2D/2D Ti3C2/Bi4O5Br2 Nanosheet Heterojunction with Enhanced Visible Light Photocatalytic Activity for NO Removal
Xiaoqing Yang , Hualin Yang , Huan Lu , Haoxuan Ding , Yanxin Tong , Fei Rao , Xin Zhang ,Qian Shen , Jianzhi Gao ,*, Gangqiang Zhu
1 School of Physics and Information Technology, Shaanxi Normal University, Xi’an 710062, China.
2 School of Geography and Tourism, Shaanxi Normal University, Xi’an 710062, China.
3 School of Physics and Astronomy, University of Birmingham, Birmingham B15 2TT, UK.
4 Key Laboratory of Flexible Electronics, Institute of Advanced Materials, Nanjing Tech University, Nanjing 211816, China.
Abstract:This study concentrated on the production of a two-dimensional and two-dimensional (2D/2D)Ti3C2/Bi4O5Br2 heterojunction with a large interface that applied as one of the novel visible-light-induced photocatalyst via the hydrothermal method. The obtained photocatalysts enhanced the photocatalytic efficiency of the NO removal. The crystal structure and chemical state of the composites were characterized using X-ray diffraction(XRD)and X-ray photoelectron spectroscopy (XPS). The results showed that Ti3C2, Bi4O5Br2, and Ti3C2/Bi4O5Br2 were successfully synthesized. The experimental results of scanning electron microscopy (SEM)and transmission electron microscopy (TEM)showed that the prepared samples had a 2D/2D nanosheet structure and large contact area. This structure facilitated the transfer of electrons and holes. The solar light absorptions of the samples were evaluated using the UV-Vis diffuse reflectance spectra (UV-Vis DRS). It was found that the absorption band of Ti3C2/Bi4O5Br2 was wider than that of Bi4O5Br2. This represents the electrons in the Ti3C2/Bi4O5Br2 nanosheet composites were more likely to be excited. The photocatalytic experiments showed that the 2D/2D Ti3C2/Bi4O5Br2 composite with high photocatalytic activity and stability. The photocatalytic efficiency of pure Bi4O5Br2 for the NO removal was 30.5%, while for the 15% Ti3C2/Bi4O5Br2 it was 57.6%. Moreover, the catalytic reaction happened in a short period. The concentration of NO decreased exponentially in the first 5 min, which approximately reached the final value. Furthermore, the stability of 15% Ti3C2/Bi4O5Br2 was favorable: the catalytic rate was approximately 50.0% after five cycles of cyclic catalysis. Finally, the scavenger experiments, electron spin resonance spectroscopy (ESR), transient photocurrent response, and surface photovoltage spectrum (SPS)were applied to analyze the photocatalytic mechanism of the composite. The results indicated that the 2D/2D heterojunction Ti3C2/Bi4O5Br2 improved the separation rate of the electrons and holes, thus enhancing the photocatalytic efficiency. In the photocatalytic reactions, the photogenerated electrons (e?)and superoxide radica were critical active groups that had a significant role in the oxidative removal of NO. The in situ Fourier-transform infrared spectroscopy (in situ FTIR)showed that the photo-oxidation products were mainly. Based on the above experimental results, a possible photocatalytic mechanism was proposed. The electrons in Bi4O5Br2 were excited by visible light. They jumped from the valence band (VB)of Bi4O5Br2 to the conduction band (CB). Then, the photoelectrons transferred from the CB of Bi4O5Br2 to the Ti3C2 surface, which significantly promoted the separation of the electron-hole pairs. Therefore, the photocatalytic efficiency of Ti3C2/Bi4O5Br2 on NO was significantly improved. This study provided an effective method for preparing 2D/2D Ti3C2/Bi4O5Br2 nanocomposites for the photocatalytic degradation of environmental pollutants, which has great potential in solving energy stress and environmental pollution.
Key Words:Ti3C2/Bi4O5Br2; 2D/2D heterojunction; Semiconductor; Photocatalyst; Photocatalytic degradation;NO removal
1 Introduction
NO is one of the significant air pollutants. It has a tremendous negative impact on the environment and human health around globe1,2. Nowadays, common NO removal technologies generally include physical adsorption3, biological filtration2, thermal catalytic reduction4and selective catalytic reduction5,6. However, these methods are complicated, costly,and inefficient for the removal of NO at the parts per billion (ppb,1 ppb = 1 × 10?9(volume fraction))level7–9. By considering the issues above, semiconductor photocatalytic technology has become a solution and attracted many attentions in recent years.It can degrade NO without introducing other pollutants through solar energy10–12. The key to photocatalytic technology is to fabricate a material which can absorb the sunlight in a broader range and separate the photo-generated carriers extensively.Therefore, it is necessary to study the high-efficient photocatalysts and use it in the field of NO removal.
According to the recent study, one of the available ways to improve the photocatalytic performance of semiconductor photocatalytic materials is combining a 2D material with another 2D material to form 2D/2D heterojunctions13,14. The reason is that 2D ultrathin nanosheets can expose a large number of internal atoms and form abundant structural defects on the surface, which can not only promote light absorption but also serve as highly active sites for photocatalytic reactions. Also, 2D materials have a large surface area, which is conducive to increasing their contact area with other reactants, reducing the transmission path of photogenerated carriers, and improving the separation rate of carriers15,16. The 2D/2D heterogeneous junction has a larger interface area compared with 0D/2D and 1D/2D heterojunctions. This characteristic can accelerate the transfer and separation efficiency of electron-hole pairs. Thus 2D composite materials have a wide application prospect in the photocatalytic region14,17,18.
