability to osmotic stress, which can result in wallerian degeneration of the corticospinal tract.
The basis pontis is anatomically unusual in both gray and white matter, and this feature is presumed to account for the vulnerability of this area to perturbations in ionic and osmotic homeostasis (Park and Jung, 2010; Hurley et al., 2011). The reduction of cerebral blood after ischemia initially causes oxygen and glucose deprivation and acute cell death, eventually leading to an infarct core.
Central myelinated axons are critically dependent on a continuous supply of oxygen and glucose (Dirnagl et al., 1999; Stys, 2004; Nanetti et al., 2008). Na+/K+-ATPase is an integral membrane protein that plays a key role in cellular osmotic regulation through the maintenance of the transmembrane Na+and K+gradients, and is responsible for the maintenance of ionic homeostasis in both astrocytes and neurons. (D’Ambrosio et al., 2002). Accumulating evidence supports a key role of energy defciency and dysfunction of the Na+/K+-ATPase in ischemia-induced cell volume changes and cell death. In a focal cerebral infarct, degeneration of associated fiber tracts and neuronal damage have been shown to be related to a disruption in ionic homeostasis resulting from reduced energy metabolism (Fuller et al., 2003).
Conclusions and perspective
During the acute phase of pontine infarction, cerebral blood fow is reduced, resulting in oxygen and glucose deprivation, which leads to neuronal necrosis. We hypothesize that the inhibition of the Na+/K+-ATPase leads to intracellular Na+overload and the perturbation of osmotic homeostasis. As a result of osmotic stress in the basis pontis, Wallerian degeneration of the corticospinal tract occurs after the onset of symptoms. This may underlie the progressive motor defcits in pontine infarctions. The hypothesis is summarized schematically in Figure 1.
Author contributions:JBL, XMY and RDC conceived the review. RDC and JBL collected the data and wrote the paper. LZ, WSW, GYZ and LT revised the paper. XMY and JBL supervised the paper. All authors approved the final version of the paper.
Conficts of interest:None declared.
Bassetti C, Bogousslavsky J, Barth A, Regli F (1996) Isolated infarcts of the pons. Neurology 46:165-175.
D’Ambrosio R, Gordon DS, Winn HR (2002) Differential role of KIR channel and Na(+)/K(+)-pump in the regulation of extracellular K(+) in rat hippocampus. J Neurophysiol 87:87-102.
Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22:391-397.
Erro ME, Gallego J, Herrera M, Bermejo B (2005) Isolated pontine infarcts: etiopathogenic mechanisms. Eur J Neurol 12:984-988.
Fisher CM, Caplan LR (1971) Basilar artery branch occlusion: a cause of pontine infarction. Neurology 21:900-905.
Forster A, Ottomeyer C, Wolf ME, Kern R, Griebe M, Gass A, Hennerici MG, Szabo K (2010) Dynamic susceptibility contrast perfusion MRI identifes persistent vessel pathology in acute pontine stroke. Cerebrovasc Dis 29:389-394.
Fuller W, Parmar V, Eaton P, Bell JR, Shattock MJ (2003) Cardiac ischemia causes inhibition of the Na/K ATPase by a labile cytosolic compound whose production is linked to oxidant stress. Cardiovasc Res 57:1044-1051.
Grassel D, Ringer TM, Fitzek C, Fitzek S, Kohl M, Kaiser WA, Witte OW, Axer H (2010) Wallerian degeneration of pyramidal tract after paramedian pons infarct. Cerebrovasc Dis 30:380-388.
Gresle MM, Jarrott B, Jones NM, Callaway JK (2006) Injury to axons and oligodendrocytes following endothelin-1-induced middle cerebral artery occlusion in conscious rats. Brain Res 1110:13-22.
Hurley RA, Filley CM, Taber KH (2011) Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci 23:369-374.
Inagaki A, Inagaki T, Hasizume Y, Ojika K (1996) Autopsy fndings of pontine lesions in the elderly. Nihon Ronen Igakkai Zasshi 33:524-531.
