Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats
Venkatashivam Shiva Kumar, Anuchandra Ramchandra Rajmane, Mohammad Adil, Amit Dattatraya Kandhare, Pinaki Ghosh, Subhash Laxman Bodhankar
Department of Pharmacology, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, Maharashtra 411038, India.
Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats
Venkatashivam Shiva Kumar, Anuchandra Ramchandra Rajmane, Mohammad Adil, Amit Dattatraya Kandhare, Pinaki Ghosh, Subhash Laxman Bodhankar
Department of Pharmacology, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, Maharashtra 411038, India.
The aim of this study was to evaluate the effect of naringin on experimentally induced inflammatory bowel disease in rats. Naringin (20, 40 and 80 mg/kg) was given orally for 7 days to Wistar rats before induction of colitis by intrarectal instillation of 2 mL of 4% (v/v) acetic acid solution. The degree of colonic mucosal damage was analyzed by examining mucosal damage, ulcer area, ulcer index and stool consistency. Intrarectal administration of 4% acetic acid resulted in significant modulation of serum alkaline phosphatase, lactate dehydrogenase, superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA) and myeloperoxidase (MPO) content along with colonic nitric oxide (NO), xanthine oxidase (XO) level and protein carbonyl content in the colonic tissue as well as in blood. Naringin (40 and 80 mg/kg) exerted a dose dependent (P < 0.05) ameliorative effect, as it significantly increased hematological parameter as well as colonic SOD and GSH. There was a significant (P < 0.05) and dose dependant inhibition of macroscopical score, ulcer area along with colonic MDA, MPO activity by the 7 days of pretreatment of naringin (40 and 80 mg/kg). Biochemical studies revealed a significant (P < 0.05) dose dependant inhibition in serum alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) levels by pretreatment of naringin. Increased levels of colonic NO, XO, protein carbonyl content and DNA damage were also significantly decreased by naringin pretreatment. The findings of the present investigation propose that naringin has an anti-inflammatory, anti-oxidant and anti-apoptotic potential effect at colorectal sites as it modulates the production and expression of oxidative mediators such as MDA, MPO, NO and XO, thus reducing DNA damage.
acetic acid, apoptosis, fluorimetric analysis of DNA unwinding, inflammatory bowel disease, myeloperoxidase, naringin, nitrite/nitrate, oxidative stress, porotein carbonyl content, vascular permeability, xanthine oxidase
INTRODUCTION
Inflammatory bowel disease (IBD) encompasses an array of conditions including chronic inflammation of the mucosal and sub-mucosal layers of the large intestine and rectum[1-7]. Intra rectal-administration of acetic acid produces a reproducible laboratory animal model for preclinical evaluation of potential drug can-didates for inflammatory bowel disease[8-11]. It mimics some characteristics of IBD, including transmural colon inflammation, granuloma formation, scarring and the fibrosis of intestinal tissue, fecal impaction, diarrhea and oxidative stress[12,13]. Increased oxidation and lipid peroxidation is a key feature of acetic acid induced colitis model[14]. Ulcerative colitis is also associated with aberrant mucosal antioxidant defense[15].
It has been demonstrated that reactive oxygen species (ROS) play an important role in the pathogenesis of IBD, with the up-regulation of ROS production resulting from the respiratory burst of infiltrating phagocytic cells[16]. The release of various inflammatory cytokines from macrophages including tumor necrosis factor-α, interleukin-1 and interleukin-8 lead to the production of oxidative mediators such as ROS[17], and activates the expression of oxidative stress-responsive genes[18].
The treatment of IBD includes drugs like 5-amino salicylic acid derivatives, broad spectrum antibiotics, steroids and immunosuppressant. However, these treatment strategies are associated with side ef fects such as nausea, anorexia cytopenia, myalgia and malfunctions of the kidney, liver and lungs[9,19]. Therefore, there is an urgent need to develop alternative therapeutic strategies[20]. Herbal drugs provide a ray of hope in the development of novel and therapeutically acceptable drugs. However, crude extract comprising an admixture of many chemical moieties have been used by researchers to treat experimentally induced inflammatory bowel disease in laboratory animals[9,10,17]. Such uncharacterized extracts and fractions of unknown composition draw a vague picture of the therapeutic profile of plant species.
The 4′,5,7-trihydroxy flavonone 7-rhamnoglucoside i.e. naringin (Fig. 1) is isolated from the grape as well as citrus fruit species and it has immense therapeutic value in the abrogation of a plethora of inflammatory maladies[21]. It has been reported that naringin is a potential metal-chelating as well as free radical scavenging agent[22]. Extensive research on naringin has shown that it has anticancer, anti-inflammatory, antioxidant, antiatherogenic, antiulcer, antidiabetic, cardioprotective and renoprotective properties[6,23-29]. It inhibits lipopolysaccharide (LPS) induced release of tumor necrosis factor-α in macrophages of mice[30]. The study of Kandhare et al. showed that naringin possesses neuroprotective effects in streptozotocin induced neuropathic pain via the modulation of various endogenous biomarkers[31].
The aim of this study was to evaluate the ameliorative effect of naringin in the healing of experimentally induced inflammatory bowel disease by assessing various behavioral, biochemical and microscopic parameter as well as DNA damage in rats.
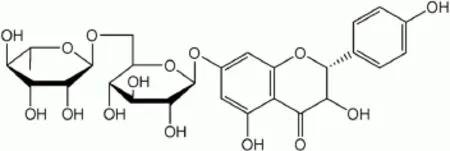
Fig. 1 Structure of flavonoid naringin.
MATERIAL AND METHODS
Animals
Male wistar rats (180-200 g) were obtained from the National Toxicological Centre, Pune, India. The animals were housed in environmentally controlled conditions (24±1°C, with a relative humidity of 45-55% and a 12 hour dark/light cycle). The animals were acclimatized for a period of two weeks before experiments. They had free access to standard laboratory chow (Pranav Agro Industries Ltd., Sangli, India) throughout the experiment with the exception of overnight fasting before induction of colitis. The research protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Poona College of Pharmacy, Pune, India.
Drugs and chemicals
Naringin was purchased from Sigma-Aldrich Co. LLC. (St Louis, MO, USA). Prednisolone was obtained as a gift from Symed Pharmaceutical Pvt. Ltd., (Hyderabad, India). Acetic acid, reduced glutathione, 5,5′-dithiobis (2-nitrobenzoic acid), bovine serum albumin, thiobarbituric acid, tris buffer, sucrose, Evans blue, trichloroacetic acid, citric acid monohydrate and Folin's phenol reagent were purchased from S.D. Fine Chemicals (Mumbai, India). The 2,4-dinitrophenylhydrazine, sulphanilamide, naphthalamine diamine HCl, phosphoric acid were obtained from LobaChemi Pvt. Ltd., (Mumbai, India). LDH and ALP kit were obtained from Accurex Biomedical Pvt. Ltd., (Mumbai, India) and Pathozyme Diagnostics (Kolhapur, India), respectively.
Induction of colitis
Colitis was induced in fasting rats as described by Mascolo et al. (1995)[32]. The animals (n = 6/group) were assigned to receive 1 mL distilled water for 12 days (group 1), 2 mL 4% acetic acid (once, intra-rectally) and 1 mL distilled water for 12 days (group 2), 20, 40, or 80 mg/kg naringin, p.o. for 12 days and 2mL 4% acetic acid intra-rectally on the 8thday (groups 3 to 5), or prednisolone (2 mg/kg, p.o.) and acetic acid (2 mL 4% solution, once, intra-rectally) for 5 days (group 6).
