A Bi-component Cu Catalyst for the Direct Synthesis of Methylchlorosilane from Silicon and Methyl Chloride
WANG Chao (汪 超), WANG Guangrun (王光潤(rùn)) and WANG Jinfu (王金福)
Beijing Key Laboratory of Green Chemical Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
A Bi-component Cu Catalyst for the Direct Synthesis of Methylchlorosilane from Silicon and Methyl Chloride
WANG Chao (汪 超), WANG Guangrun (王光潤(rùn)) and WANG Jinfu (王金福)*
Beijing Key Laboratory of Green Chemical Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
A bi-component catalyst comprising CuCl and metallic copper was used in the direct synthesis of methylchlorosilane to study the catalytic synergy between the different copper sources. The catalyst exhibited high activity and high selectivity of dimethyldichlorosilane (M2) in the stirred bed reactor. The effect of the proportion of CuCl used was studied and 10%-30% CuCl gave the best yield of M2. The use of CuCl decreased the induction period of reaction, improved the selectivity in the induction stage, and gave a longer stable stage. These results suggest that bi-component catalyst has advantages in the direct synthesis reaction.
methylchlorosilane, direct synthesis, bi-component catalyst, synergy
1 INTRODUCTION
Methylchlorosilane (MCS), especially dimethyldichlorosilane [M2, (CH3)2SiCl2], is the basic monomer to produce silicone polymers. Commercial production of MCS is accomplished by direct synthesis process, which is a synthetic reaction between metallurgical silicon and methyl chloride (MeCl) [1]. The reaction mainly gives the most desired product M2, yet a number of by-products are also generated, including methyltrichlorosilane (M1, CH3SiCl3), methyldichlorosilane (MH, CH3HSiCl2) and many others [2].
Rochow first discovered that the reaction can be very selective to M2 when copper is used as the catalyst [3]. Since then, there were numerous investigations in this field [4-12]. In practice, various copper compounds have been used and two of them are successful. One is CuCl, which is reactive and mostly used in laboratory [6-8, 14, 15]. The other is the metallic copper (sometimes oxidized partially or completely), which is also widely used in the silicone industry [16, 17]. The metallic copper can maintain high selectivity in a long period, nevertheless its activity is not always satisfactory.
What if the two copper catalysts coexist in the reaction? Will it lead to a balance between the activity and the selectivity? Kalchauer et al. [18] have described a process in which CuCl and copper oxide were used alternately in a fluid bed reactor, into which fresh catalyst was supplied continuously. CuCl was added for high activity, and it was replaced with copper oxide when the reaction selectivity deteriorated. The practice on the industrial-scale reactor proved that the alternation of catalysts increased the yield of M2. Herein, we propose that a bi-component catalyst comprising both CuCl and copper (or copper oxide) has more advantages over the traditional mono-component catalyst, yet the conclusive evidences are still needed.
In this work, a bi-component catalyst was prepared by physically mixing CuCl and a special metallic copper catalyst. We studied the catalytic performance of bi-component catalyst in a stirred-bed reactor and compared with the mono-component ones. The influence of the composition of the mixture was also studied.
2 CATALYTIC MECHANISMS
Due to its complexity, the direct synthesis of MCS is not yet completely understood. It is confirmed that the catalyst in the process undergoes a thorough transformation to form the active site [4, 5, 19]. It is widely accepted that the copper catalysts first interact with silicon to form various Cu-Si alloys, especially Cu3Si, which is the actual active component. The significance of Cu3Si has been discussed by Voorhees [3], Ward [4], and many others [6-8, 19-22]. It was proposed that finely dispersed Cu3Si will result in high activity.
The Cu3Si alloy can be formed by the following reactions:
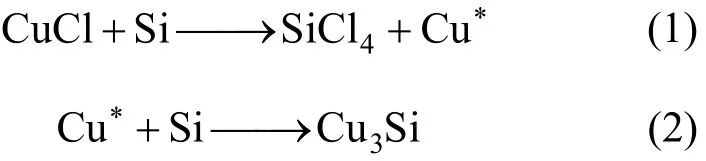
The asterisk on Cu denotes that the copper produced is activated and dispersed on the surface of silicon. Then, it diffuses into the silicon matrix to form the alloy. Eq. (1) is rapid and explains the high effectiveness of CuCl catalyst. However, it is also proposed that too rapid reduction of CuCl will cause the deposition of excess copper on the surface of the alloy, which leads to side reactions.
