Overexpression of microRNA-124 promotes the neuronal differentiation of bone marrow-derived mesenchymal stem cells
Defeng Zou, Yi Chen, Yaxin Han, Chen Lv, Guanjun Tu
1 Department of Orthopedics, First Af fi liated Hospital of China Medical University, Shenyang, Liaoning Province, China
2 Department of Orthopedics, Jinhua Central Hospital of Zhejiang University, Jinhua, Zhejiang Province, China
Overexpression of microRNA-124 promotes the neuronal differentiation of bone marrow-derived mesenchymal stem cells
Defeng Zou1, Yi Chen2, Yaxin Han1, Chen Lv1, Guanjun Tu1
1 Department of Orthopedics, First Af fi liated Hospital of China Medical University, Shenyang, Liaoning Province, China
2 Department of Orthopedics, Jinhua Central Hospital of Zhejiang University, Jinhua, Zhejiang Province, China
microRNAs (miRNAs) play an important regulatory role in the self-renewal and differentiation of stem cells. In this study, we examined the effects of miRNA-124 (miR-124) overexpression in bone marrow-derived mesenchymal stem cells. In particular, we focused on the effect of overexpression on the differentiation of bone marrow-derived mesenchymal stem cells into neurons. First, we used GeneChip technology to analyze the expression of miRNAs in bone marrow-derived mesenchymal stem cells, neural stem cells and neurons. miR-124 expression was substantially reduced in bone marrow-derived mesenchymal stem cells compared with the other cell types. We constructed a lentiviral vector overexpressing miR-124 and transfected it into bone marrow-derived mesenchymal stem cells. Intracellular expression levels of the neuronal early markers β-III tubulin and microtubule-associated protein-2 were signi fi cantly increased, and apoptosis induced by oxygen and glucose deprivation was reduced in transfected cells. After miR-124-transfected bone marrow-derived mesenchymal stem cells were transplanted into the injured rat spinal cord, a large number of cells positive for the neuronal marker neurofilament-200 were observed in the transplanted region. The Basso-Beattie-Bresnahan locomotion scores showed that the motor function of the hind limb of rats with spinal cord injury was substantially improved. These results suggest that miR-124 plays an important role in the differentiation of bone marrow-derived mesenchymal stem cells into neurons. Our fi ndings should facilitate the development of novel strategies for enhancing the therapeutic ef fi cacy of bone marrow-derived mesenchymal stem cell transplantation for spinal cord injury.
nerve regeneration; microRNA-124; lentivirus; overexpression; bone marrow-derived mesenchymal stem cells; neural stem cells; spinal cord injury; neurogenesis; GeneChip; motor function; NSFC grant; neural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 81070971.
Zou DF, Chen Y, Han YX, Lv C, Tu GJ. Overexpression of microRNA-124 promotes the neuronal differentiation of bone marrow-derived mesenchymal stem cells. Neural Regen Res. 2014;9(12):1241-1248.
Introduction
Injury to the spinal cord leads to loss of function, such as movement, sensation and autonomic control, in the regions innervated below the site of damage. Transplantation of neural stem cells to treat spinal cord injuries is currently one of the hottest research fi elds in biology (Paspala et al., 2009; Xu et al., 2012). However, neural stem cells are mainly obtained from embryos, which raise ethical and legal issues (Robertson, 1999). Bone marrow-derived mesenchymal stem cells (BMSCs) are a potentially promising source of cells for use in regenerative medicine because they are abundantly available, are easy to isolate from the patient themselves, are an autologous tissue and there is no ethical dispute over their use (Eftekharpour et al., 2007; Eftekharpour et al., 2008).
BMSCs can be isolated and differentiated into a variety of cell lineages in vitro, including osteoblasts, myofibroblasts, chondrocytes, adipocytes and nerve cells (Jung et al., 2005; Roese-Koerner et al., 2013). Cultured BMSCs are hypoimmunogenic and capable of homing, and thus have great potential for various clinical applications (Abdallah and Kassem, 2009). However, the differentiation of BMSCs into neurons or neural stem cells remains limited. In this study, we sought to improve the differentiation ef fi ciency of transplanted BMSCs into neurons.