Bi4O5Br2is a 2D graphene-like material that can be made into an ultrathin structure by controlling the experimental conditions and their unique (Bi2O2)2+structure, double halide atomic layer and the internal electric field between cations and anions can facilitate the separation of electrons and holes19–22. Besides,Bi4O5Br2has suitable band gap (~2.26 eV)and visible-lightresponsive ability23,24. However, although Bi4O5Br2has these advantages, its practical application is limited by the high recombination probability of photogenerated electron-hole pairs25.Recently, Bi4O5Br2-based hybrid materials have been widely studied to improve the photocatalytic activity of Bi4O5Br2, such as g-C3N4/Bi4O5Br225, Bi4O5Br2/Bi24O31Br10/Bi2SiO526, h-BN-Bi4O5Br227,etc. Ti3C2is a typical member of two dimensional(2D)layer MXenes which have extraordinary ability in metallic conductivity, structural stability and light capture28–30. On the other hand, the ultrahigh electron mobility and large surface area of Ti3C2make it one of the most promising 2D co-catalysts31,32.These strengths make it possible for Ti3C2acting as a potential material for heterojunction formation. It is expected that heterojunction based on Bi4O5Br2 and Ti3C2 can effectively improve the separation and transfer rate of photo-generated carriers.
In this work, we successfully synthesized the Ti3C2/Bi4O5Br2composite with a simple and feasible hydrothermal method.Furthermore, the photocatalytic activity of Ti3C2/Bi4O5Br2 for oxidative removal of NO was evaluated at ppb level under simulated visible light irradiation. Complex Ti3C2/Bi4O5Br2showed superior photocatalytic activity compared to pure Bi4O5Br2. The mechanism of photocatalytic degradation of NO was discussed according to the transient photocurrent response,SPS and other experimental results. The results show that Ti3C2as a co-catalyst accelerates the transmission of photocarriers,increases the separation rate of electron-hole pairs, and improves the photocatalytic efficiency.
2 Experimental
2.1 Preparation of Ti3C2/Bi4O5Br2 (TC/BOB)composite
Different proportions of TC/BOB samples were synthesized using hydrothermal method. The two dimensional layered Ti3C2(TC)was prepared by selectively exfoliating Al from Ti3AlC2using aqueous HF28. 1.9935 g Bi(NO3)3·5H2O, 0.5 g cetyltrimethyl ammonium bromide and a certain amount of TC was introduced into 60 mL deionized water and stirred continuously (when the proportions of Ti3C2is 5%, 10%, 15%,20% and 25% (w, mass fraction), the mass of TC was 0.043,0.086, 0.1288, 0.172 and 0,215 g). Then the pH value of the uniform suspension solution was adjusted to 8.5 by adding an appropriate amount of 2 mol?L?1NaOH aqueous solution. The solution was continuously stirred until all the solution was mixed thoroughly, and the pH was adjusted to 8.5 again. Then the uniform suspension solution was transferred into 100 mL Teflonlined stainless steel autoclaves, heated to 160 °C for 18 h, and cooled to room temperature naturally. The resulting samples were collected after discarding the supernatant and were washed with deionized water and ethyl alcohol several times. Finally, the TC/BOB samples were dried in vacuum at 70 °C for 12 h. Pure Bi4O5Br2(BOB)was prepared in the same way, without adding TC.
2.2 Photocatalytic test
The photocatalytic performance of the prepared samples was evaluated by the removal efficiency of NO at ppb levels. The sample was performed in a continuous flow reactor at surroundings temperature under simulated solar irradiation (300 W xenon lamp with an ultraviolet-cut filter,λ> 420 nm). The rectangular stainless steel chamber of the reactor has a quartz glass panel on top, with a total volume of 4.5 L. Dispersing 0.108 g TC/BOB in 15 mL deionized water at first. Then the mixed solution was oscillated with ultrasonic for about 20 min, poured into an evaporative glass dish with a diameter of 10 cm, and dried in an oven at 80 °C for 12 h to form a film. Then the sample was exposed to NO gas. When the concentration of NO was stable, the light kept on for 30 min to ensure the equilibrium of adsorption-desorption, and the change of NO concentration during this period was recorded.
3 Results and discussion
3.1 Crystal structures and surface compositions
The crystal structure of prepared catalysts was thoroughly investigated by X-ray diffraction (XRD). As exhibited in Fig. 1,the phase structure analysis of TC, BOB and TC/BOB composite photocatalysts were performed. The prominent diffraction peaks at 2θ= 18.1°, 27.2° and 61.0° belong to TC33,34. For the XRD pattern of the BOB, the diffraction peaks at 2θ =24.29°, 29.54°,31.80°, 43.35°, 45.49° could be assigned to the (112), (113ˉ ),(020), (105), and (422)crystal planes of BOB (ICDD PDF #37-0699)respectively, and it has a standard monoclinic structure. In the XRD pattern of TC/BOB composite, the typical characteristic diffraction peaks of BOB were observed, but there were no notable characteristic diffraction peaks of TC. It can be attributed to the fact that the intensity of the diffraction peak of TC is too weak to be seen when compared with the high-intensity diffraction peak of BOB. Further X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM)and transmission electron microscopy (TEM)experiments helped us confirm that the synthesized composite was TC/BOB indeed.