The above-mentioned reactions also describe the activation of other catalysts besides CuCl. It is believed that there is the conversion to CuCl firstly and then the activation via CuCl occurs [7, 8]. This conversion is reasonable since the metallic copper or copperoxide hardly react with silicon under the condition of the direct synthesis (typically 290 °C-340 °C). The conversion is affected by the cracking of MeCl, which is the source of chlorine in the system. Limited by this step, the CuCl concentration will be low, thus the activity is limited but the side reactions are also suppressed.
The present view of the catalytic mechanism does not emphasize the synergy between copper precursors. This work is based on the speculation that the coexistence of CuCl and metallic copper will lead to the moderate concentration of CuCl on the silicon. The performance of bi-component catalyst in the synthetic experiments suggested the existence of catalytic synergy between CuCl and metallic copper.
3 EXPERIMENTAL
3.1 Chemicals
The silicon used in experiments was chemical grade, with the impurity (mainly Fe and Al) below 0.5%. Before reaction, the particles were sieved to the size <120 μm.
The bi-component catalyst was prepared with two copper sources. CuCl (analytical reagent A.R., purity≥97%) was purchased from Sinopharm Chemical Reagent Corporation (Shanghai, China). The other was an industrial catalyst which was provided by Dongyue Silicone Corporation (Huantai, China) and consisted mainly of metallic copper, with some other elements such as Zn, P and Sn as promoters. The detailed chemical composition of the industrial catalyst was listed in Table 1. The structure of the catalyst was characterized by X-ray diffraction (BRUKER D8 Advance Diffractometer using Cu Kαradiation), scanning electron microscopy (JSM 7401F), laser particle size analysis (Malvern Mastersizer Micro) and the Brunauer-Emmett-Teller nitrogen (N2BET) adsorption (Autosorb-1-C from Quantachrome Instrument).
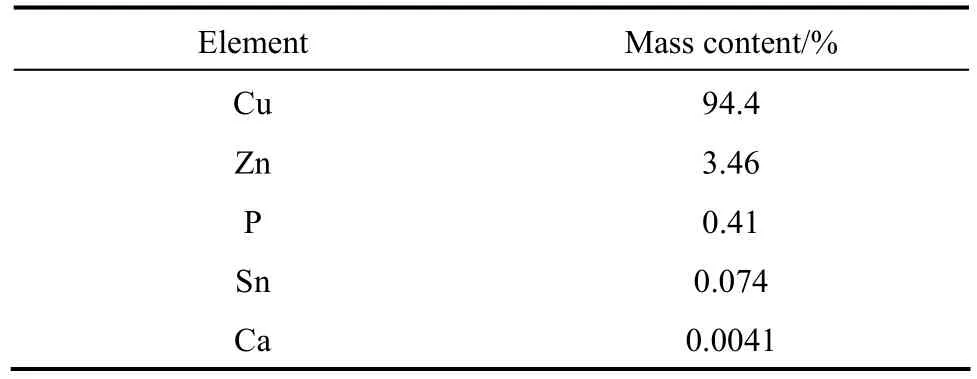
Table 1 Chemical composition of the industrial catalyst
The bi-component catalysts were physical mixtures of two copper sources with different proportions. The mixtures were ground and sieved to a size <80 μm, kept dry in the dark before the reaction.
3.2 Apparatus and procedure
Evaluation of the catalyst was performed in a stirred bed reactor, which was made of stainless steel and with a diameter of 45 mm and height of 150 mm. Silicon particles and catalyst were blended and packed in the reactor, typically 50 g and with a Cu/Si ratio of 1︰20. The reactor was heated to 120 °C in a flow of 4.5 mmol·min?1N2and lasted for 2 h to dry the reactants. When the temperature was finally raised to the set value, i.e. 320 °C, MeCl vapor was introduced from the bottom of the reactor and passed upward through the bed. The moisture in MeCl should be strictly removed with concentrated H2SO4and molecular sieve before the reaction. The reactor was kept at the pressure of 0.2 MPa (absolute pressure) and stirring at 120 r·min?1. 5 min after the reaction started, the analysis of the effluent began and repeated at intervals of 30-60 min.