Food and water intake were recorded by placing the animals in metabolic cage (Techniplast, Varese, Italy). On the 12th day, blood was drawn via the retro-orbital plexus and the animals were then sacrificed by cervical dislocation. The colon and spleen from each animal were dissected and weighed. Colitis was evaluated grossly as described Morris et al. (1989)[12]. Stool consistency (0: normal; 2: loose; 4: diarrhea) was graded as previously reported[33]. The ulcer area and ulcer index were determined as described by Dengiz and Gursan[34]. The ulcer index was calculated by using the following formula:
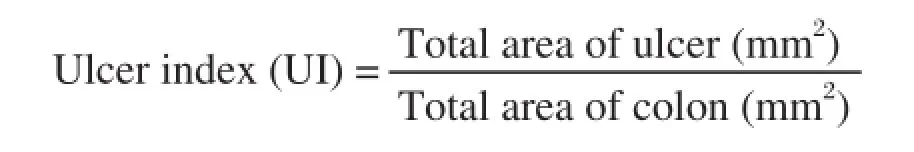
and the inhibition rate was calculated using the formula:
% Inhibition= [(UIcontrol-UItreated/UIcontrol)] × 100
Biochemical assays
Serum LDH and ALP contents were measured by spectrophotometry (JASCO-V-530, JASCO Corp., Tokyo, Japan) using commercially available reagent kits (LDH: Accurex Biomedical Pvt. Ltd., and ALP: Pathozyme Diagnostics, India). Additionally, 500 mg colon tissues were homogenized in chilled 10 mM Tris buffer (pH 7.4) and the homogenate was employed for biochemical assays. The SOD content was determined as previously described by Misra and Fridovich[35]and SOD activity was expressed as U/mg protein. Glutathione (GSH) assay was performed according to the method of Moron et al. (1979)[36]and the amount of reduced GSH was expressed as μg of GSH/mg protein. Myeloperoxidase (MPO) content was measured using the method of Krawisz et al. (1984)[37]and MPO activity was defined as the quantity of enzyme degrading 1 μmol of peroxide per minute at 25°C and was expressed in units per gram (U/g) of wet scrapings. Malondialdehyde (MDA) levels in the colon tissue were determined by the method of Slater and Sawyer[38]and were expressed as nanomoles of MDA/mg protein. Nitrite/nitrate (NO) levels were determined by the acidic Griess reaction by reducing nitrate to nitrite by vanadium trichloride according to Miranda et al. (2001)[39]and were expressed as μg/mg of wet tissue. Tissue xanthine oxidase (XO) activity was measured by spectrophotometry[40]at 293 nm and expressed as U/ mg protein. The extent of protein oxidation was determined by measuring protein carbonyl content. Briefly, the intestinal homogenate (1 mg protein) was incubated with dinitrophenylhydrazine for 1 hour followed by precipitation with TCA. Following centrifugation, the pellet was washed with ethanol-ethyl acetate (1:1) to remove excess dinitrophenylhydrazine, dissolved in 6M guanidine hydrochloride and absorbance was measured at 366 nm. The carbonyl content was calculated using a molar extinction coefficient value of 22,000/M·cm and expressed as nmol/mg protein[41].
Determination of colonic vascular permeability
The vascular permeability of the colon was measured by a modified method of Erickson et al. (1992)[42]. The amount of extravagated dye was calculated from the standard curve and expressed as microgram per gram wet weight of tissue.
Quantification of DNA damage
Fluorimetric analysis of DNA unwinding was performed as described by Birnboim, Percy and Chipman[43,44]. The percentage of double-stranded DNA (dsDNA) was determined by measuring the fluorescence of samples after the addition of ethidium bromide at 520 nm and 590 nm. The percentage of dsDNA remaining after the unwinding process was computed by the ratio (fluorescence of unwound DNA - fluorescence of denatured DNA)/(fluorescence of native DNA - fluorescence of denatured DNA).
Microscopic evaluation
Freshly excised colon of one animal from each group was washed with saline and preserved in 10% formaldehyde solution for histopathological studies. It was processed for 12 hours using isopropyl alcohol, xylene and paraffin embedded for light microscopic study (Nikon E200, Tokyo, Japan). Tissue sections (5 μm thickness) were prepared and stained after deparaffinization by using hematoxylin and eosin stain (H & E). Photomicrographs were captured at a magnification of 40 ×.
Statistical analysis
All the results were expressed as mean±S.E.M. Data analysis was performed by using GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA). Statistical comparisons were made between drugtreated groups and colitis control animals. Data of body weight, food intake and water intake were analyzed using two way ANOVA followed by Bonferroni's test. Data of biochemical parameters were analyzed using one way ANOVA followed by Tukey's multiple range test. Data of macroscopical score and stool consistency was analyzed using nonparametricKruskal-Wallis ANOVA. A value of P < 0.05 was considered to be statistically significant.
RESULTS
Naringin attenuated acetic acid-induced colitis
Mucosa necrosis, erosion, bleeding, ulceration and inflammation, which are key features of ulcerative colitis, were observed after intra-rectal 4% acetic acid (Fig. 2B). Naringin (80 mg/kg) suppressed acetic acidinduced ulceration, necrosis and mucosal bleeding (Fig. 2D). Intra-rectal acetic acid resulted in a significant decrease (P < 0.05) in body weight, food intake and water intake compared to normal rats (Fig. 3), which, however, was markedly attenuated by naringin (40 and 80 mg/kg) in a dose-dependent manner (P <0.05). As shown in Fig. 2, intrarectal administration of 4% acetic acid resulted in the formation of deep erosion along with necrosis in the colon. Rats treated with naringin significantly (P < 0.05) and dose dependently (40 and 80 mg/kg) decreased ulcer area as well as ulcer index as compared to acetic acid control rats and thus showed significant protection against ulcer formation. When compared with acetic acid control rats, the ulcer area as well as ulcer index in prednisolone (2 mg/kg) treated rat was also significantly decreased (P < 0.05) (Table 1).
Effects of naringin on wet weight of the colon

Fig. 2 Morphological representation of colons. (A) Normal, (B) acetic acid treated, (C) prednisolone (2 mg/kg) treated and (D) naringin (80 mg/kg) treated rats.
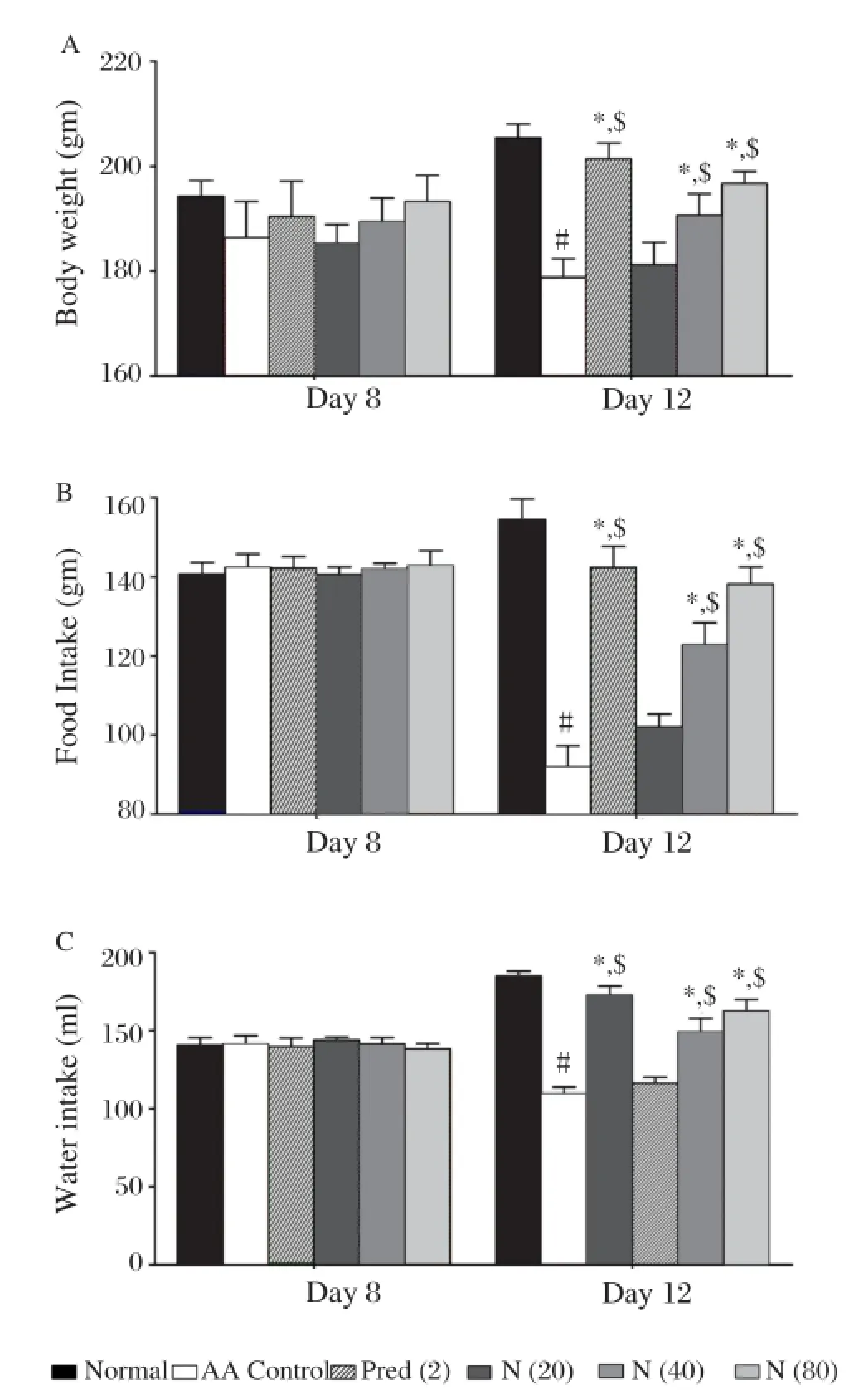
Fig. 3 Effect of naringin on (A) body weight, (B) water intake and (C) food intake in acetic acid induced colitis. Data are expressed as mean±S.E.M. (n = 6) and analyzed by two way ANOVA followed by Bonferroni's test.*P < 0.05 as compared to the acetic acid control group,#P < 0.05 as compared to normal group and$P < 0.05 as compared to one another. AA control: Acetic acid control rats; Pred (2): Prednisolone (2 mg/kg) treated rats; N (20): naringin (20 mg/kg, p.o.) treated rats; N (40): naringin (40 mg/kg, p.o.) treated rats; N (80): naringin (80 mg/kg, p.o.) treated rats.