Fig. 1 XRD patterns of TC, BOB and TC/BOB.

Fig. 2 (a)XPS survey spectrum and high-resolution orbital scans for (b)Ti 2p, (c)C 1s, (d)Bi 4f, (e)Br 3d of the TC/BOB composite.
In order to further understand the chemical composition and elements valence states of the prepared TC/BOB samples, XPS analysis was investigated. Fig. 2a shows the survey spectrum of XPS, indicating that elements Bi, O, Br, Ti and C existed in the samples. As shown in Fig. 2b, four peaks can be observed,among which 459.1 eV (Ti 2p3/2)and 461.6 eV (Ti 2p1/2)can be attributed to Ti-C bond of TC sample, while 457.9 eV (Ti 2p3/2)and 464.4 eV (Ti 2p1/2)belong to Ti-O bond of TC sample35,36,which comes from O adsorbed on TC surface. After compounding TC and BOB, the peaks of Ti-O disappeared.This indicated that the Ti-O bond was broken during the composite process. There are four peaks appeared in Fig. 2c:288.5, 285.8, 284.5 and 281.3 eV, corresponding to C-F, C=O, C-C and C-Ti bonds, respectively35,37. The surface of TC was negatively charged with abundant surface terminal groups attached on32, which makes the connection between TC and BOB easier. The high-resolution spectrum of Fig. 2d can be fitted to two peaks: 164.4 eV (Bi 4f5/2)and 159.3 eV (Bi 4f7/2),which confirmed the existence of Bi3+in BOB23. In Fig. 2e, it shows that the binding energies of BOB and TC/BOB are 68.2 and 69.4 eV, corresponding to Br 3d3/2and Br 3d5/2respectively38.Furthermore, due to the interaction between TC and BOB, there is some peak offset in the XPS survey spectrum. From the spectrum of XPS, we can draw the conclusion that we have successfully prepared the compound of TC and BOB indeed.

Fig. 2 (a)XPS survey spectrum and high-resolution orbital scans for (b)Ti 2p, (c)C 1s, (d)Bi 4f, (e)Br 3d of the TC/BOB composite.
3.2 Morphology
To reveal the surface morphology of the prepared TC, BOB and TC/BOB samples, SEM was studied. As shown in Fig. 3a,b,BOB and TC materials are constructed of typical nanosheets. TC has an apparent hierarchical structure, showing the characteristics of 2D MXenes. And in Fig. 3c,d, the BOB is attached to the surface of TC, forming 2D/2D heterojunction.This structure can facilitate the photo-generated charge carriers transfer to the surface of TC and extend the life of carriers.