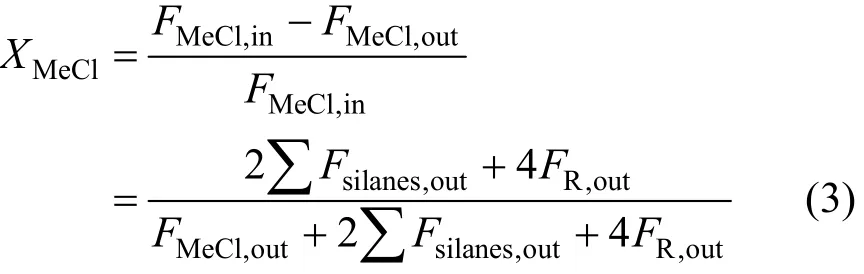
The reactor was connected to an on-line gas chromatograph (GC7890, Techcomp Corporation, China) with a thermal conductor detector. An OV-210 packed column was used for the separation of MeCl, various silanes (including M2), and high boiling compounds (R, mainly disilanes). The calculation of MeCl conversion was based on the composition of effluent by wherein F stands for the mole flow rate of MeCl and products, and subscripts “in” and “out” denote the inlet and outlet. It was taken that 1 mole of silanes consumed 2 mole of CH3Cl, and 1 mole disilanes consumed 4. A trace of HCl was also observed as the result of MeCl cracking, but it was not considered in the calculation because of its trace level.
The selectivity to M2 was defined as Eq. (4) and the activity of the catalyst was measured by the space time yield of M2 as Eq. (5), which describes the mass of M2 produced per kilogram silicon per hour.
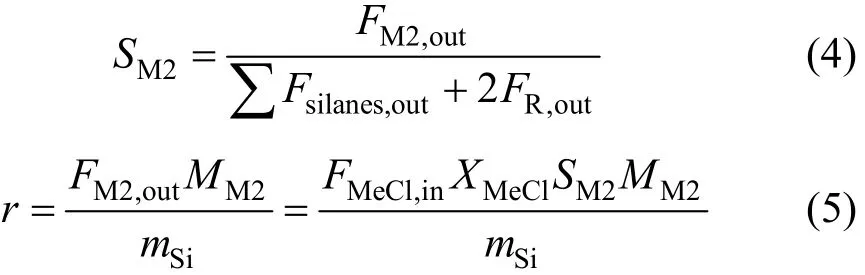
4 RESULTS AND DISCUSSION
4.1 Characterization of the industrial catalyst
The X-ray diffraction (XRD) pattern in Fig. 1 showed that metallic copper is the bulk phase of the industrial catalyst. The sharp peaks demonstrated a high degree of crystalline for Cu phase. Cu-Zn alloy phase was also detected and a trace of Cu3P was affirmed by the weak peaks at 2θ=36.0°, 45.1° and 46.2°. The scanning electron microscope (SEM) image in Fig. 2 shows that the catalyst is in the form of flake. It seems to be loose and easy to split. N2BETmeasurement gave the BET surface of 0.9 m2·g?1. Laser particle analysis showed the size distributed in the range of 2.3-100 μm with the mean value of 9.4 μm.
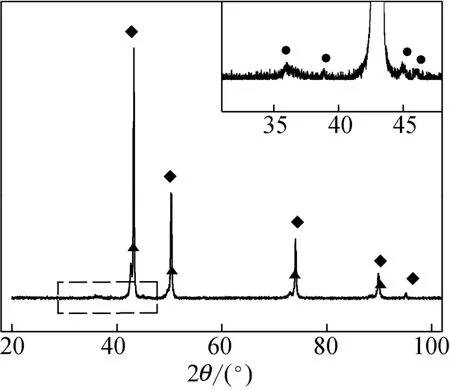
Figure 1 XRD pattern of the industrial catalyst

Figure 2 SEM image of the industrial catalyst
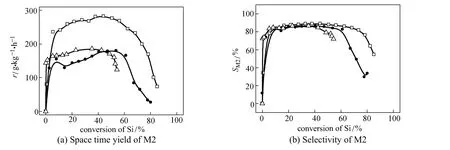
Figure 3 The comparative results of M2 over various catalysts (reaction conditions: temperature 320 °C, pressure 0.2 MPa, molar ratio of Cu︰Si is 1︰20)
4.2 Synthesis of MCS over the bi-component catalyst
In the direct synthesis process, silicon was consumed and the catalyst was gradually deactivated simultaneously, so the activity and selectivity varied with the conversion of silicon. Typically an inverted“U” shape curve as in Fig. 3 was observed. Initially the activity was low and increased rapidly. This “induction period” lasted for 60-120 min and converted 5%-10% of silicon. The highest activity and selectivity to M2 appeared in the middle period and maintained steady for a while. But before the silicon was completely utilized, the reaction was ceased due to the decay of the activity and selectivity. To delay the deactivation is also important for improving the efficiency of silicon utilization.