Compared to normal rats, rats given intra-rectal 4% acetic acid had a significant increase in the weight of the colon (P < 0.05). Naringin dose-dependently ameliorated acetic acid-induced increase in colon weight (P < 0.05). Prednisolone (2 mg/kg) also caused a significant inhibition of acetic acid-induced increase in colon weight (P < 0.05) (Table 1). The colon weight to length ratio was significantly (P < 0.05) increased in the acetic acid control rats as compared to normal rats. Treatment with naringin showed significant (P <0.05) and dose dependent (40 and 80 mg/kg) reduction of weight to length ratio as compared to acetic acid control rats. Treatment with prednisolone (2 mg/ kg) also significantly (P < 0.05) decreased weight to length ratio as compared to acetic acid control rats. (Table 1)
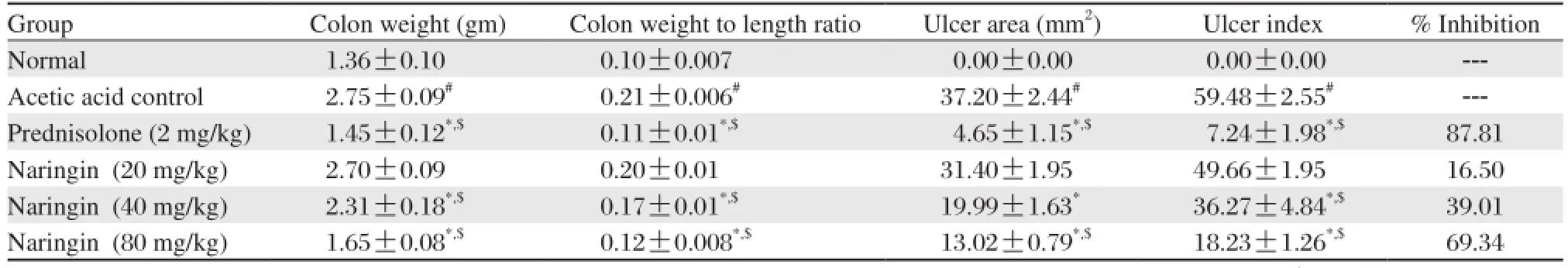
Table 1 Effects of naringin on colon weight, colon weight to length ratio, ulcer area and ulcer index of rats in acetic acid induced colitis
Effects of naringin on macroscopic scores
There was a significant (P < 0.05) increase in the macroscopic score of the colon in acetic acid control rats as compared to normal rats. Treatment with naringin (40 and 80 mg/kg) significantly and dose dependently decreased (P < 0.05) ulcer production and thereby decreased macroscopic score as compared to acetic acid control rats. Prednisolone (2 mg/kg) treatment for 5 days significantly attenuated (P < 0.05) ulcer formation according to macroscopic score as compared to acetic acid control rats. (Table 2)
Effects of naringin on spleen weight
Spleen weight in acetic acid treated rats was significantly increased (P < 0.05) as compared to the weight of spleen in normal rats. Pretreatment with naringin (40 and 80 mg/kg) for 7 days significantly and dose dependently (P < 0.05) decreased the increased weight of the spleen as compared to rats with induced colitis. Prednisolone (2 mg/kg) treatment for 5 days significantly decreased (P < 0.05) spleen weight as compared to acetic acid control rats. (Table 2)
Effects of naringin on stool consistency
As shown in the Table 2, when compared with normal rats, the stool consistency of acetic acid control rats was significantly increased (P < 0.05) after intrarectal administration of 4% acetic acid. Treatment with naringin (40 and 80 mg/kg) for 12 days significantly and dose dependently decreased (P < 0.05) the increased stool consistency level as compared to acetic acid control rats. Prednisolone (2 mg/kg) treated rats also showed a significant decrease (P < 0.05) in stool consistency as compared to acetic acid control rats.
Effects of naringin on hematology
As shown in Table 3, the levels of WBC, RBC, Hb and platelet were significantly decreased (P < 0.05) in acetic acid treated rats after intrarectal administration of 4% acetic acid as compared to normal rats. The intrarectal administration of 4% acetic acid did not produce any significant change in the levels of MCV, MCH and MCHC. Rats pretreated with naringin (40 and 80 mg/kg) for 7 days showed significant increase (P < 0.05) in the level of WBC, RBC, Hb and platelet as compared to acetic acid control rats. Treatment with prednisolone (2 mg/kg) for 5 days also showed significant increase (P < 0.05) in the level of WBC, RBC, Hb and platelet as compared to acetic acid control rats.
Effects of naringin on serum LDH and ALP levels
Acetic acid-induced colitis resulted in significant increase (P < 0.05) in serum LDH and ALP levels in acetic acid control rats as compared to normal rats.
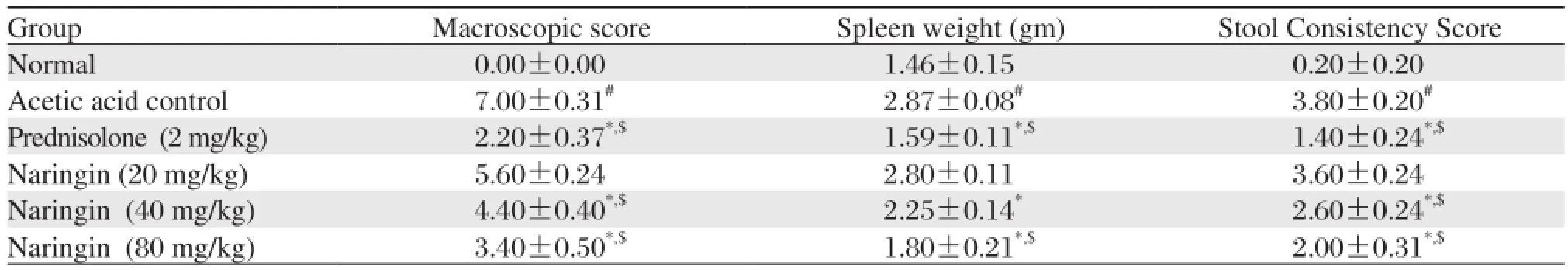
Table 2 Effects of naringin on macroscopic score, spleen weight and stool consistency score of rats in acetic acid induced colitis
Data are expressed as mean±S.E.M. (n = 6). Data of macroscopical score and stool consistency was analyzed using nonparametric Kruskal-Wallis ANOVA whereas data of spleen weight was analyzed by one way ANOVA followed by Tukey's multiple range test.*P < 0.05 as compared to the acetic acid control group,#P < 0.05 as compared to normal group and$P < 0.05 as compared to one another.Administration of naringin (40 and 80 mg/kg) for 12 days significantly and dose dependently decreased (P < 0.05) the increased level of serum LDH whereas naringin (80 mg/kg) significantly attenuated (P <0.05) the increased level of serum ALP as compared to acetic acid control rats. When compared with acetic acid control rats, treatment with naringin (20 and 40 mg/kg) failed to produce any significant reduction in the level of serum ALP. Prednisolone (2 mg/kg) also provided significant protection (P < 0.05) against the increase in the level of serum LDH and ALP as compared to acetic acid control rats. (Table 4)
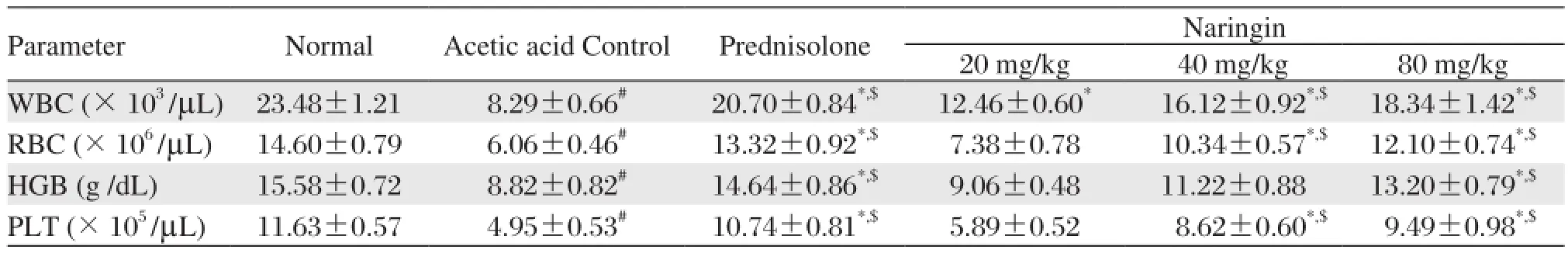
Table 3 Effects of naringin on hematological parameters of rats in acetic acid induced colitis
Effects of naringin on colonic vascular permeability
In acetic acid control rats, the level of Evans blue was significantly increased (P < 0.05) as compared to normal rats. Naringin (40 and 80 mg/kg) treated rats showed significant and dose dependant inhibition (P <0.05) of the increased concentration of Evans blue as compared to acetic acid control rats. Treatment with prednisolone (2 mg/kg) also significantly attenuated (P < 0.05) the increased levels of Evans blue as compared to acetic acid control rats. (Table 4)
Effects of naringin on colonic SOD and GSH levels
Fig. 4A and Fig. 4B demonstrate significantly decreased (P < 0.05) SOD and GSH concentration in colonic mucosa of acetic acid control rats as compared to normal rats. Treatment with naringin (40 and 80 mg/ kg) significantly and dose dependently inhibited (P <0.05) the decrease in the level of SOD and GSH as compared to acetic acid control rats. When compared with acetic acid control rats, treatment with prednisolone (2 mg/kg) for 5 days significantly increased (P < 0.05) the level of SOD and GSH.