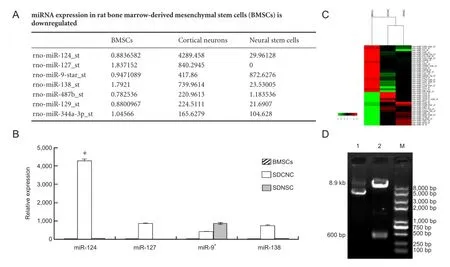
Figure 1 Detection of rat miR-124 (rno-miR-124) expression in rat bone marrow-derived mesenchymal stem cells (BMSCs), cortical neurons (SDCNC) and neural stem cells (SDNSC).
MicroRNAs (miRNAs) are 20-24-nt endogenous, evolutionarily conserved, RNA molecules that negatively regulate the translation of their target mRNAs by binding to their 3′-untranslated region (3′-UTR) (Bartel, 2004). Recent research has shown that miRNAs play essential roles in neural development and neuronal function (Bartel, 2009; Shi et al., 2010), and in the differentiation of stem cells (Krichevsky, 2007; Bak et al., 2008; Schoolmeesters et al., 2009; Liu et al., 2012; McNeill and Van Vactor, 2012; Akerblom and Jakobsson, 2013). However, the role of miRNAs in the neurogenesis of BMSCs remains unclear. For example, the brain-enriched miRNAs miR-9/9? and miR-124, which promote the assembly of neuron-specific BAF complexes (ATP-dependent chromatin remodeling complexes), are able to convert non-neuronal human dermal fibroblasts into post-mitotic neurons (Sun et al., 2013). In this study, we compared the miRNA profiles of BMSCs with cortical neurons and neural stem cells using GeneChip. We observed that miR-124 was one of the most downregulated miRNAs in BMSCs compared with cortical neurons and neural stem cells. We then overexpressed miR-124 in BMSCs using a lentiviral vector, and evaluated the effects of overexpression on the differentiation of BMSCs. We also assessed the effect of treatment with miR-124-overexpressing BMSCs in an animal model of spinal cord injury.
Materials and Methods
Materials
Adult rat neural stem cells, cortical neurons from 18.5-dayold rats, and adult rat BMSCs (all from Sprague-Dawley rats) were purchased from Cyagen Biosciences Inc., Guangzhou, Guangdong Province, China. The adult male Sprague-Dawley rats used for in vivo experiments (body weight: 230-250 g) were purchased from the Chinese Medical University Laboratory Animal Center (license No. SYXK (Liao) 2008-0013). All rats were housed in a temperature and light-cycle-controlled animal laboratory and allowed free access to food and water. This study was approved by the Animal Research Committee of China Medical University, China.
Cell culture, identi fi cation and GeneChip miRNA array
Total RNA, containing miRNAs, was isolated from sorted cells with an miRNeasy kit (Qiagen, Frankfurt, Germany). GeneChip microarray assay was then performed by a third-party service provider (Affymetrix, CA, USA). qRTPCR was used to validate miRNA expression in the BMSCs, neural stem cells and neurons.
Vector construction and transfection of BMSCs with miR-124
The lentiviral vector pLVX-EN-rno-miR-124 was constructed at Yingrun Biotechnologies Inc. (Changsha, China). 293FT cells were then transiently transfected with pLVXEN-rno-miR124 (10 μg), pLP1 (6.5 μg), pLP/VSVG (3.5 μg) and pLP2 (2.5 μg) using Lipofectamine 2000 (Life Technologies, CA, USA). The virus titer was evaluated by counting the number of GFP-positive cells. We then transfected BMSCswith the pLVX-EN-rno-miR124 pseudovirion.

Figure 2 miR-124 expression in lentiviral vector-infected BMSCs.
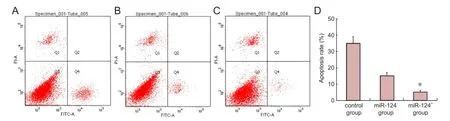
Figure 4 Analysis of the effects of miR-124 on apoptosis in bone marrow-derived mesenchymal stem cells (BMSCs) following oxygen and glucose deprivation by annexin V-FITC/PI double staining.
RT-PCR analysis of miR-124 expression in transfected BMSCs
We performed RNA extraction using Trizol reagent (Invitrogen, Guangzhou, China). Reverse transcriptases (Life Technologies) were used to prepare complementary DNA (cDNA) according to the instructions provided by Fermentas Corporation. Expression levels were quantitatively determined with the ABI 7500 system using the SYBR GreenI dye method (TOYOBO, Shanghai, China). U6 was used as the internal reference for miRNA detection. PCR protocol: 95°C for 3 minutes; 40 cycles of 95°C for 20 seconds, 60°C for 30 seconds; and 95°C for 10 seconds (to obtain the melting curve).