Figure 3 (a) shows the activity enhancement with the bi-component catalyst containing 5% CuCl. The results are compared with both the metallic copper (industrial catalyst) and the pure CuCl. The advantage of bi-component catalyst is rather remarkable. The value in the steady period exceeded 250 g·kg?1·h?1, which was 60% higher than those of the mono-component catalysts.
Figure 3 (b) exhibits a comparison of the selectivity between different catalysts. The use of bi-component catalyst almost did not affect the selectivity to M2 in the steady period. However, it is noteworthy that the utilization of silicon is obviously improved by the bi-component catalyst, over which almost 70% of the silicon was transformed before the reaction deteriorated (the criterion was set as the M2 selectivity dropped below 80%). In comparison, the utilization of silicon was 60% over industrial catalyst and even less over pure CuCl.
Figure 4 shows the difference in the distribution of products between different catalysts. The comparison is based on the data obtained from the steady period, with the silicon conversion around 20%-30%. The value of Me/Cl is also listed in the chart. Me/Cl is the average ratio of methyl group and chlorine in the products. Ideally, this index should be 1, the same asthe reactant. However, it is always lower than 1 because of the cracking of methyl groups. The value here was calculated according to the composition of product, in which the high boilers (about 1% in products) is supposed to have the average Me/Cl value.
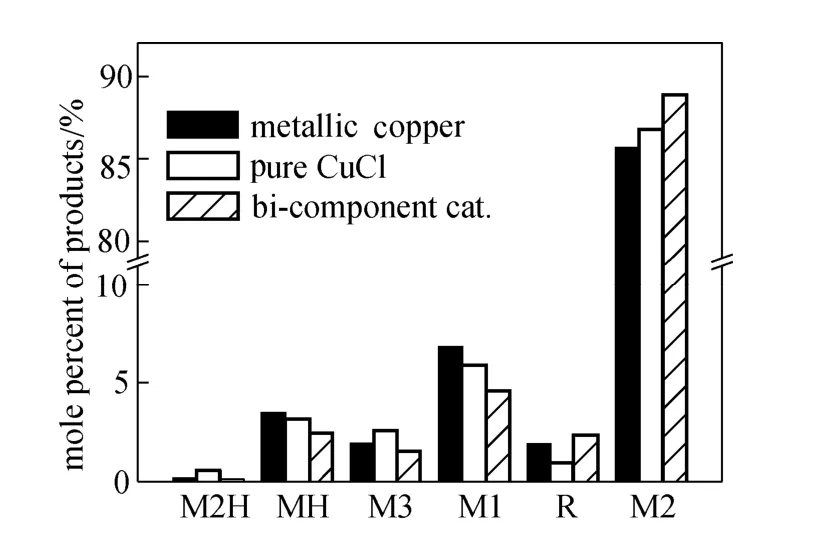
Me/Cl:0.945;0.966;0.965
Compared with the different catalysts, the metallic copper favored the production of methyldichlorosilane (MH, CH3HSiCl2) and methyltrichlorosilane (M1, CH3SiCl3). It was characterized with the lowest value of Me/Cl, which suggested the cracking of methyl groups was encouraged. Pure CuCl gave a Me/Cl value even higher than that of the bi-component catalyst. However, it was attributed to the balance from trimethylchlorosilane [M3, (CH3)3SiCl] and dimethylchlorosilane [M2H, (CH3)2HSiCl] rather than the reduction of MH and M1. The bi-component catalyst reduced the selectivity of most by-products, except for the high boilers, which mainly comprised of various disilanes such as (CH3)2ClSi-SiCH3Cl2. It was supposed that the bi-component catalyst would enhance the production of M2 in the competitive reactions between mono silanes, but the disilanes are consecutive products.
4. 3 The effect of CuCl content
The superior behavior of the bi-component illustrates the catalytic synergy between CuCl and metallic copper. It is reasonable to explore the effect of its proportion in the catalyst. Fig. 5 shows that the effect of CuCl proportion in the bi-component catalysts varied in the range of 2% to 80% by molar fraction, together with pure CuCl (100%) and the metallic copper (0%). It can be seen that with the amount of CuCl below 2%, there was no visible change comparing with the metallic copper. But 5% was quite enough to give an enhancement in catalytic performance. The highest activity and selectivity to M2 were obtained in the range of 10%-30%.