Effects of naringin on colonic LPO levels
Lipid peroxidation level in the acetic acid control rats was significantly increased (P < 0.05) after intrarectal administration of 4% acetic acid as compared to normal rats. Naringin (40 and 80 mg/kg) treatment for 12 days significantly and dose dependently (P < 0.05) inhibited the increase in the levels of LPO as compared to acetic acid control rats. Prednisolone (2 mg/ kg) treated rats also showed significant reduction (P <0.05) in the level of LPO when compared with acetic acid control rats. (Fig. 4C)
Effects of naringin on colonic MPO and NO levels
We further assessed colonic MPO activity which is a marker of neutrophil infiltration. Intrarectal administration of 4% acetic acid resulted significant increase (P < 0.05) in the level of MPO and NO in acetic acid control rats as compared to normal rats. This increased levels of MPO as well as NO was significantly anddose dependently (P < 0.05) decreased by 7 days pretreatment of naringin (40 and 80 mg/kg) as compared to normal rats. When compared with acetic acid control rats, prednisolone (2 mg/kg) treated rats showed significant decrease (P < 0.05) in the level of MPO and NO. (Fig. 4D and 5A)
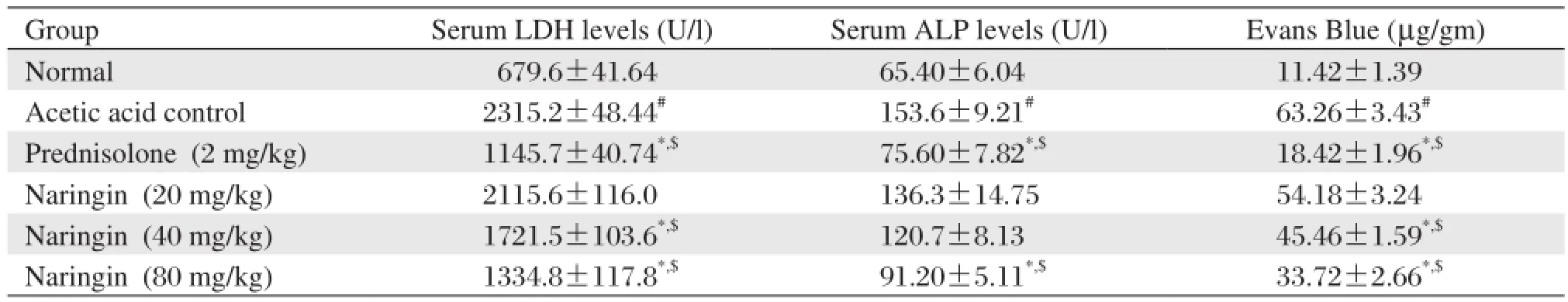
Table 4 Effects of naringin on serum LDH, serum ALP and colonic vascular permeability of rats in acetic acid induced colitis

Fig. 4 Effect of naringin on colonic (A) SOD, (B) GSH, (C) MDH and (D) MPO in acetic acid induced colitis. Data are expressed as mean±S.E.M. (n = 6) and analyzed by one way ANOVA followed by Tukey's multiple range test.*P < 0.05 as compared to the acetic acid control group,#P < 0.05 as compared to the normal group and$P < 0.05 as compared to one another. AA control: Acetic acid control rats; Pred (2): Prednisolone (2 mg/kg) treated rats; N (20): naringin (20 mg/kg, p.o.) treated rats; N (40): naringin (40 mg/kg, p.o.) treated rats; N (80): naringin (80 mg/ kg, p.o.) treated rats.
Effects of naringin on xanthine oxidase (XO) level
Fig. 5B demonstrates a significant increase (P < 0.05) in mucosal XO concentration in colonic mucosa of acetic acid control rats following intrarectal administration of acetic acid as compared to normal rats. Pretreatment with naringin (40 and 80 mg/kg) produced a significant and dose dependent (P < 0.05) reduction in XO activity as compared to the acetic acid group. Prednisolone (2 mg/kg) treated rats also showed a significant (P < 0.05) reduction in the increased levels of XO as compared to acetic acid control rats. The post treatment of prednisolone (2 mg/kg) showed similar effect in the reduction of the increased levels of XO as compared to naringin (80 mg/kg) post treatment.
Effects of naringin on protein carbonyl content
Effect of the intrarectal administration of 4% acetic acid on protein carbonyl content is shown in Fig. 5C. A significant increase (P < 0.05) in the level of protein carbonyl content was found in acetic acid control rats as compared to normal rats. Naringin (40 and 80 mg/kg) pretreatment for 7 days dose dependently and significantly decreased (P < 0.05) the increased levels of protein carbonyl content as compared to acetic acid control rats. When compared to acetic acid control rats, prednisolone (2 mg/kg) treated rats also showed significant inhibition (P < 0.05) in the increased protein carbonyl content.
Effects of naringin on DNA damage
As shown in Fig. 5D, there was a significant decrease (P < 0.05) in the number of unwinded double strand DNA in acetic acid control rats as compared to normal rats. Naringin (40 and 80 mg/kg) treated rats significantly and dose dependently increased (P <0.05) the number of unwinded double strand DNA ascompared to acetic acid control rats. Treatment with prednisolone (2 mg/kg) also significantly increased the number of unwinded double strand DNA as compared to acetic acid control rats (P < 0.05).