Fig. 3 SEM images of (a)BOB, (b)TC, (c), (d)TC/BOB composite.
In order to further understand the microstructure and the formation of 2D/2D heterojunction of composite samples, TEM was performed. As shown in Fig. 4a, transparent ultra-thin nanosheets can be observed. The corresponding enlarged image Fig. 4b shows the distinguishable interface between TC and BOB, which is indicated by the blue dash area and the red dash area respectively. At the scale of 50 nm, it is evident that a large number of small ultra-thin nanocrystals adhere to a large ultrathin nanocrystal. The area circled by the yellow dotted line in Fig. 4b is magnified to Fig. 4c. As can be seen from Fig. 4c, the light-colored area is TC, and the dark-colored area is BOB. In the high-power TEM image Fig. 4c, both kinds of nanosheets generate lattice phases. Meanwhile, by measuring the width of the lattice diffraction fringes on different nanosheets, twodvalues of 0.281 and 0.266 nm can be obtained, which correspond to the lattice distance of the (020)facet of the BOB and the(010)facet of TC39,40. In order to further prove the element composition of TC/BOB composite structure, the mapping diagram of Bi, Br and Ti elements is given in Fig. 4d. It can be seen that Bi, Br and Ti elements distributed in TC/BOB uniformly.

Fig. 4 TEM images of (a)(b)TC/BOB, (c)HRTEM image of TC/BOB, (d)EDS mapping: Bi, Br and Ti of TC/BOB composite.
3.3 The specific surface area and Optical properties
The specific BET surface area (SBET)of a photocatalyst is an important factor that affects the photocatalytic efficiency. Fig. 5a shows the N2 adsorption-desorption isotherms of the synthesized samples. TheSBET values of the TC, BOB and TC/BOB were 13.14, 19.67 and 24.73 m2?g?1, respectively. TC and BOB are both layered structures, with superposition between layers.When TC/BOB heterojunction is formed, the TC layer and the BOB layer will interpenetrate each other and more areas will be exposed. Therefore, the composite compound TC/BOB has the largest specific surface area, which is conducive to the occurrence of photocatalytic reaction.