The proportion was calculated on the basis of the Cu element and the total amount of Cu in the catalyst was constant. Thus, the increase of CuCl means the promoter content was reduced. This was not a significant change when CuCl is a minor part. However, the disturbance of promoter was estimated via adding the extra promoter in the catalyst. The additive consists of Zn, Sn, P and maintains the similar amount as the original catalyst. As shown in Fig. 5 (a), the addition of promoter does influence the catalyst, but in a minor degree.
4.4 The induction period
The “induction period” is a universal phenomenon in the direct synthesis process. It will help us to understand the active of catalyst. Early kinetic studies showed that the initial rate and selectivity follow the sigmoid curves. An empirical expression as Eq. (6) proposed by Decooker et al. [19] can be used to describe the kinetic behavior concerning the induction period of the direct synthesis:
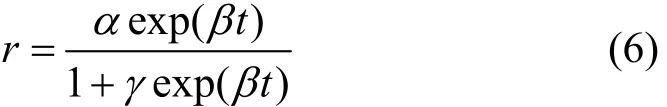
wherein r is the reaction rate and t is the time, α, β, γ are parameters. Obviously, the rate increases exponentially at first, and it will reach the asymptotic value α at last. In fact, Eq. (6) is the solution of the Logistic growth model:
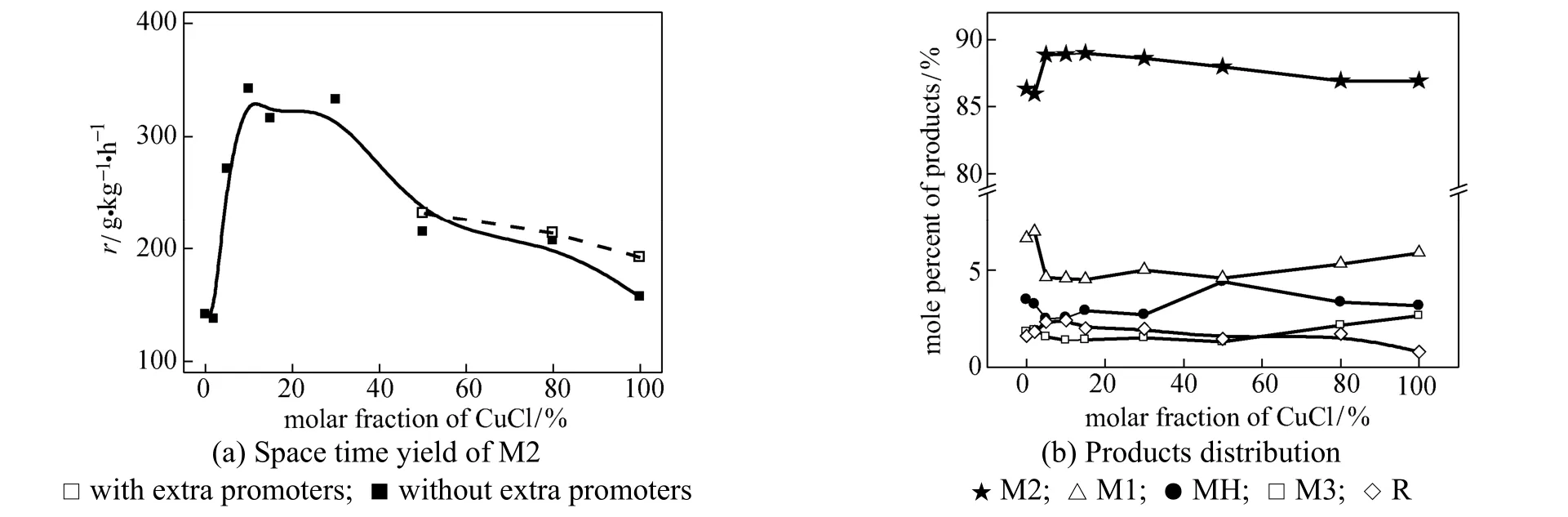
Figure 5 Influence of molar fraction of CuCl in the bi-component catalyst (reaction conditions: temperature 320 °C, pressure 0.2 MPa, molar ratio of Cu︰Si is 1︰20)
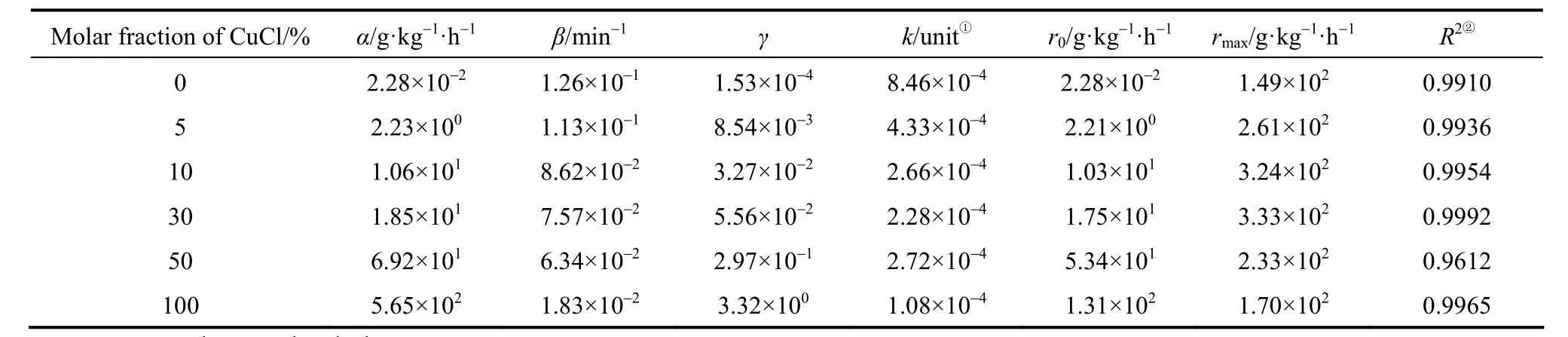
① Unit=min?1·(g·kg?1·h?1)?1. ② Coefficient of correlation.
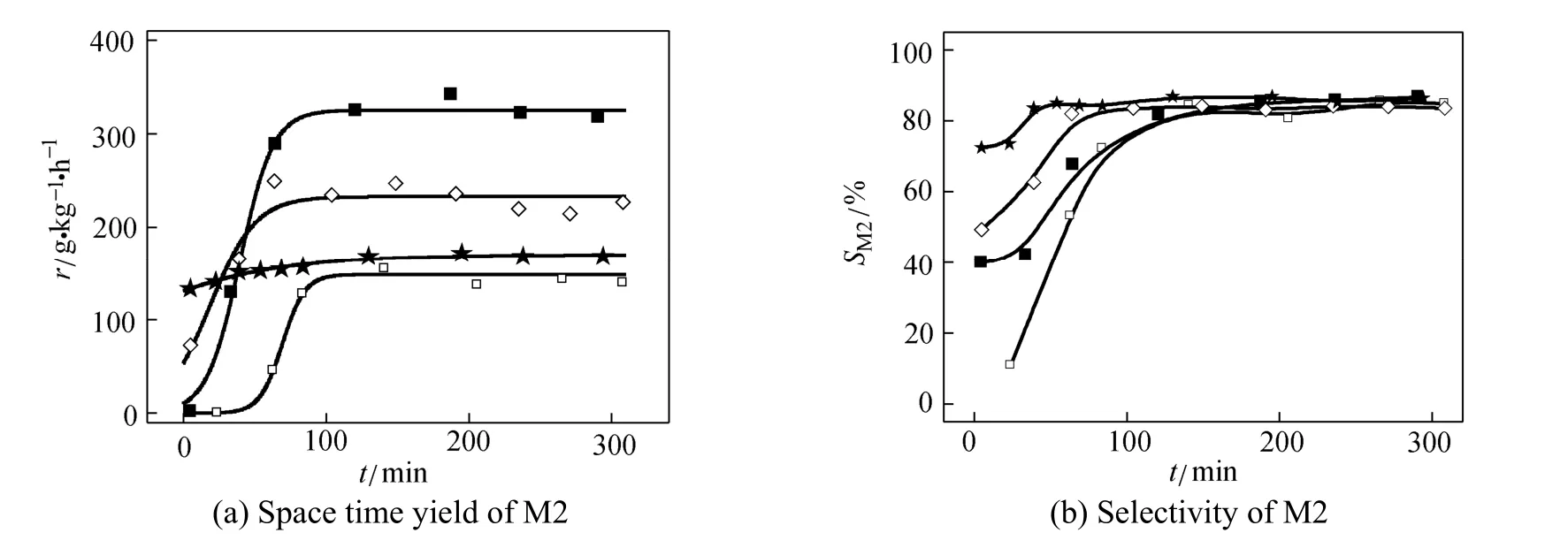
Figure 6 The induction period over various catalysts (reaction conditions: temperature 320 °C, pressure 0.2 MPa, molar ratio of Cu/Si is 1︰20)
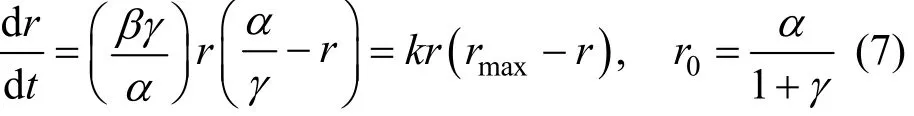
molar fraction of CuCl/%: □ 0; ■ 10; ◇ 50; ★ 100
wherein k, r0and rmaxrepresent the growth factor, initial rate and maximum rate, respectively.