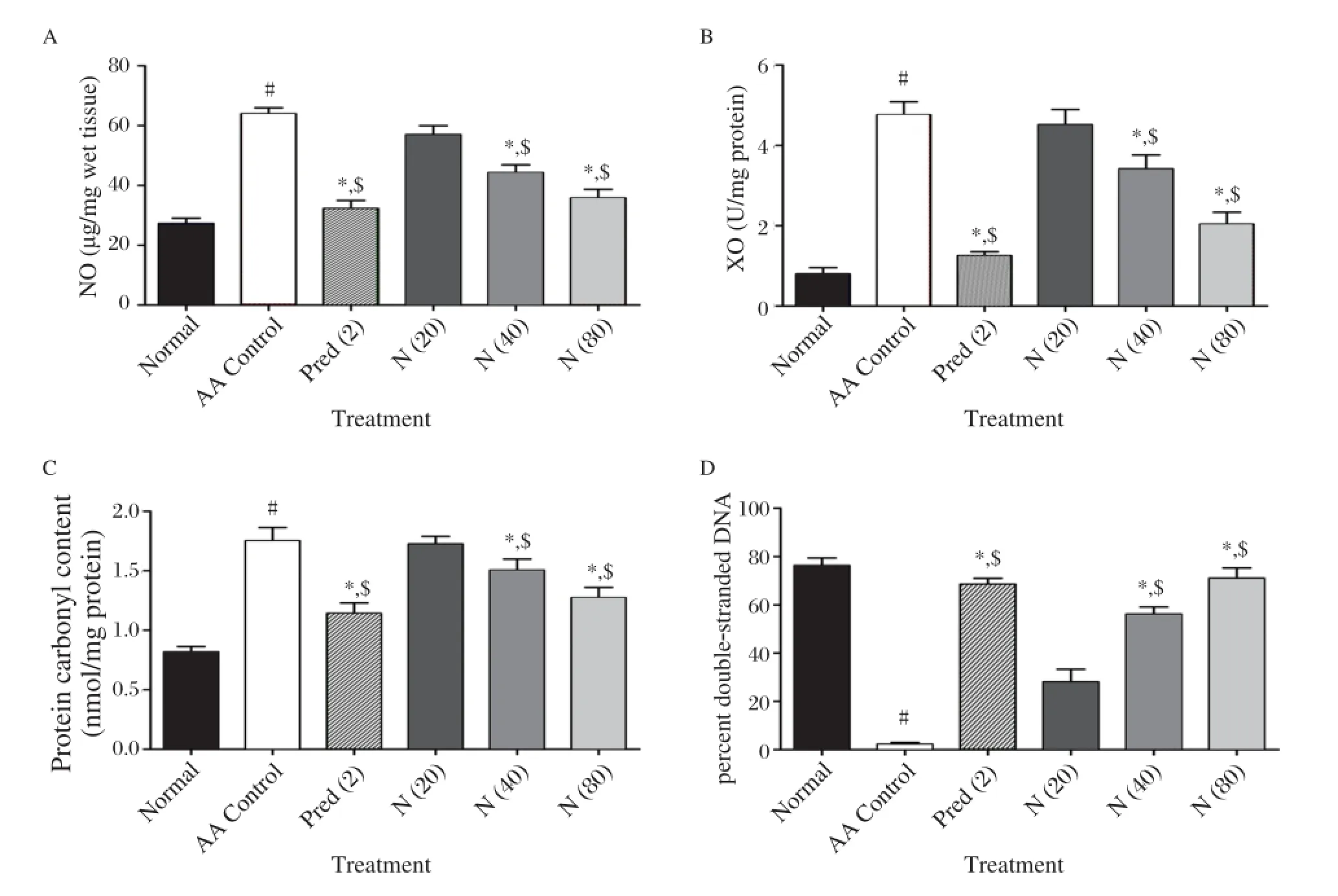
Fig. 5 Effect of naringin on colonic (A) NO, (B) XO, (C) PCC and (D) DNA damage in acetic acid induced colitis. Data are expressed as mean±S.E.M. (n = 6) and analyzed by one way ANOVA followed by Tukey's multiple range test.*P < 0.05 as compared to the acetic acid control group,#P < 0.05 as compared to the normal group and$P < 0.05 as compared to one another. AA control: Acetic acid control rats; Pred (2): Prednisolone (2 mg/kg) treated rats; N (20): naringin (20 mg/kg, p.o.) treated rats; N (40): naringin (40 mg/kg, p.o.) treated rats; N (80): naringin (80 mg/kg, p.o.) treated rats. NO, ; PCC, protein carbonyl content; XO, NO: Nitric Oxide; XO: Xanthine Oxidase.
Effects of naringin on histological alteration induced by acetic acid
In normal rats, the epithelial crypts of the mucosal layer were in tact and there was no infiltration of inflammatory cells (Fig. 6A). The histopathological features of untreated animals included transmural necrosis, oedema and diffuse inflammatory cell infiltration in the mucosa, desquamated areas and loss of the epithelium. An infiltrate consisted of mixed inflammatory cells was observed (Fig. 6B). Pretreatment of rats with naringin (80 mg/kg) (Fig. 6D) or treatment with prednisolone (2 mg/kg) (Fig. 6C) attenuated the extent and severity of the histological signs of cell damage that were associated with intrarectal instillation of acetic acid. There were no inflammatory cells in the lamina propria and the epithelium remained intact (Fig. 6C and 6D) (Table 5).
DISCUSSION
The underlying mechanisms involving acetic acid induced localized inflammation, desquamation and loss of mucosal integrity leading to epithelial injury is due to conversion of acetic acid to protonated form which diffused into epithelium and later dissociates to form protons[7]. This leads to the acidification of intracellular architecture and the induction of inflammatory bowel disease. Luminal acetic acid has been studied to instigate lipid peroxidase, cyclooxygenase pathways and thus precipitating inflammatory bowel disease[32].
Intrarectal administration of the acetic acid resulted in significantly decreased body weight, food intake and water intake. These findings are in congruence with the finding of Patil et al.(2012)[8]. In this study, colitis induced decrease in body weight was significantly reduced by the treatment of naringin. These observations may be attributed to cellular biosynthesis and metabolism restoration in animals treated with naringin.
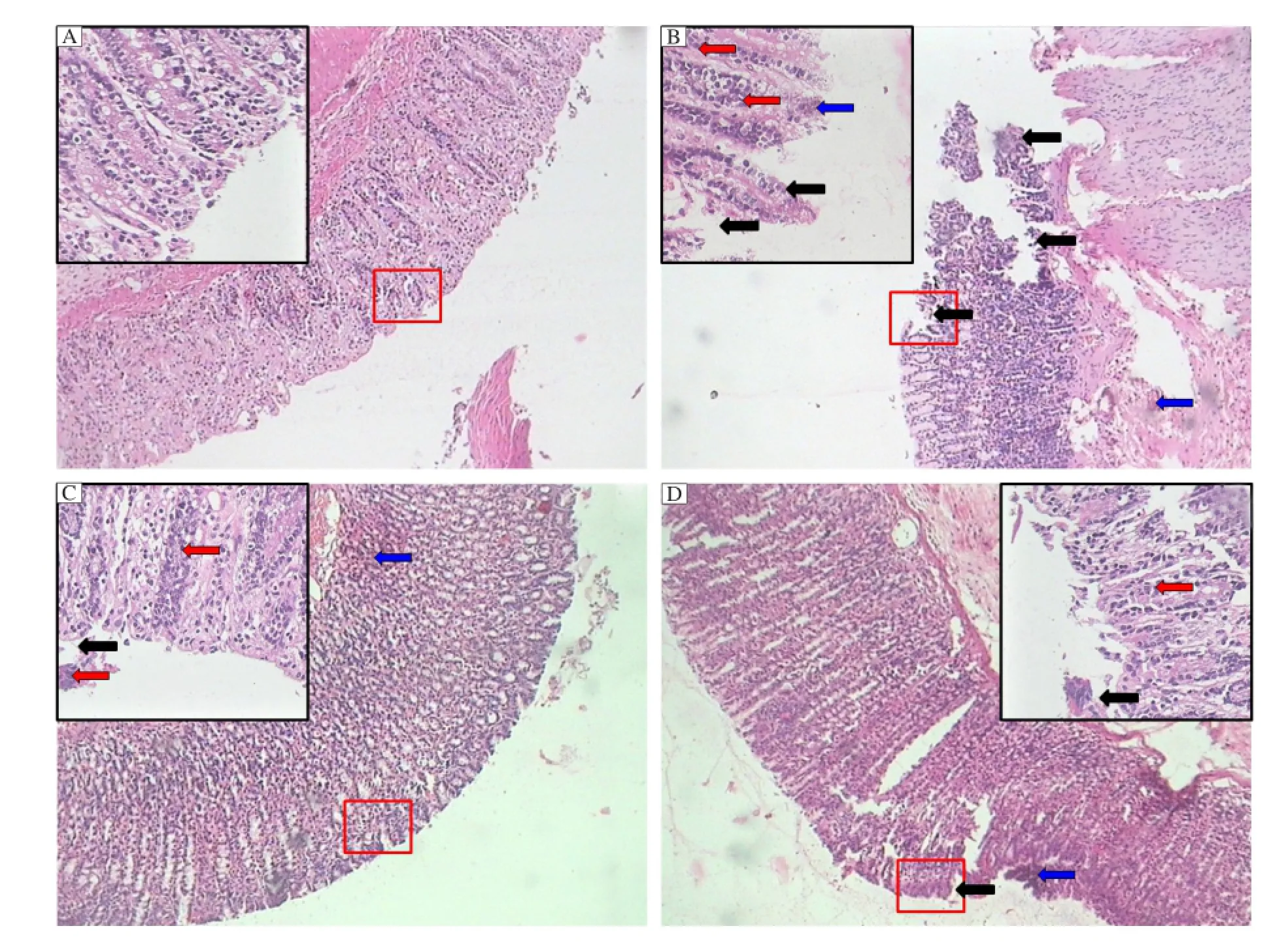
Fig. 6 Photomicrographs of sections of colons from rats stained with H & E. Colon microscopic image of (A) normal rat; (B) acetic acid induced colitis rat; (C) prednisolone (2 mg/kg, p.o.) treated rat; (D) naringin (80 mg/kg) 7 days pretreated rat. Necrosis (black arrow), oedema (blue arrow) and infiltration of inflammatory cells (red arrow). Images (100 × and respective inset 400 × magnification) are typical and representative of each study group.