Western blot analysis
The BMSCs were separated into three groups: (1) control (untransfected), (2) miR-124+(pLVX-EN-rno-miR124-transfected) and (3) miR-124-(pLVX-EN-rno-transfected). Western blotting was carried out using standard protocols. The cells were lysed on ice with PMSF lysis buffer (Applygen Technologies Inc, Beijing, China) for 30 minutes. Lysed cells were collected by centrifugation at 12,000 × g for 5 minutes at 4°C to obtain total protein, which was then quanti fi ed using the bicinchoninic acid (BCA) protein assay (ShineGene, Shanghai, China). A total of 50 μg of protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 5% to 10% gels and then transferred to nitrocellulose membranes. The membranes were blocked with 5% skimmed milk powder in TBST (10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.05% Tween-20) and incubated overnight with mouse anti-β-III tubulin, anti-MAP-2 (1:1,000, Abgent Biotechnology, San Diego, CA, USA), anti-synaptophysin or anti-β-actin (1:3,000, Abgent Biotechnology) antibody at 4°C. After washing, the membranes were incubated with the secondary antibody, horseradish peroxidase-labeled IgG (goat anti-mouse IgG/HRP, KPL Biotechnology, Gaithersburg, MD, USA), for 1 hour and visualized with an ECL chemiluminescent reagent system (Pierce Biotechnology, Rockford, IN, USA). Gray scale densitometric scanning of the protein bands was performed with Quanti Scan software using β-actin as the control. Data were expressed as mean ± SD of the percentage ratio of the control.
Immuno fl uorescence detection of neuronal markers
Three groups of cells were cultured in vitro 6 days after transfection, and were fixed with 4% paraformaldehyde on coverslips and then rinsed with PBS, blocked with 10% goat serum for 1 hour at room temperature, and incubated at 4°C overnight. Sections were rinsed with PBS, and incubated with mouse anti-β-III tubulin (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse anti-MAP-2 (1:50, Cell Signaling, Boston, MA, USA) for 24 hours at 4°C. Sections were then rinsed with PBS, followed by incubation with secondary antibodies (Dy-Light488 green fl uorescence-labeled goat anti-mouse IgG [1:250, Abcam, Cambridge, MA, USA]; Texas Red-labeled rabbit anti-mouse IgG [1:250, Merck Millipore, Billerica, MA, USA]) for 1 hour in the dark at 37°C. After rinsing in PBS, the sections were observed under a fl uorescence microscope (Olympus, Tokyo, Japan).
Apoptosis assay
Oxygen and glucose deprivation (OGD) was performed on cells according to Sun et al. (2013). After 12 hours, apoptosis was quanti fi ed with an annexin V-FITC/PI double staining kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Apoptosis was measured using a flow cytometer (BD Pharmingen, Franklin, NJ, USA).
Spinal cord injury model and transplantation of BMSCs
Sprague-Dawley rats were randomly divided into three groups: (1) spinal cord injury group (spinal cord injury, treated only with 10 μL saline, n = 20), (2) miR-124--BMSCs group (spinal cord injury followed by transplantation of 10 μL (1 × 105) miR-124--BMSCs, n = 20), and (3) miR-124+-BMSCs group (spinal cord injury followed by transplantation of 10 μL (1 × 105) miR-124+-BMSCs, n = 20). The rats were then subjected to a contusion injury of the spinal cord using a 20-g weight dropped from a height of 10 cm onto the surface of the spinal cord at T10-11exposed by laminectomy (Allen, 1911). Following this, either saline, miR-124--BMSCs or mir-124+-BMSCs were transplanted into the damaged area within 30 minutes of the spinal cord injury and were also injected intraperitoneally at a dose of 20 μL/100 g four times with an interval of 3 hours.