Fig. 5 (a)N2 gas adsorption-desorption isotherms of different samples and (b)UV-Vis diffuse reflectance spectra of different samples.
The solar light absorptions of samples were evaluated by UVVis diffu se reflectance spectra (UV-Vis DRS)in Fig. 5b. Since the TC is black, it exhibited the highest visible light absorption from 400 to 800 nm31. Meanwhile, it can be observed that pure BOB exhibited an optical response at wavelengths shorter than 480 nm. Typically, the absorption edges of different proportions of TC/BOB was located at around 500 nm and the absorbance ability for visi ble light got better as the amount of TC increased,which indicated that the TC nanosheets were successfully coupled with BOB. Furthermore, stronger absorbance ability ensures that the TC/BOB can generate enough photogenerated electron-hole pairs under visible light irradiation and might give rise to a photo-thermal effect during the photocatalytic reaction.The red shift of absorption band and enhancement of optical absorption capacity would promote the visible-light photocatalytic performance of TC/BOB41,42.
3.4 photocatalytic performance
The photocatalytic activities of the TC, BOB, and TC/BOB composite photocatalysts for oxidative removal of NO were evaluated at ppb levels under simulated visible light irradiation(λ> 420 nm)in a continuous reactor. Fig. 6a displays the change of NO concentration with irradiation time after adding a certain amount of photocatalyst (Ct/C0, %). Here,C0is the initial concentration of NO at 420 ppb, andCtrepresents the concentration of NO after exposing to the light for a period of timet.
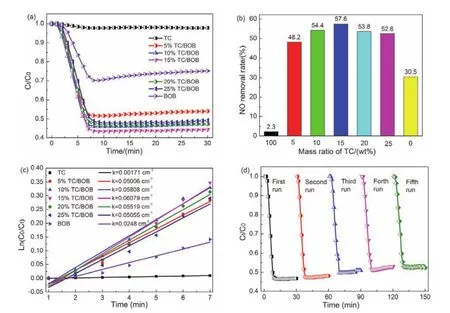
Fig. 6 (a)Photocatalytic activity, (b)NO removal efficiency, (c)the kinetic curves of photo-degradation of samples under visible light irradiation; (d)photocatalytic activity of 15%TC/BOB for five cycles under visible light irradiation.
The pure TC has no photolysis for NO even after exposing to irradiation for 30 min, indicating that TC have no intrinsic catalytic ability. In Fig. 6b, the removal ratio of pure BOB was 30.5% exhibiting a suboptimal photocatalytic performance. The poor photocatalytic ability of BOB is caused by various inherent properties of this material, for example, the rapid recombination of electron-hole pairs25. Inspiringly, all TC/BOB composite exhibited better photocatalytic activity than the pure BOB which may be attributed to the enhanced light absorption, effective charge transfer and close contact between TC and BOB. After 30 min of visible light irradiation (λ> 420 nm), the removal ratios of NO by 5%TC/BOB, 10%TC/BOB, 15%TC/BOB,20%TC/BOB, and 25%TC/BOB are 48.2%, 54.4%, 57.6%,53.8%, and 52.6%, respectively.
Amongst the samples, the 15%TC/BOB composite photocatalyst exhibited the highest NO removal effect. The photocatalytic rate constant (k)is showed in Fig. 6c. Thekvalue of 15%TC/BOB composite is nearly 5.8 times and 1.7 times higher than that of pure TC and BOB, respectively. However, as the amount of TC continued to increase the photocatalytic efficiency of TC/BOB gradually deteriorated, which suggested that the appropriate amount of TC would make the photocatalytic efficiency of BOB reach optimization.
BOB and TC/BOB are vital in the photocatalytic process. The concentration of NO decreased exponentially in the first 5 min,which approximately reach to the final value. After that, the catalytic effects of all TC/BOB composite showed no attenuation during the process of NO oxidative removal. This showed the high photo-stability of the photocatalysts, which is in good agreement with the results of recyclability test. The catalytic stability of 15%TC/BOB composite was investigated by multiple photocatalytic reactions. The results are shown in Fig. 6d. After five times photocatalytic reactions, the photocatalytic activity of 15%TC/BOB decreased slightly, indicating the excellent stability of the composite.
3.5 Possible mechanism of the enhancement
The scavenger experiments and electron spin resonance spectroscopy (ESR)are useful to demonstrate the photocatalytic mechanism of the photocatalytic oxidation of NO. The type and contribution of functional groups in the photocatalytic reaction were determined by trap experiments and ESR tests.KI, K2Cr2O7, isopropyl alcohol (IPA), and p-benzoquinone(PBQ)were prepared for trapping holes (h+), electrons (e?),hydroxide radical (·OH), and superoxide radicalspecies separately43,44. As shown in Fig. 7a,b, when K2Cr2O7 andpbenzoquinone (PBQ)were added to the sample, the NO removal efficiency prominently reduced. Comparing the suppression effects of KI and IPA with K2Cr2O7and PBQ, the KI and IPA were less effective, which indicated that the photogenerated e?andare significant for oxidative removal of NO. Since the photogenerated electrons and holes could provoke ·OH andgenerated from H2O and O2, it is necessary to understand the role of these active oxygen species in the oxidative removal of NO.In Fig. 7c,d, the electron spin resonance spectroscopy (ESR)indicated that the 15%TC/BOB can produceand ·OH, which is in agreement with the result of the trapping experiments.

Fig. 7 (a), (b)Photocatalytic activity of 15%TC/BOB composite in the presence of various scavengers,(c)ESR spectra of DMPO- and (d)DMPO-·OH of 15%TC/BOB photocatalyst.
The separation rate of photocatalytic electron-hole pairs is vital to assess the photocatalytic performance of a sample. A practical and common way to illustrate the transfer and separation efficiency of the photogenerated charge carriers is the transient photocurrent response. In order to evaluate the effect of 2D/2D heterojunction for carrier separation rate, the transient photocurrent response was studied. Fig. 8a shows the transient photocurrent response of BOB and 15%TC/BOB electrodes. The photocurrent density of pure BOB electrode was 1.47 μA·cm?2,while that of 15%TC/BOB electrode was 2.55 μA·cm?2. In other words, the photocurrent of 15%TC/BOB electrode is 1.7 times higher than that of the pure BOB electrode, which indicates the 15%TC/BOB has more effective charge separation and transfer rate.