Increased weight of colon along with colon weight/ length ratio is a hallmark of the degree of local inflammation, swelling, atrophy or hypertrophy as well as edema and wall thickening[33]. Increased weight of colon reflected the severity and extent of the disease which may be mediated by the release of inflammatory mediators[34]. Treatment with naringin reduced colonweight along with colon weight/length ratio depicting its anti-inflammatory potential as well as colitis healing property.
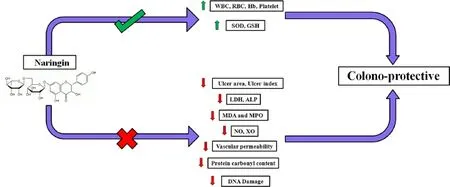
Fig. 7 Graphical representation: Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxidonitrosative balance and DNA damage.
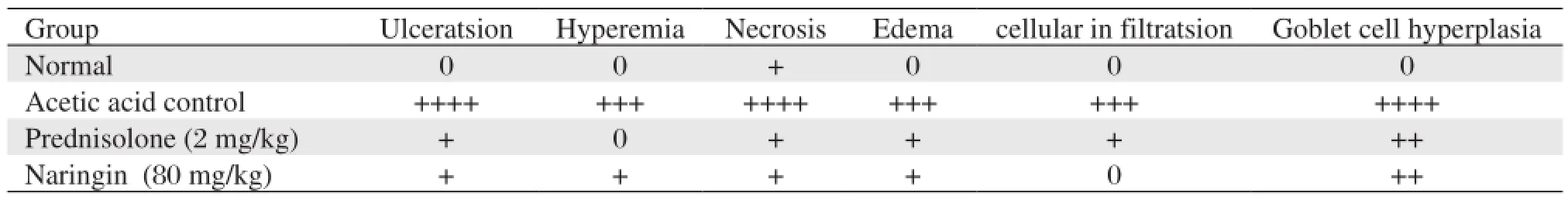
Table 5 Effects of naringin on pathological changes of rat colon in acetic acid induced inflammatory bowel disease
The procedure for quantitative determination of colonic lesion by morphometric evaluation was expressed as ulcer area and ulcer index[35]. The significant and dose dependant reduction of ulcer area and ulcer index by naringin reflected its antiulcer potential via the protection of colonic microflora from the corrosive effect of acetic acid.
Imbalance between hematological parameters including RBC, WBC, hemoglobin and platelet due to tissue damage is an important clinical manifestation of IBD. The assessment of hematological parameters can be used to determine the extent of disease state of colitis[13]. TNF-α, IL-1β, IL-6 and IL-8 like cytokines were released via the activation of white blood cells (including macrophages) via neutrophils[36]. This may have resulted in diarrhoea and malena. The above alterations in clinical features of IBD were reversed by naringin. These findings were in congruence with findings of the Choe et al.(2001)[22].
It has been well documented that IBD is associated with increased levels of RBC[7,8,37]. Cho et al. (2001) have reported that spleen destroys the increased amount of red blood cells which intern plays a vital role in the regulation of immune system[22]. Intrarectal administration of acetic acid may lead to atrophication of spleen. This increase in spleen weight was significantly reduced by the treatment with naringin depicting its immunomodulatory property.
Lactate dehydrogenase is an enzyme which is present in cell cytoplasm and plays a pivotal role in energy metabolism. Increase in the activities of LDH suggests cellular damage[38]. The level of LDH was significantly increased due to epithelial damage by intrarectal administration of acetic acid. Naringin treated rats showed significant decrease in the increased levels of LDH indicating its colonoprotective effect. This finding confirmed the results of Choe et al.[22].
It has been documented that phosphohydrolase enzyme like alkaline phosphatase is attached with glycosyl phosphatidyl inositol anchors to the cell wall, and its activity is considered to be an important hallmark for inflammation during the ulcerative colitis, as activity of this enzyme is elevated after intrarectal administration of acetic acid[39]. The elevated level of alkaline phosphatase enzyme was significantly attenuated by the treatment of naringin by virtue of its anti-inflammatory potential. This result confirmed the findings of Choe et al.[22].
The extent of mucosal damage is dependent upon the vascular permeability of the colonic mucosa. Increased vascular permeability is a rate limiting step in mucosal damage produced by acetic acid[40]. Hence decreased vascular permeability may play a vital role the mechanism of mucosal protection. Decreased spread of Evans blue by the treatment of naringin explored its ulcer protective potential.
Superoxide dismutase is an enzyme existing in mitochondria as well as cytosol which is responsible for the maintenance of redox balance in the tissue as well as vascular endothelial damage[41,42]. Electron transport chain is an important physiological component maintaining the mitochondrial energy metabolism. The increased level of reactive oxygen species caused dysregulation of cellular redox balance[43]. SOD reduces O2
-to H2O thus playing a pivotal role in scavenging of noxious free radicals[44]. In this study, SOD was found to be diminished in the acetic acid control group of animals reflecting uncontrolled oxidative stress. Naringin treatment demonstrated an increase on the levels of SOD thus restoring oxidative balance in the colonic mucosa.
GSH is an indispensible member of antioxidant free radical scavenger family, which converts H2O2to H2O[45]. GSH shields cells and tissues against free radical generation during stressful conditions arising after the administration of acetic acid[46]. GSH plays an important role in electrophile detoxification, transport of amino acid and synthesis of DNA[47]. Hence, lower levels of GSH show the progression of oxidative stress and the depletion of balance between pro and oxidative enzymes in the cells[48]. Treatment with naringin significantly increased the decreased level of GSH and halting the progression of oxidative stress.
Enhanced lipid peroxidation is a hallmark of free radical induced tissue damage. The β-oxidation oflipid peroxides in the cells lead to double bond rearrangement of unsaturated fatty acids of the membrane which cause destruction of lipid membrane culminating into tissue damage[49]. This activity is enhanced due to stress exerted by free radicals[7,8]. Naringin down regulated the levels of MDA corroborating its role in down regulation of lipid peroxidation which indirectly correspond to healing of ulcerative colitis.
Myloperoxidase represents the degree of neutrophil infiltration in a tissue[15,50]. Under normal physiological conditions, MPO is released from azurophillic storage granules[51]. When an inflammatory insult leads to the formation of reactive species, release of this enzyme increases MPO of colonic mucosa scraping which is a manifestation of increased infiltration[8,52]. Naringin was found to reduce the level of MPO in a dose dependent manner. The inhibition of neutrophil infiltration in colonic tissue by naringin is also evident in the histopathological observations of colonic tissue. The reversal of neutrophil infiltration by naringin established its anti-inflammatory activity.
Under stressful conditions, nitric oxide levels are increased in colonic mucosal cells, thus promoting the generation of reactive oxygen metabolites. It is caused due to increased iNOS production leading to exacerbation of nitrostative stress in the colonic mucosa[53]. Production of nitric oxide is invariably coupled with deficient level of SOD resulting in production of peroxynitrite[54,55]. These toxic NO metabolites lead to free radical formation and initiate the vicious cascade of stress induced lipid peroxidation[56], which then impair the colonic mucosa[40]. The increase in nitric oxide levels was substantially reduced in rats treated with naringin.