Immunocytochemistry
Animals were euthanized 7 days after injury (n = 4 for each group). A 10-mm segment of the spinal cord encompassing the injury site was then harvested. After fi xation, the tissue blocks were embedded in paraf fi n, and sectioned at 5 μm thickness. After paraffin sections were deparaffinized and rehydrated, antigen retrieval was performed in sodium citrate buffer heated to 92-98°C for 20 minutes. Endogenous peroxidase was inactivated by incubation with 3% hydrogen peroxide for 20 minutes. Non-specific binding sites were blocked by 10% normal goat serum (Zsbio, Beijing, China) for 30 minutes. Sections were incubated with primary antibody in PBS at 4°C overnight, and the following antibody was used: mouse anti-neuro fi lament 200 (NF-200, 1:100, Santa Cruz Biotechnology). After rinsing in PBS, sections were incubated with goat anti-mouse IgG for 30 minutes followed by avidin-peroxidase complex solution containing avidin-peroxidase conjugate for 30 minutes. Staining was developed by incubating in 50% 3,3-diaminobenzidine (DAB) and 3% hydrogen peroxide in 0.1 mol/L PBS. Then, the sections were dehydrated, cleared, and coverslipped. PBS instead of primary antibody was used in the negative control.
Behavioral testing
Locomotor activity was evaluated at 1, 4, 7, 14, 21, 28, 35 and 42 days post-injury using the Basso, Beattie and Bresnahan (BBB) score to measure locomotor ability over 4 minutes. Two independent and well-trained investigators observed movement and scored locomotor function according to the BBB scale as described previously (Caggiano et al., 2005).
Statistical analysis
The data were analyzed using SPSS 17.0 statistical software. Values are presented as mean ± SD. Student’s t-test was performed for statistical evaluation. Differences with a level of P < 0.05 were considered statistically signi fi cant.
Results
Differential expression of miRNAs in BMSCs, cortical neurons and neural stem cells
GeneChip data revealed that compared with cortical neurons and neural stems cells, miR-124 was signi fi cantly downregulated in BMSCs (Figure 1A, C), which was confirmed by qRT-PCR (Figure 1B). Previous studies showed that miR-124 has an important role in the neurogenesis of non-neuronal cell types (Cheng et al., 2009). Therefore, we chose miR-124 for further study.
miR-124 expression is upregulated in BMSCs after transfection with pLVX-EN-rno-miR-124
The sequence of the lentiviral vector pLVX-EN-rno-miR-124 was confirmed by restriction enzyme digestion and DNA sequencing (Figure 1D). We observed three GFP-positive 293FT cells following addition of a 10-6dilution of the virus, indicating that there were at least three pseudovirion-transfected 293FT cells (Figure 2N). We calculated the virus titer at 1.5 × 109TU/mL. We transfected miR-124+cells with the pLVX-EN-rno-miR-124 pseudovirion and miR-124-cells with pLVX-EN-rno. Following this, the expression profiles of the three cell groups were assessed using RT-PCR. Quantitation of miR-124 was estimated based on measured Ct values. qRT-PCR revealed that miR-124 expression in miR-124+cells was significantly higher than in control or miR-124-cells (Figure 2O; ΔΔCt values 141.60 ± 8.51, 1.00 ± 0.05, 0.54 ± 0.02, respectively). This result indicated that miR-124 was upregulated after BMSCs were transfected with pLVXEN-rno-miR-124.
Overexpression of miR-124 increases expression ofβ-III tubulin, MAP-2 and synaptophysin
To investigate neurogenesis, we performed immuno fl uorescence for β-III tubulin and MAP-2 in the BMSCs during the earlier stages of the neural differentiation process. We observed a strong signal for β-III tubulin (a marker for neurons in the earlier stage; Figure 3A, red fl uorescence) and MAP-2 (Figure 3B, green fl uorescence) in the miR-124+neurons compared with the control or miR-124-group on the 6thday of in vitro culture. β-III tubulin and MAP-2 expression were clearly found in both the cell soma and the neurite-like structures under high magni fi cation (200 ×;Figure 3A, B) on the 6thday of differentiation. BMSCs developed dendrites and neurites, similar to neurons (Figure 3A2, A4, B2, B4).
Overexpression of miR-124 reduces apoptosis in BMSCs following oxygen and glucose deprivation
The effect of miR-124 on apoptosis of BMSCs following oxygen and glucose deprivation was analyzed by annexin V-FITC/PI double staining. Quanti fi cation of apoptosis was performed 6 days post-injury (n = 4/group). Early apoptosis was determined (Figure 4A-C). The rate of apoptosis in the miR-124+group was signi fi cantly lower than in the control or miR-124-group (5 ± 1% vs. 35 ± 4% or 15 ± 2%, respectively; P < 0.05,Figure 4D).