Ino T, Nakai R, Azuma T, Yamamoto T, Tsutsumi S, Fukuyama H (2007) Somatotopy of corticospinal tract in the internal capsule shown by functional MRI and diffusion tensor images. Neuroreport 18:665-668.
Jang SH (2011) Somatotopic arrangement and location of the corticospinal tract in the brainstem of the human brain. Yonsei Med J 52:553-557.
Ju Y, Hussain M, Asmaro K, Zhao X, Liu L, Li J, Wang Y (2013) Clinical and imaging characteristics of isolated pontine infarcts: a one-year follow-up study. Neurol Res 35:498-504.
Kaps M, Klostermann W, Wessel K, Bruckmann H (1997) Basilar branch disease presenting with progressive pure motor stroke. Acta Neurol Scand 96:324-327.
Karepov VG, Gur AY, Bova I, Aronovich BD, Bornstein NM (2006) Stroke-in-evolution: infarct-inherent mechanisms versus systemic causes. Cerebrovasc Dis 21:42-46.
Kataoka S, Hori A, Shirakawa T, Hirose G (1997) Paramedian pontine infarction. Neurological/topographical correlation. Stroke 28:809-815.
Kim JS, Lee JH, Im JH, Lee MC (1995) Syndromes of pontine base infarction. A clinical-radiological correlation study. Stroke 26:950-955.
Kim JS, Pope A (2005) Somatotopically located motor fbers in corona radiata: evidence from subcortical small infarcts. Neurology 64:1438-1440.
Kim JS, Cho KH, Kang DW, Kwon SU, Suh DC (2009) Basilar artery atherosclerotic disease is related to subacute lesion volume increase in pontine base infarction. Acta Neurol Scand 120:88-93.
Kim SK, Song P, Hong JM, Pak CY, Chung CS, Lee KH, Kim GM (2008) Prediction of progressive motor defcits in patients with deep subcortical infarction. Cerebrovasc Dis 25:297-303.
Klein IF, Lavallee PC, Schouman-Claeys E, Amarenco P (2005) High-resolution MRI identifes basilar artery plaques in paramedian pontine infarct. Neurology 64:551-552.
Kobayashi S, Hasegawa S, Maki T, Murayama S (2005) Retrograde degeneration of the corticospinal tract associated with pontine infarction. J Neurol Sci 236:91-93.
Konishi J, Yamada K, Kizu O, Ito H, Sugimura K, Yoshikawa K, Nakagawa M, Nishimura T (2005) MR tractography for the evaluation of functional recovery from lenticulostriate infarcts. Neurology 64:108-113.
Kumral E, Bayulkem G, Evyapan D (2002) Clinical spectrum of pontine infarction. Clinical-MRI correlations. J Neurol 249:1659-1670.
Kunz S, Griese H, Busse O (2003) Etiology and long-term prognosis of unilateral paramedian pontine infarction with progressive symptoms. Eur Neurol 50:136-140.
Kwon HM, Kim JH, Lim JS, Park JH, Lee SH, Lee YS (2009) Basilar artery dolichoectasia is associated with paramedian pontine infarction. Cerebrovasc Dis 27:114-118.
Liang Z, Zeng J, Zhang C, Liu S, Ling X, Wang F, Ling L, Hou Q, Xing S, Pei Z (2009) Progression of pathological changes in the middle cerebellar peduncle by diffusion tensor imaging correlates with lesser motor gains after pontine infarction. Neurorehabil Neural Repair 23:692-698.
Maeshima S, Osawa A, Miyazaki Y, Takeda H, Tanahashi N (2012) Functional outcome in patients with pontine infarction after acute rehabilitation. Neurol Sci 33:759-764.
Matsusue E, Sugihara S, Fujii S, Kinoshita T, Ohama E, Ogawa T (2007) Wallerian degeneration of the corticospinal tracts: postmortem MR-pathologic correlations. Acta Radiol 48:690-694.