Xanthine oxidase (XO) is the primary biological source of SOD and is considered an important mediator in supplying active oxygen radical in free radical induced lesions. The level of XO increased in stressful conditions. Oxygen free radicals produced by the degradation of adenine nucleotides in which xanthine oxidase plays a pivotal role[57]. Acetic acid instillation increased the production of oxygen free radicals owing their origin to increased XO activity. In healthy colonic mucosa, dehydrogenase form of XO does not produce superoxide anion radicals. However, under stressful conditions, enzyme is converted into its oxidative form, which is the major source of oxygen radicals[58]. The results showed that increased XO was reduced by naringin, which is proportional to its antioxidant and anti-inflammatory properties.
The increased level of protein carbonyl content is a hallmark of oxidation induced damage. Under oxidative conditions, ROS mediated protein oxidation leads to high levels of protein carbonyl content (PCC) in the tissues[29]. This corresponds to uncontrolled protein oxidation and reduced repair activity. Increased oxidative stress leads to increased PCC content in colonic mucosa of acetic acid control animals[59]. Naringin was found to reduce PCC levels in the colonic mucosa, hence establishing restoration of cellular integrity and amelioration of colonic lesions.
DNA damage has been investigated to be an important underlying mechanism of IBD[14]. It has been reported that inflammatory mediators play vital role in oxidative DNA damage via the generation of ROS caused colon carcinogenesis[60]. It is accentuated due to necrotic and apoptotic transformations in the mucosal cells. DNA damage also leads to shedding of the rectal mucosa cells[61,62]. These DNA lesions can be quantified by fluorometric analysis of DNA unwinding, which detects unwound and native DNA fluorescence compared with native DNA fluorescence. It reports the % of ds-DNA remaining after the unwinding process. Increased oxidative stress was found to be associated with the reduced levels of ds-DNA, and depicted cellular damage[61]. Naringin was found to restore the content of DNA and maintained cellular integrity in the colonic epithelial cell.
The pharmacotherapy of inflammatory bowel disease has not advanced substantially and the approved therapies possess side effects. Recent treatment regiment for IBD includes various natural anti-oxidant therapies. The plant including Plantago ovate and Boswellia serrata[63,64]along with bioflavonoid moieties like bilberries, scutellaria and curcumin[65,66]possessing antioxidant potential have been proven clinically for the treatment of IBD.
In conclusion, the results demonstrate healing activity of naringin on inflamed tissue in acetic acid induced colitis, an effect that is associated with amelioration in the production of some of the mediators involved in the oxido-inflammatory response of the intestine, such as MDA, MPO, NO and XO, thus reducing DNA damage via anti-inflammatory, antioxidant and anti-apoptotic potential.
Acknowledgements
The authors would like acknowledge Dr. S. S. Kadam, Vice-Chancellor and Dr. K. R. Mahadik, Principal, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, India, for providing necessary facilities to carry out the study. We are also thankful to the All India Council of Technical Education (AICTE), India for financial support for the research work.
[1] Ghosh P, Kandhare AD, Raygude KS, Gauba D, Gosavi TP, Bodhankar SL. Cigarette smoking and H. pylori infection: A meta-analysis of literature. Der Pharmacia Lettre 2012; 4: 128-134.
[2] Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am 1999; 28: 255-81, vii.
[3] Raygude KS, Kandhare AD, Ghosh P, Gosavi TP, Bodhankar SL. Consumption of Alcohol and H. pylori infection: A Cumulative Meta-Analysis of Literature. Asian J Biochem Pharm Res 2011; 3: 338-345.
[4] Ghosh P, Kandhare AD, Gauba D, Raygude KS, Bodhankar SL. Determination of efficacy, adverse drug reactions and cost effectiveness of three triple drug regimens for the treatment of H. pylori infected acid peptic disease patients. Asian Pac J Trop Dies 2012: 2(2): S783-S789.
[5] Singh D, Chander V, Chopra K. Protective effect of naringin, a bioflavonoid on glycerol-induced acute renal failure in rat kidney. Toxicology 2004; 201: 143-51.
[6] Kandhare AD, Raygude KS, Ghosh P, Gosavi TP, Bodhankar SL. Patentability of Animal Models: India and the Globe. Int J Pharm Biol Arc 2011; 2: 1024-1032.
[7] Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Gosavi TP, Badole SL, et al. Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. leaves in experimental colitis in rats. Asian Pac J Trop Biomed 2012; 5: 337-344.
[8] Patil MVK, Kandhare AD, Bhise SD. Anti-inflammatory effect of Daucus carota root on experimental colitis in rats. Int J Pharm Pharm Sci 2012; 4: 337-343.
[9] Kandhare AD, Ghosh P, Ghule AE, Zambare GN, Bodhankar SL. Protective effect of<i> Phyllanthus amarus</ i> by modulation of endogenous biomarkers and DNA damage in acetic acid induced ulcerative colitis: Role of phyllanthin and hypophyllanthin. Apollo Medicine 2013; 10(1): 87-97.
[10] Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989; 96: 795-803.
[11] Qin HY, Wu JC, Tong XD, Sung JJ, Xu HX, Bian ZX. Systematic review of animal models of post-infectious/ post-inflammatory irritable bowel syndrome. J Gastroenterol 2011; 46: 164-74.
[12] Millar AD, Rampton DS, Chander CL, Claxson AW, Blades S, Coumbe A, et al. Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut 1996; 39: 407-15.
[13] Jagtap AG, Niphadkar PV, Phadke AS. Protective effect of aqueous extract of Bombax malabaricum DC on experimental models of inflammatory bowel disease in rats and mice. Indian J Exp Biol 2011; 49: 343-51.
[14] D'Odorico A, Bortolan S, Cardin R, D'Inca R, Martines D, Ferronato A, et al. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol 2001; 36: 1289-94.
[15] Patil MVK, Kandhare AD, Bhise SD. Pharmacological evaluation of ameliorative effect of aqueous extract of Cucumis sativus L. fruit formulation on wound healing in Wistar rats. Chron Young Sci 2011; 2: 207-13.
[16] Larrick JW, Wright SC. Cytotoxic mechanism of tumor necrosis factor-alpha. FASEB J 1990; 4: 3215-23.
[17] Joshi R, Kumar S, Unnikrishnan M, Mukherjee T. Free radical scavenging reactions of sulfasalazine, 5-aminosalicylic acid and sulfapyridine: mechanistic aspects and antioxidant activity. Free Radic Res 2005; 39: 1163-72.
[18] Gosavi TP, Ghosh P, Kandhare AD, Bodhankar SL. Unwrapping Homeopathic Principles in the Wake of Research: Serendipity, Placebo or True Therapeutic Milestones. Pharmacologyonline 2011; 1: 894-906.
[19] Jagetia GC, Reddy TK. The grapefruit flavanone naringin protects against the radiation-induced genomic instability in the mice bone marrow: a micronucleus study. Mutat Res 2002; 519: 37-48.
[20] Chen YT, Zheng RL, Jia ZJ, Ju Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic Biol Med 1990; 9: 19-21.
[21] Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem 2008; 56: 6185-205.
[22] Choe SC, Kim HS, Jeong TS, Bok SH, Park YB. Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. J Cardiovasc Pharmacol 2001; 38: 947-55.
[23] Martin MJ, Marhuenda E, Perez-Guerrero C, Franco JM. Antiulcer effect of naringin on gastric lesions induced by ethanol in rats. Pharmacology 1994; 49: 144-50.
[24] Gonzalez-Gallego J, Sanchez-Campos S, Tunon MJ. Anti-inflammatory properties of dietary flavonoids. Nutr Hosp 2007; 22: 287-93.
[25] Rajadurai M, Prince PS. Preventive effect of naringin on isoproterenol-induced cardiotoxicity in Wistar rats: an in vivo and in vitro study. Toxicology 2007; 232: 216-25.
[26] Kawaguchi K, Kikuchi S, Hasegawa H, Maruyama H, Morita H, Kumazawa Y. Suppression of lipopolysaccharide-induced tumor necrosis factor-release and liver injury in mice by naringin. Eur J Pharmacol 1999; 368: 245-50.
[27] Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia 2012; 83: 650-9.
[28] Prajda N, Weber G. Malignant transformation-linked imbalance: decreased xanthine oxidase activity in hepatomas. FEBS Lett 1975; 59: 245-9.
[29] Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 1990;186: 464-78.
[30] Erickson RA, Chang K, Lifrak E, Rivera N, Stachura J. 16,16-Dimethyl prostaglandin E2 reduces bile acid-mediated intestinal vascular injury in rats. Gastroenterology 1992; 102: 1295-305.