Axonal growth assessed with NF-200 immunohistochemistry
Six days after spinal cord injury, axonal regeneration was assessed by NF-200 immunostaining. In the miR-124+group, NF-200 immunoreactivity could be detected in a large number of cells in the area of spinal cord injury. In contrast, only a small number of NF-200-immunoreactive cells were observed in the injured area in the injury and miR-124-groups (Figure 5A-D).
Overexpression of miR-124 promotes functional recovery after spinal cord injury
As shown inFigure 5E, compared to the control or miR-124-group, recovery was significantly greater in the miR-124+group from day 14 after injury (P < 0.05), indicating that overexpression of miR-124 in BMSCs promotes functional recovery after spinal cord injury.
Discussion
miR-124 is one of the best characterized and most abundantly-expressed neuronal miRNAs (Krichevsky et al., 2003; Kim et al., 2004). Overexpression of miR-124 results in upregulation of the expression of neuronal markers, as well as morphological changes, including enhanced neurite outgrowth and complexity (Yoo et al., 2011). Some overexpression studies in vertebrates have identi fi ed miR-124 as a promoter of neuronal differentiation and an inhibitor of progenitor self-renewal (Maiorano et al., 2009; Clark et al., 2010; Liu et al., 2011; Sanuki et al., 2011; Akerblom et al., 2012; Weng and Cohen, 2012; Xia et al., 2012). However, whether miR-124 can regulate neurogenesis in BMSCs remains unknown. In this study, we succeeded in constructing a lentiviral vector for the overexpression of miR-124 in BMSCs. Overexpression of miR-124 was associated with increased expression of the proteins β-III tubulin, MAP-2 and synaptophysin a■er 6 days of in vitro culture.
Tubulin is an important structural protein in neurons and is a marker of differentiated neurons. β-III tubulin is expressed by the neuroepithelium during embryogenesis and is widely used as a speci fi c marker of neurons (von Bohlen und Halbach, 2011). MAP-2 is a dendrite-specific protein that plays an important role in the development, formation and regeneration of the nervous system (Czikk et al., 2014). We investigated the rate of cell differentiation of transfected BMSCs into neurons based on their expression of β-III tubulin and MAP-2. In BMSCs overexpressing miR-124, the number of β-III tubulin and MAP-2-positive neurons were remarkably elevated. This result is consistent with the fi ndings of Roese-Koerner et al. (2013). However, these two markers do not demonstrate that BMSCs that have undergone neurogenesis are functionally neurons. Synaptophysin is a synaptic protein that is used as a marker of synapse formation (Czikk et al., 2014). We observed that synaptophysin expression was substantially increased in miR-124+cells compared with miR-124-or control cells. This indicates that miR-124 not only promotes neurogenesis in BMSCs, but also commits them to the development of synapses, which is essential for recovery of nerve function. After spinal cordinjury, secondary damage is triggered by multiple processes. Usually, transplanted BMSCs undergo apoptosis as a result of in fl ammatory and oxidative damage. Recently, a number of miRNAs were found to be decreased after spinal cord injury. In particular, miR-124a expression was signi fi cantly decreased 1 to 7 days after spinal cord injury (Nakanishi et al., 2010). Here, we found that BMSCs overexpressing miR-124 were relatively protected from oxygen and glucose deprivation-induced apoptosis in vitro.
