Expression of human hepatic lipase negatively impacts apolipoprotein A-I production in primary hepatocytes from Lipc-null mice
Michelle Bamji-Mirza,Wandong Zhang,Zemin Yao
aDepartment of Biochemistry,Microbiology&Immunology,andbDepartment of Pathology and Laboratory Medicine, University of Ottawa,Ottawa,Ontario,K1H 8M5,Canada;
cHuman Health Therapeutics Portfolio,Life Sciences Division,National Research Council Canada,Ottawa,Ontario, K1A 0R6,Canada;
dOttawa Institute of Systems Biology,University of Ottawa,Ottawa,Ontario,K1H 8M5,Canada.
Expression of human hepatic lipase negatively impacts apolipoprotein A-I production in primary hepatocytes from Lipc-null mice
Michelle Bamji-Mirzaa,b,c,Wandong Zhangb,c,Zemin Yaoa,d,?
aDepartment of Biochemistry,Microbiology&Immunology,andbDepartment of Pathology and Laboratory Medicine, University of Ottawa,Ottawa,Ontario,K1H 8M5,Canada;
cHuman Health Therapeutics Portfolio,Life Sciences Division,National Research Council Canada,Ottawa,Ontario, K1A 0R6,Canada;
dOttawa Institute of Systems Biology,University of Ottawa,Ottawa,Ontario,K1H 8M5,Canada.
This study aimed to examine whether expression of human hepatic lipase(hHL)exerted an intracellular effect on hepatic production of apolipoprotein(apo)A-I.The levels of secreted and cell-associated apoA-I were contrasted between primary hepatocytes isolated from Lipc-null and C57BL/6 mice,and between Lipc-null hepatocytes transfected with either hHL-encoding or control adenovirus.An HSPG-binding deficient hHL protein(hHLmt)was used to determine the impact of cell surface binding on HL action.Accumulation of apoA-I in conditioned media of primary hepatocytes isolated from Lipc-null mice was increased as compared to that from C57BL/6 mice. Metabolic labeling experiments showed that secretion of35S-apoA-I from Lipc-null cells was significantly higher than that from C57BL/6 cells.Expression of hHL in Lipc-null hepatocytes,through adenovirus-mediated gene transfer,resulted in decreased synthesis and secretion of35S-apoA-I,but not35S-apoE,as compared with cells transfected with control adenovirus.Expression of HSPG-binding deficient hHLmt in Lipc-null cells also exerted an inhibitory effect on apoA-I production,even though hHLmt displayed impaired exit from the endoplasmic reticulum as compared with hHL.Subcellular fractionation revealed that expression of hHL or hHLmt led to increased microsome-association of apoA-I relative to non-transfected control.Expression of hHL negatively impacts hepatic production of apoA-I.
high density lipoprotein,heparan sulphate proteoglycans,ABCA1,endoplasmic reticulum,adenovirus
INTRODUCTION
A strong inverse correlation exists between the level of plasma high density lipoprotein(HDL)-associated cholesterol and the incidence of coronary heart disease[1].Genome-wide association studies have shown that the HL gene(LIPC)is a genetic determinant associated with plasma HDL-cholesterol concentrations[2-4]. A dual function has been ascribed to hHL-it acts as a triacylglycerol(TAG)hydrolase and phospholipase, and also acts as a ligand(independent of its catalytic activity)for cell surface anchorage/uptake of various lipoproteins[5].Deficiency of HL in humans is characterized by an elevation in plasma concentrations of cholesterol and TAG as well as large,buoyant HDL particles[6-8].The Lipc-null mice exhibited elevatedplasma cholesterol(including HDL-cholesterol),phospholipid,and apoA-I(the major apolipoprotein of HDL)[9,10].On the other hand,infection of Lipc-null mice with hHL-encoding adenovirus resulted in decreased cholesterol,TAG,phospholipids,HDL-cholesterol and apoA-I[11,12].Mature hHL(476 amino acids)secreted from hepatocytes is mainly associated with HSPG on the cell surface[13]whereas secreted mouse HL is mostly blood borne[13,14].Structure-function analyses with HL and lipoprotein lipase(LPL) chimeric proteins suggested that the carboxyl-terminal domain of HL was important for HSPG-binding[15],and more recent systematic analysis of hHL showed that HSPG-binding domains span amino acids 301 through 320 and the carboxyl-terminal amino acids 465 through 476[16].A chimeric hHL protein(designated hHLmt),in which the carboxyl terminus of hHL (amino acid residues 406 through 476)was replaced with the corresponding mouse sequence,exhibited reduced HSPG binding activity and yet retained catalytic activity[13].Expression of the HSPG-binding deficient hHLmt in C57BL/6 mice resulted in decreased apoA-I,HDL-cholesterol,and HDL-phospholipid in pre-heparin plasma relative to hHL-expressing mice[17].