[31] Percy AJ, Chipman JK. The measurement of DNA strand breaks in rat colonic mucosa by fluorometric analysis of DNA unwinding. Toxicol Lett 1991; 56: 69-77.
[32] Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology 1995; 109: 1344-67.
[33] Thippeswamy BS, Mahendran S, Biradar MI, Raj P, Srivastava K, Badami S, et al. Protective effect of embelin against acetic acid induced ulcerative colitis in rats. Eur J Pharmacol 2011; 654: 100-5.
[34] Rachmilewitz D, Simon PL, Schwartz LW, Griswold DE, Fondacaro JD, Wasserman MA. Inflammatory mediators of experimental colitis in rats. Gastroenterology 1989; 97: 326-37.
[35] Yuan H, Ji WS, Wu KX, Jiao JX, Sun LH, Feng YT. Anti-inflammatory effect of Diammonium Glycyrrhizinate in a rat model of ulcerative colitis. World J Gastroenterol 2006; 12: 4578-81.
[36] Paunovic B, Deng X, Khomenko T, Ahluwalia A, Tolstanova G, Tarnawski A, et al. Molecular mechanisms of basic fibroblast growth factor effect on healing of ulcerative colitis in rats. J Pharmacol Exp Ther 2011; 339: 430-7.
[37] Patil MVK, Kandhare AD, Bhise SD. Effect of aqueous extract of Cucumis sativus Linn. fruit in ulcerative colitis in laboratory animals. Asian Pacific Journal of Tropical Biomedicine 2012; 2: S962-S969.
[38] Schreiber S, Hamling J, Zehnter E, Howaldt S, Daerr W, Raedler A, et al. Renal tubular dysfunction in patients with inflammatory bowel disease treated with aminosalicylate. Gut 1997; 40: 761-6.
[39] Venkataranganna MV, Rafiq M, Gopumadhavan S, Peer G, Babu UV, Mitra SK. NCB-02 (standardized Curcumin preparation) protects dinitrochlorobenzene- induced colitis through down-regulation of NFkappa-B and iNOS. World J Gastroenterol 2007; 13: 1103-7.
[40] Boughton-Smith NK, Evans SM, Hawkey CJ, Cole AT, Balsitis M, Whittle BJ, et al. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet 1993; 342: 338-40.
[41] Kandhare AD, Raygude KS, Ghosh P, Bodhankar SL. The ameliorative effect of fisetin, a bioflavonoid, on ethanol-induced and pylorus ligation-induced gastric ulcer in rats. Int J Green Pharm 2011; 5: 236-43.
[42] Abreu MT. The pathogenesis of inflammatory bowel disease: translational implications for clinicians. Curr Gastroenterol Rep 2002; 4: 481-9.
[43] Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci Lett 2012; 511: 18-22.
[44] Kruidenier L, Kuiper I, Van Duijn W, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, et al. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol 2003; 201: 17-27.
[45] Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med 1999; 27: 916-21.
[46] Sanchez de Medina F, Galvez J, Romero JA, Zarzuelo A. Effect of quercitrin on acute and chronic experimental colitis in the rat. J Pharmacol Exp Ther 1996; 278: 771-9.
[47] Raygude KS, Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacol 2012; 20:331-341.
[48] Hagar HH, El-Medany A, El-Eter E, Arafa M. Ameliorative effect of pyrrolidinedithiocarbamate on acetic acidinduced colitis in rats. Eur J Pharmacol 2007; 554: 69-77.
[49] Cheesman KH. Lipid peroxidation in biological systems. In: Halliwell B, Aruoma OI (Eds.), DNA and Free Radicals, Ellis Horwood, London 1993: 12-17.
[50] Patil MVK, Kandhare AD, Bhise SD. Anti-arthritic and anti-inflammatory activity of Xanthium srtumarium L. ethanolic extract in Freund's complete adjuvant induced arthritis. Biomedicine & Aging Pathology 2012; 2: 6-15.
[51] Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984; 87: 1344-50.
[52] Tahan G, Aytac E, Aytekin H, Gunduz F, Dogusoy G, Aydin S, et al. Vitamin E has a dual effect of antiinflammatory and antioxidant activities in acetic acidinduced ulcerative colitis in rats. Can J Surg 2011; 54: 333-8.
[53] Grisham MB, Pavlick KP, Laroux FS, Hoffman J, Bharwani S, Wolf RE. Nitric oxide and chronic gut inflammation: controversies in inflammatory bowel disease. J Investig Med 2002; 50: 272-83.
[54] Raygude KS, Kandhare AD, Ghosh P, Bodhankar SL. Anticonvulsant effect of fisetin by modulation of endogenous biomarkers. Biomedicine & Preventive Nutrition 2012; 2: 215-222.
[55] Gosavi TP, Kandhare AD, Ghosh P, Bodhankar SL. Anticonvulsant activity of Argentum metallicum, a homeopathic preparation. Der Pharmacia Lettre 2012; 4: 626-637.
[56] Patil M, Kandhare A, Ghosh P, Bhise S. Determination of role of GABA and nitric oxide in anticonvulsant activity of Fragaria vesca L. ethanolic extract in chemically induced epilepsy in laboratory animals. Oriental Pharmacy and Experimental Medicine 2012: 12(4):1-10.
[57] Fadillioglu E, Yilmaz HR, Erdogan H, Sogut S. The activities of tissue xanthine oxidase and adenosine deaminase and the levels of hydroxyproline and nitric oxide in rat hearts subjected to doxorubicin: protective effect of erdosteine. Toxicology 2003; 191: 153-8.
[58] Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis:clinical considerations and pathogenetic concepts. Pediatr Dev Pathol 2003; 6: 6-23.
[59] Bhor VM, Raghuram N, Sivakami S. Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int J Biochem Cell Biol 2004; 36: 89-97.
[60] Bancroft LK, Lupton JR, Davidson LA, Taddeo SS, Murphy ME, Carroll RJ, et al. Dietary fish oil reduces oxidative DNA damage in rat colonocytes. Free Radic Biol Med 2003; 35: 149-59.
[61] Boirivant M, Marini M, Di F, G., Pronio AM, Montesani C, Tersigni R, et al. Lamina propria T cells in Crohn's disease and other gastrointestinal inflammation show defective CD2 pathway-induced apoptosis. Gastroenterology 1999; 116: 557-65.
[62] Gosavi TP, Ghosh P, Kandhare AD, Shiva KV, Rajmane AR, Mohammad A, et al. Therapeutic effect of H. pylori nosode in healing of chronic H. pylori infected ulcers in laboratory animals. Asian Pac J Trop Dis 2012: D152
[63] Holtmeier W, Zeuzem S, Preiss J, Kruis W, Bohm S, Maaser C, et al. Randomized, placebo-controlled, double-blind trial of Boswellia serrata in maintaining remission of Crohn's disease: good safety profile but lack of efficacy. Inflamm Bowel Dis 2011; 17: 573-82.
[64] Madisch A, Miehlke S, Eichele O, Mrwa J, Bethke B, Kuhlisch E, et al. Boswellia serrata extract for the treatment of collagenous colitis. A double-blind, randomized, placebo-controlled, multicenter trial. Int J Colorectal Dis 2007; 22: 1445-51.
[65] Hoensch HP, Oertel R. Emerging role of bioflavonoids in gastroenterology: Especially their effects on intestinal neoplasia. World J Gastrointest Oncol 2011; 3: 71-4.
[66] Guilliams TG. Inflammatory Bowel Disease & Irritable Bowel Syndrome: Understanding, Distinguishing and Addressing. Edited by Thomas G. Guilliams 2010; 10: 9-11.
Received 14 August 2012, Revised 15 October 2012, Accepted 27 February 2013, Epub 15 August 2013
The authors reported no conflict of interests.
10.7555/JBR.27.20120082
 THE JOURNAL OF BIOMEDICAL RESEARCH2014年2期
THE JOURNAL OF BIOMEDICAL RESEARCH2014年2期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Rapid progression of nonculprit coronary lesions six weeks after successful primary PCI in culprit artery:a case report
- IRE1α is essential for Xenopus pancreas development
- Application of the back-error propagation artificial neural network (BPANN) on genetic variants in the PPAR-γ and RXR-α gene and risk of metabolic syndrome in a Chinese Han population
- Clopidogrel improves aspirin response after off-pump coronary artery bypass surgery
- Diagnostic and prognostic value of minor elevated cardiac troponin levels for percutaneous coronary intervention-related myocardial injury:a prospective,single-center and double-blind study
- A clinical perspective on mucoadhesive buccal drug delivery systems