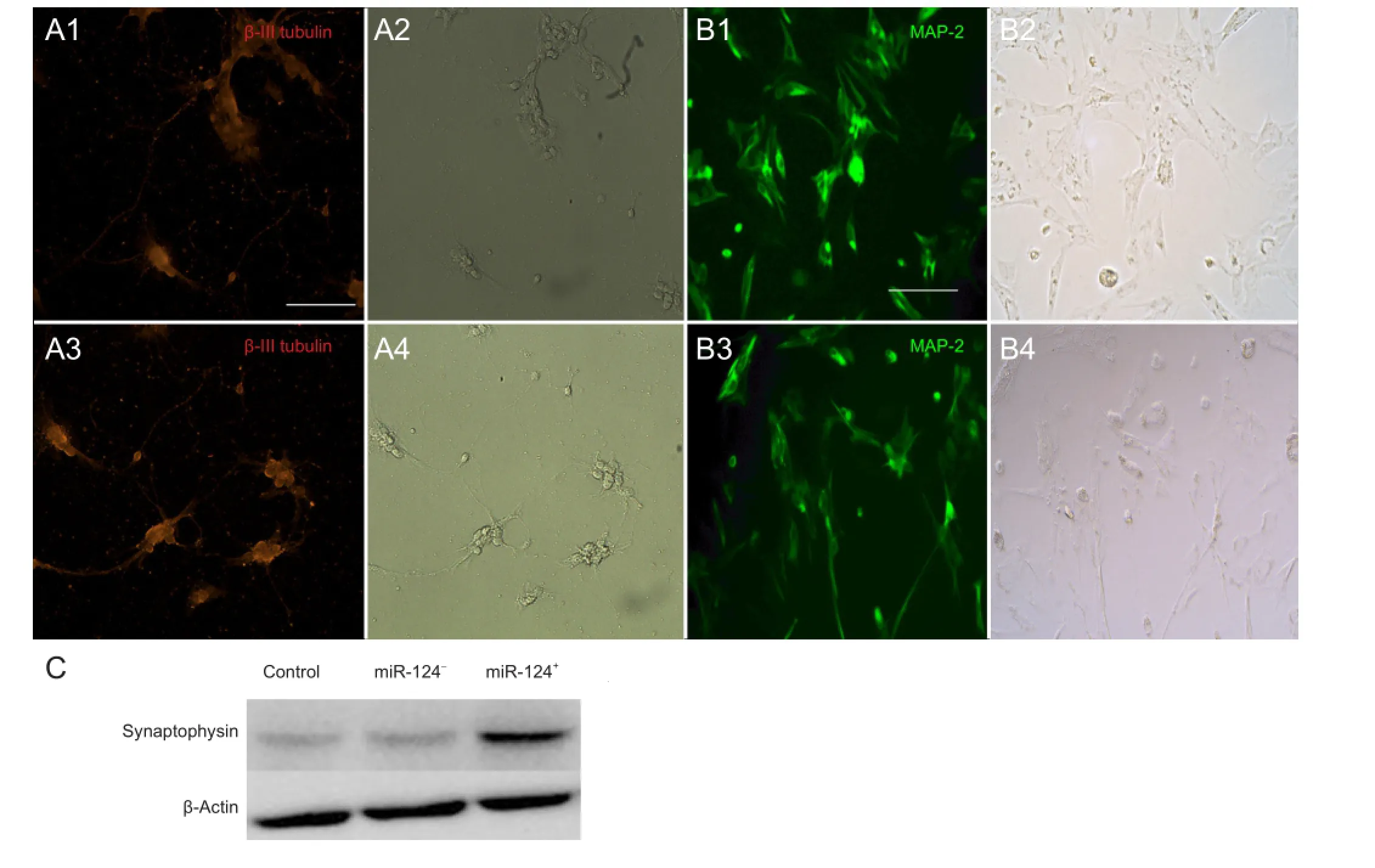
Figure 3 Neural differentiation of BMSCs on the 6thday of in vitro transfection.

Figure 5 NF-200 immunohistochemistry in the injured rat spinal cord following miR-124-transfected bone marrow-derived mesenchymal stem cell (BMSC) transplantation.
Although we initially showed that BMSCs could be induced to differentiate into neurons in vitro by overexpressing miR-124, we needed to confirm if they could differentiate into functional neurons in vivo. We found that overexpression of miR-124 in BMSCs not only enhances the ability of the cells to survive, but also raises the rate of differentiation of the transplanted BMSCs into neurons in the region of spinal cord injury. Our NF-200 immunohistochemistry results indicated that there were more NF-200-positive cells in the miR-124+group than in the miR-124-or control group. This result is in agreement with those of Sun et al. (2013). Our behavioral data revealed that miR-124-overexpressing BMSCs promote functional locomotor recovery after spinal cord injury.
Previous studies demonstrated that miR-124 represses the expression of proteins with anti-neuronal activities, including repressor-element-1-silencing transcription factor (Qureshi et al., 2010; Baudet et al., 2011), small c-terminal domain phosphatase 1 (SCP1) (Visvanathan et al., 2007) and Sox9 (Sanuki et al., 2011). A study by Doeppner et al. (2013) found that miR-124 reduces expression of the target deubiquitinating enzyme Usp14, thereby increasing repressor-element-1-silencing transcription factor degradation. These observations provide insight into the molecular mechanisms underlying the differentiation of BMSCs into neurons.