Moncayo J (2012) Pontine infarcts and hemorrhages. Front Neurol Neurosci 30:162-165.
Nanetti L, Vignini A, Raffaelli F, Moroni C, Silvestrini M, Provinciali L, Mazzanti L (2008) Platelet membrane fluidity and Na+/K+ATPase activity in acute stroke. Brain Res 1205:21-26.
Oh S, Bang OY, Chung CS, Lee KH, Chang WH, Kim GM (2012) Topographic location of acute pontine infarction is associated with the development of progressive motor defcits. Stroke 43:708-713.
Ohara T, Yamamoto Y, Tamura A, Ishii R, Murai T (2010) The infarct location predicts progressive motor deficits in patients with acute lacunar infarction in the lenticulostriate artery territory. J Neurol Sci 293:87-91.
Park S, Jung Y (2010) Combined actions of Na/K-ATPase, NCX1 and glutamate dependent NMDA receptors in ischemic rat brain penumbra. Anat Cell Biol 43:201-210.
Qin W, Zhang M, Piao Y, Guo D, Zhu Z, Tian X, Li K, Yu C (2012) Wallerian degeneration in central nervous system: dynamic associations between diffusion indices and their underlying pathology. PLoS One 7:e41441.
Saia V, Pantoni L (2009) Progressive stroke in pontine infarction. Acta Neurol Scand 120:213-215.
Schmahmann JD, Ko R, MacMore J (2004) The human basis pontis: motor syndromes and topographic organization. Brain 127:1269-1291.
Silverstein A (1964) Acute infarctions of the brain stem in the distribution of the basilar artery. Confn Neurol 24:37-61.
Stys PK (2004) White matter injury mechanisms. Curr Mol Med 4:113-130.
Sylaja RN, Goyal M, Watson T, Hill MD (2007) Wallerian-like degeneration after ischemic stroke revealed by diffusion--weighted imaging. Can J Neurol Sci 34:243-244.
Vemmos KN, Spengos K, Tsivgoulis G, Manios E, Zis V, Vassilopoulos D (2005) Aetiopathogenesis and long-term outcome of isolated pontine infarcts. J Neurol 252:212-217.
Watson SR, Colebatch JG (2002) Focal pathological startle following pontine infarction. Mov Disord 17:212-218.
Yamamoto H, Bogousslavsky J, Melle G (1998) Different predictors of neurological worsening in different causes of stroke. Arch Neurol 55:481-486.
Yamamoto Y, Ohara T, Hamanaka M, Hosomi A, Tamura A, Akiguchi I (2011) Characteristics of intracranial branch atheromatous disease and its association with progressive motor deficits. J Neurol Sci 304:78-82.
Yamamoto Y, Ohara T, Hamanaka M, Hosomi A, Tamura A, Akiguchi I, Ozasa K (2010) Predictive factors for progressive motor defcits in penetrating artery infarctions in two different arterial territories. J Neurol Sci 288:170-174.
Yu C, Zhu C, Zhang Y, Chen H, Qin W, Wang M, Li K (2009) A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage 47:451-458.
Copyedited by Patel B, Raye W, Li CH, Song LP, Zhao M
*
10.4103/1673-5374.153703
http://www.nrronline.org/
Accepted: 2014-12-10
- 中國神經(jīng)再生研究(英文版)的其它文章
- A new look at auranofn, dextromethorphan and rosiglitazone for reduction of glia-mediated infammation in neurodegenerative diseases
- Appearance of a neural bypass between injured cingulum and brainstem cholinergic nuclei of a patient with traumatic brain injury on follow-up diffusion tensor tractography images
- Compensatory recombination phenomena of neurological functions in central dysphagia patients
- Curcumin pretreatment and post-treatment both improve the antioxidative ability of neurons with oxygen-glucose deprivation
- Connecting the P300 to the diagnosis and prognosis of unconscious patients
- Ulinastatin suppresses endoplasmic reticulum stress and apoptosis in the hippocampus of rats with acute paraquat poisoning