Fig. 8 (a)Transient photocurrent response and (b)SPS spectra of BOB and TC/BOB.
To further test the effect of 2D/2D TC/BOB heterojunction on charge separation and transfer, surface photo-voltage spectrum(SPS)was studied. As is known to all that the stronger SPS signal means the higher electron-hole separation rate. BOB and 15%TC/BOB composites generated significant positive SPS signals with the stimulation of 300–550 nm light, which shows in Fig. 8b. Furthermore, the SPS signal of 15%TC/BOB is much stronger than that of pure BOB, which indicates that there is a strong interaction between TC and BOB nanosheets, promoting the transfer efficiency of photogenerated charges.
In order to further understand the reaction pathway and mechanism of TC/BOB in NO removal,in situFouriertransform infrared spectroscopy (in situFTIR)was carried out.As shown in Fig. 9,in situFTIR spectrum presents both the performance of 15%TC/BOB in the dark and under visible light irradiation. It can be seen that with the extension of the illumination time, almost all the peaks are strengthened,indicating that the reaction products are increasing. When light hits the surface of TC/BOB photocatalyst, e?and h+(Eq. (1))can be excited, and then e–can react with O2to form(Eq. (2)).According to the result of trapping experiment and ESR measurement, the photocatalytic e?andare the main active groups for NO photocatalytic oxidation. The two peaks,appearing at 1457 and 1635 cm?1, are the characteristic peaks of NO22,45, which was produced after NO oxidized by oxygen (Eq.(3))and was the initial reaction products. The generated NO2 can be further converted into N2O4 (Eq. (4)). As a result, there is a characteristic peak belonging to N2O4at 1275 cm?123. Two peaks, 1489 and 1558 cm?1, indicate the presence ofThe remaining four characteristic peaks 870, 1009, 1475, 1541 cm?1were all attributed to23,45,47,48. Photogenerated e?reacts with NO2, leading to the product of(Eq. (5)). NO reacts withto produce(Eq. (6)). Besides, NO and NO2react with h+and water in the air to form(Eq. (7),Eq. (8)). According to the above analyses, all reaction paths are shown by Eqs. (1)–(8).

Fig. 9 In situ FT-IR spectra of photocatalytic oxidation process of NO by 15%TC/BOB.


Since the nitrite ions and nitric acid ions generated by the reaction ads orb on the surface of TC/BOB, the photocatalytic NO removal ability of TC/BOB reduces slightly in cycle experiments.
Based on the experimental results, the mechanism of the photocatalytic degradation NO by 2D/2D TC/BOB heterojunction was proposed. As shown in Fig. 10, the electrons in BOB are excited by visible light, which jump from the valence band (VB)to the conduction band (CB). Since the CB energy level of BOB (?0.34 eV)is more negative than Fermi energy level of TC (?0.04 eV)and the energy difference between them is subtle, it is easy for photoelectrons to transfer from CB of BOB to TC surface, which significantly improves the separation rate of electron-hole pairs23,30. Subsequently, the photogenerated electrons gather on the surface of TC and react with the adsorbed NO. The electrons on TC also reacts with the surface-trapped O2to generatedradicals, which play a crucial role in photocatalytic degradation of NO. Furthermore,the huge surface area of TC/BOB guarantees abundant surface activity sites and improves the adsorption ability of NO.Therefore, the 2D/2D TC/BOB composites show excellent photocatalytic performance.

Fig. 10 Schematic illustration of the separation and transfer of photogenerated electron in the TC/BOB heterojunction.
4 Conclusions
In general, TC/BOB with 2D/2D heterostructure was synthesized by a simple hydrothermal method to improve the photocatalytic activity of BOB for NO removal in the range of visible light. With the increase of TC, the photocatalytic efficiency of the composite was lifted initially and then reduced,among which 15%TC/BOB showed the best catalytic performance. The results of transient photocurrent response, SPS and other experiments showed that the TC as a co-catalyst could promote both the transfer of photogenerated electron-hole pairs and the carrier separation rate. After conducting the capture experiments and ESR, it was found that functional groups such as e?, h+andplay an important role in photocatalytic reaction. It is expected that this promising 2D/2D composite material will have a great prospect in controlling the air pollution.
- 物理化學(xué)學(xué)報的其它文章
- Photocrosslinking-Immobilized Polymer Vesicles for Lowering Temperature Triggered Drug Release
- Poly(ε-caprolactone)-Polypeptide Copolymer Micelles Enhance the Antibacterial Activities of Antibiotics
- ReaxFF MD局部區(qū)域反應(yīng)追蹤與物理性質(zhì)可視化分析
- 稀土-天然皮革可穿戴X射線防護(hù)材料的合成及性能
- 石墨烯玻璃透明薄膜加熱特性
- Single-Molecule Field-Effect Transistors with Graphene Electrodes and Covalent Pyrazine Linkers