In conclusion, the transplantation of BMSCs overexpressing miR-124 may be an effective therapeutic strategy for promoting regeneration and functional recovery following spinal cord injury.
Acknowledgments:We would like to thank the staff of the Central Laboratory of the First Affiliated Hospital of China Medical University (Shenyang, Liaoning Province, China) for their technical assistance.
Author contributions:Zou DF performed the experiments, conducted statistical analysis, and wrote the manuscript. Chen Y and Han YX assisted in the experiments. Lv C was responsible for statistical analysis. Tu GJ directed the research and supervised manuscript writing. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Abdallah BM, Kassem M (2009) The use of mesenchymal (skeletal) stem cells for treatment of degenerative diseases: current status and future perspectives. J Cell Physiol 218:9-12.
?kerblom M, Sachdeva R, Barde I, Verp S, Gentner B, Trono D, Jakobsson J (2012) MicroRNA-124 is a subventricular zone neuronal fate determinant. J Neurosci 32:8879-8889.
Akerblom M, Jakobsson J (2013) MicroRNAs as neuronal fate determinants. Neuroscientist 20:235-242.
Allen A (1911) Surgery for experimental lesions of spinal cord equivalent to crush injury of fracture dislocation of spinal column: a preliminary report. JAMA 57:878-880
Bak M, Silahtaroglu A, M?ller M, Christensen M, Rath MF, Skryabin B, Tommerup N, Kauppinen S (2008) MicroRNA expression in the adult mouse central nervous system. RNA 14:432-444.
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281-297.
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215-233.
Baudet ML, Zivraj KH, Abreu-Goodger C, Muldal A, Armisen J, Blenkiron C, Goldstein LD, Miska EA, Holt CE (2011) miR-124 acts through CoREST to control onset of Sema3A sensitivity in navigating retinal growth cones. Nat Neurosci 15:29-38.
Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA (2005) Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma 22: 226-239.
Cheng LC, Pastrana E, Tavazoie M, Doetsch F (2009) MiR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci 12:399-408.
Clark AM, Goldstein LD, Tevlin M, Tavaré S, Shaham S, Miska EA (2010) The microRNA miR-124 controls gene expression in the sensory nervous system of Caenorhabditis elegans. Nucleic Acids Res 38:3780-3793.
Czikk MJ, Totten S, Hammond R, Richardson BS (2014) Microtubule-associated protein 2 and synaptophysin in the preterm and near-term ovine fetal brain and the effect of intermittent umbilical cord occlusion. Reprod Sci. doi:10.1177/1933719114529371
Doeppner TR, Doehring M, Bretschneider E, Zechariah A, Kaltwasser B, Müller B, Koch JC, B?hr M, Hermann DM, Michel U (2013) MicroRNA-124 protects against focal cerebral ischemia via mechanisms involving Usp14-dependent REST degradation. Acta Neuropathol 126:251-265.
Eftekharpour E, Karimi-Abdolrezaee S, Wang J, El Beheiry H, Morshead C, Fehlings MG (2007) Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction. J Neurosci 27: 3416-3428.
Eftekharpour E, Karimi-Abdolrezaee S, Fehlings MG (2008) Current status of experimental cell replacement approaches to spinal cord injury. Neurosurg Focus 24:E19.
Jung DI, Ha J, Kang BT, Kim JW, Quan FS, Lee JH, Woo EJ, Park HM (2009) A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci 285:67-77.
Kim J, Krichevsky A, Grad Y, Hayes GD, Kosik KS, Church GM, Ruvkun G (2004) Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci U S A 101:360-365.
Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS (2003) A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 9:1274-1281.
Krichevsky AM (2007) MicroRNA pro fi ling: from dark matter to white matter, or identifying new players in neurobiology. Sci World J 7: 155-166.
Liu J, Githinji J, Mclaughlin B, Wilczek K, Nolta J (2012) Role of miRNAs in neuronal differentiation from human embryonic stem cell-derived neural stem cells. Stem Cell Rev 8:1129-1137.
Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, Zhang ZG (2011) MicroRNA pro fi ling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One 6: e23461.