Equation (6) was used to describe the variation of activity versus time on stream (TOS) in the case as shown in Fig. 6 (a). All the experimental data was confined in the first 300 min to avoid the deterioration of the reaction. The parameters were estimated with nonlinear least square method. The results are listed in Table 2.
Figure 6 (b) shows the difference of selectivity in the induction period over various catalysts. It is clear that the induction period was influenced by the composition of catalyst significantly. The pure CuCl catalyst exhibited high initial rate and better selectivity in the induction period. By comparison, the metallic copper had the very low initial rate and the poor selectivity in first 2 h. However, the growth factor of the metallic copper catalyst is high, that means the increase of activity will be accelerated if the initial rate is elevated, that may be the reason that the reaction rate will be enhanced by addition of CuCl.
5 CONCLUSIONS
Direct synthesis of MCS by a bi-component catalyst was investigated using a stirred bed reactor. The coexistence of CuCl and metallic copper gave the enhanced catalytic performance in the reaction. As Compared with traditional catalysts, the bi-component catalyst exhibited remarkable activity, better selectivity to M2 and more effective utilization of silicon. The optimized molar percentage of CuCl in the catalyst was 10%-30%. The highest activity was above 300 g·kg?1·h?1and selectivity was above 88% (under the condition of 0.1 MPa and 320 °C). The behavior during the induction period was strongly affected by the addition of CuCl, and increasing the CuCl proportion in the mixture results in a more rapid start of the reaction. A synergistic effect in this bi-component catalyst is demonstrated by the present experiments.
NOMENCLATURE
Siselectivity of component i, %
mSimass of silicon powder initially, kg
M molecular mass, g·mol?1
Fimole flow rate of component i, mol·h?1
r activity (space time yield of M2) , g·kg?1·h?1t time on stream, min
X conversion, %
REFERENCES
1 Seyferth, D., “Dimethyldichlorosilane and the direct synthesis of methylchlorosilanes: The key to the silicones industry”, Organometallics,20, 4978-4992 (2001).
2 Friedrich, H.B., Sevenich, D.M., Gaspergalvin, L.D., Rethwisch, D.G., “Infrared spectroscopic determination of methylchlorosilanes from the direct process reaction”, Anal. Chim. Acta, 222 (1), 221-234 (1989).
3 Rochow, E.G., “Preparation of organ-silicon halides”, U.S. Pat., 2380995 (1945).
4 Voorhoeve, R.J.H., “Mechanism and kinetics of the metal-catalyzed synthesis of methylchlorosilanes: III The catalytically active form of the copper catalyst”, J. Catal., 4 (2), 123-133 (1965).
5 Banholzer, W.F., Lewis, N., Ward, W., “Active site formation in the direct process for methylchlorosilanes”, J. Catal., 101 (2), 405-415 (1986).
6 Ward, W.J., Ritzer, A., Carroll, K.M., Flock, J.W., “Catalysis of the Rochow direct process”, J. Catal., 100 (1), 240-249 (1986).
7 Gasper-Galvin, L.D., Sevenich, D.M., Friedrich, H.B., Rethwisch, D.G., “Role of metallic promoters in the direct synthesis of methylchlorosilanes”, J. Catal., 128 (2), 468-478 (1991).
8 Floquet, N., Yilmaz, S., Falconer, J.L., “Interaction of copper catalysts and Si(100) for the direct synthesis of methylchlorosilanes”, J. Catal., 148 (1), 348-368 (1994).