Maiorano NA, Mallamaci A (2009) Promotion of embryonic cortico-cerebral neuronogenesis by miR-124. Neural Dev 4:40.
McNeill E, Van Vactor D (2012) MicroRNAs shape the neuronal landscape. Neuron 75:363-379.
Nakanishi K, Nakasa T, Tanaka N, Ishikawa M, Yamada K, Yamasaki K, Kamei N, Izumi B, Adachi N, Miyaki S, Asahara H, Ochi M (2010) Responses of microRNAs 124a and 223 following spinal cord injury in mice. Spinal Cord 48:192-196.
Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B (2009) Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine (Phila Pa 1976) 34:328-334.
Paspala SA, Balaji AB, Nyamath P, Ahmed KS, Khan AA, Khaja MN, Narsu ML, Devi YP, Murthy TV, Habibullah CM (2009) Neural stem cells & supporting cells--the new therapeutic tools for the treatment of spinal cord injury. Indian J Med Res 130:379-391.
Qureshi IA, Gokhan S, Mehler MF (2010) REST and CoREST are transcriptional and epigenetic regulators of seminal neural fate decisions. Cell Cycle 9:4477-4486.
Robertson JA (1999) Ethics and policy in embryonic stem cell research. Kennedy Inst Ethics J 9:109-136.
Roese-Koerner B, Stappert L, Koch P, Brüstle O, Borghese L (2013) Pluripotent Stem Cell-Derived Somatic Stem Cells as Tool to Study the Role of microRNAs in early human neural development. Curr Mol Med 13:707-722.
Sanuki R, Onishi A, Koike C, Muramatsu R, Watanabe S, Muranishi Y, Irie S, Uneo S, Koyasu T, Matsui R, Chérasse Y, Urade Y, Watanabe D, Kondo M, Yamashita T, Furukawa T (2011) miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat Neurosci 14:1125-1134.
Schoolmeesters A, Eklund T, Leake D, Vermeulen A, Smith Q, Force Aldred S, Fedorov Y (2009) Functional pro fi ling reveals critical role for miRNA in differentiation of human mesenchymal stem cells. PLoS One 4:e5605
Shi Y, Zhao X, Hsieh J, Wichterle H, Impey S, Banerjee S, Neveu P, Kosik KS (2010) MicroRNA regulation of neural stem cells and neurogenesis. J Neurosci 30:14931-14936.
Sun AX, Crabtree GR, Yoo AS (2013) MicroRNAs: regulators of neuronal fate. Curr Opin Cell Biol 25:215-221.
Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan JL, Liu X (2013) MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci Ther 19:813-819.
Visvanathan J, Lee S, Lee B, Lee JW, Lee SK (2007) The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev 21:744-749.
von Bohlen und Halbach O (2011) Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res 345:1-19.
Weng R, Cohen SM (2012) Drosophila miR-124 regulates neuroblast proliferation through its target anachronism. Development 139:1427-1434.
Xia H, Cheung WK, Ng SS, Jiang X, Jiang S, Sze J, Leung GK, Lu G, Chan DT, Bian XW, Kung HF, Poon WS, Lin MC (2012) Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells. J Biol Chem 87:9962-9971.
Xu W, Li P, Qin K, Wang X, Jiang X (2012) MiR-124 regulates neural stem cells in the treatment of spinal cord injury. Neurosci Lett 529:12-17.
Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR (2011) MicroRNA-mediated conversion of human fi broblasts to neurons. Nature 476:228-231.
Copyedited by Patel B, Li CH, Song LP, Zhao M
10.4103/1673-5374.135333
Guanjun Tu, M.D., Department of Orthopedics, First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China,
tu188@sina.com.
http://www.nrronline.org/
Accepted: 2014-05-23
 中國(guó)神經(jīng)再生研究(英文版)2014年12期
中國(guó)神經(jīng)再生研究(英文版)2014年12期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- 1111
- Assessment on self-care, mobility and social function of children with spina bi fi da in Turkey
- Recovery of the corticospinal tracts injured by subfalcine herniation: a diffusion tensor tractography study
- Diffuse axonal injury after traumatic cerebral microbleeds: an evaluation of imaging techniques
- Autophagy: a double-edged sword for neuronal survival after cerebral ischemia
- Prolonged electrical stimulation causes no damage to sacral nerve roots in rabbits