9 Brookes, K.H., Siddiqui, M.R.H, Rong, H.M., Joyner, R.W., Hutchings, G.J., “Effect of Al and Ca addition on the copper catalyzed formation of silanes from Si and CH3Cl”, Appl. Catal. A Gen., 206 (2), 257-265 (2001).
10 Rethwisch, D.G., Olakangil, J., “Production of silanes by the direct process: Overview of the reaction mechanism and kinetics”, In: Proceedings of Silicon for the Chemical Industry VIII, Oye, H.A., eds., Norwegian University of Science and Technology, Norway, 103-120 (2006).
11 Gordon, A.D., Hinch, B.J., Strongin, D.R. “Effects of individual promoters on the direct synthesis of methylchlorosilanes”, J. Catal., 266 (2), 291-298 (2009).
12 Gordon, A.D., Hinch, B.J., Strongin, D.R., “Effects of multiple promotion of the direct synthesis contact mass with P, Zn, and Sn on the synthesis of methylchlorosilanes”, Catal. Lett., 133 (2), 291-298 (2009).
13 Acker, J., Bohmhammel, K., “Thermodynamic assessment of the copper catalyzed direct synthesis of methylchlorosilanes”, J. Organomet. Chem., 693 (8), 2483-2493 (2008).
14 Acker, J., Kother, S., Lewis, K.M., Bohmhammel, K., “The reactivity in the system CuCl-Si related to the activation of silicon in the direct synthesis”, Silicon Chem., 2, 195-206 (2003).
15 Weber, G., Viale, D., Souha, H., Gillot, B., Barret, P., “Activity of silicon with copper chloride-influence of various additives”, Solid State Ionics, 26 (2), 180-180 (1988).
16 Ritzer, A., “Catalyst for a process for producing silicones”, U.S. Pat., 4450282 (1986).
17 Aramata, M., “Metallic copper catalyst and process for the synthesis of organohalosilanes”, U.S. Pat., 6365766 (2002).
18 Kalchauer, W., Straussberger, H., Streckel, W., Gross, J., “Process for the preparation of organochlorosilanes”, U.S. Pat., 6211394 (2001).
19 Lewis, K.M., McLeod, D., Kanner, B., Falconer, J.L., Frank, T.,“Surface-chemical studies of the mechanism of the direct synthesis of methylchlorosilanes”, In: Catalyzed Direct Reactions of Silicon, Lewis, K.M., Rethwisch, D.G., eds., Elsevier Science Publishers, Amsterdam, 333-440 (1993).
20 Lieske, H., Fichtner, H., Kretzschmar, H., Zimmermann, R.,“η-Cu3Si percentage and particle size in various Rochow contact masses”, Appl. Organomet. Chem., 9 (8), 657-666 (1995).
21 Luo, W.X., Wang, G.R., Wang, J.F., “Effect of CuCl particle size on the reduction reaction by silicon in preparation of contact mass used for methylchlorosilane synthesis”, Ind. Eng. Chem. Res., 45 (1), 129-133 (2005).
22 Bablin, J.M., Lewis, L.N., Bui, P., Gardner, M., “Mechanism of the methylchlorosilane reaction: Improved lab reactor design and kinetic data”, Ind. Eng. Chem. Res., 42 (15), 3532-3543 (2003).
CATALYSIS, KINETICS AND REACTION ENGINEERING
Chinese Journal of Chemical Engineering, 22(3) 299—304 (2014)
10.1016/S1004-9541(14)60034-3
2012-04-09, accepted 2013-02-25.
*To whom correspondence should be addressed. E-mail: wangjfu@tsinghua.edu.cn
 Chinese Journal of Chemical Engineering2014年3期
Chinese Journal of Chemical Engineering2014年3期
- Chinese Journal of Chemical Engineering的其它文章
- Determination of Transport Properties of Dilute Binary Mixtures Containing Carbon Dioxide through Isotropic Pair Potential Energies
- Preparation and Characterization of Sodium Sulfate/Silica Composite as a Shape-stabilized Phase Change Material by Sol-gel Method*
- Superparamagnetic Supported Catalyst H3PW12O40/γ-Fe2O3for Alkylation of Thiophene with Olefine*
- A Contraction-expansion Helical Mixer in the Laminar Regime*
- Effects of Shape and Quantity of Helical Baffle on the Shell-side Heat Transfer and Flow Performance of Heat Exchangers*
- Hydrodynamics and Mass Transfer of Oily Micro-emulsions in An External Loop Airlift Reactor