While most studies have described the extracellular roles of HL in the catabolism of plasma lipoproteins, some data have suggested that HL may also play an intracellular role.Lipolytic activity of HL has been detected within the ER/Golgi secretory pathway in transfected Chinese hamster ovary cells(CHO)and rat primary hepatocytes[18,19].We documented that expression of hHLmt resulted in decreased levels of secreted apoA-I from mouse primary hepatocytes[17], and that expression of hHL in McA-RH7777 cells, either in the catalytically-active or-inactive form(in which Ser-145 at the catalytic site of the enzyme was substituted with Gly),impaired the secretion of TAG-rich very low density lipoproteins(VLDL)[20]. Recently,Erickson et al.[21]also showed that endoplasmic reticulum-localized hepatic lipase in McARH7777 cells decreased TAG storage and VLDL secretion.In McA-RH7777 cells,TAG utilized for VLDL maturation is present in the microsomal lumen in the form of lipid droplets[22].Expression of hHL, independent of its catalytic activity,diminished the newly synthesized TAG associated with the lumenal lipid droplets[20].Thus,expression of the hHL protein within the microsomal lumen appears to negatively affect formation of the lumenal TAG pool utilized for VLDL assembly/secretion.Whether or not hHL expression in hepatic cells also exerts an effect on HDL formation or secretion has not been examined.
Recent in vivo and cell culture studies have shown that the liver is the major organ for murine HDL production,and that the apoA-I/HDL production is dependent on the activity of the transmembrane protein ATP-binding cassette transporter A1(ABCA1)[23]. Studies with cultured mouse primary hepatocytes have shown that most secreted apoA-I was in association with phospholipid and cholesterol,suggesting intracellular assembly of nascent HDL(or minimally lipidated apoA-I)[24].However,from human hepatoblastoma HepG2 cells,apoA-I was secreted in the form of lipid-poor protein in addition to HDL particles[24-26]. The cellular location where the ABCA1-dependent apoA-I lipidation occurs remains unclear.In hepatic cells,lipidation of apoA-I may take place within the ER/Golgi secretory pathway[24].In non-hepatic cells,the ABCA1-dependent apoA-I lipidation may occur on the plasma membrane[27]as well as at the endosome[28].
In the present study,we tested the hypothesis that expression of hHL in hepatic cells may attenuate the production and secretion of apoA-I.Because apoA-I lipidation is a membrane-associated event,we also determined the potential role of hHL-membrane association(through HSPG binding)by comparing the action of hHL with the HSPG-binding deficient variant hHLmt.Data obtained from the present transfection studies suggested that transient expression of either hHL or hHLmt negatively impacted apoA-I production from Lipc-null primary hepatocytes.
MATERIALS AND METHODS
Materials
Dulbecco′s modified Eagle′s medium(DMEM), Met/Cys-free DMEM,Williams Medium E, Hepatozyme medium,fetal bovine serum(FBS),and antibiotic/antimycotic were purchased from Invitrogen Canada(Burlington,ON).Heparin,fibronectin,and fumed silica were purchased from Sigma-Aldrich(Oakville,ON).The Primaria dishes were from BD Biosciences(Mississauga,ON),and the[35S]Met/ Cys was obtained from MP Biochemicals(Solon,OH). Protease inhibitor cocktail and chemiluminescent substrates were purchased from Roche Diagnostics(Laval, PQ).The antibody to detect hHL(by immunoblot analysis)was obtained from Santa Cruz Biotechnology, Inc.(Santa Cruz,CA,USA),the antibodies to detect mouse apoE and apoA-I(for immunoblot and immunoprecipitation)were purchased from BioDesign International(Saco,ME,USA),and the antibodies to detect mouse calnexin and giantin were obtained from Stressgen Bioreagents(Ann Arbor,MI,USA)andAbcam(Cambridge,MA,USA),respectively.The antihHL antibody used for immunoprecipitation of HL was a kind gift from Dr.Ann White(University of Texas Southwestern Medical Center).
Mice
C57BL/6(obtained from Charles River Laboratories (Wilmington,MA,USA)and C57BL/6 Lipc-null (obtained from Jackson Laboratory Bar Harbor,ME, USA)mice were maintained on a normal chow diet and a 12-hour light/12-hour dark cycle.Female C57BL/6 or Lipc-null mice(aged 8-14 weeks)were used for hepatocyte isolation.All experiments were performed in accordance with the guidelines and ethical standards laid down by the Canadian Council of Animal Care regarding the care and use of animals for experimental procedures.All animal protocols were approved by the University of Ottawa Animal Care Committee.
Primary hepatocyte isolation
Primary hepatocytes were isolated from mice by collagenase liver perfusion as previously described[29,30], and plated at a density of 1×106cells/well on fibronectin-coated 6-well Primaria dishes in Williams medium E containing 10%FBS and 100 U/mL antibiotic/antimycotic(Williams complete media).The viability of the cell preparation was at least 85%(as determined by trypan blue exclusion prior to plating cells).The primary cells were harvested or infected with adenovirus 4 hours after plating(refer to section below).
Cell culture
The HepG2 cell line was obtained from the American Type Culture Collection and maintained in DMEM supplemented with 10%FBS.
Adenovirus generation and cell transfection
The adenoviruses encoding hHL,hHLmt and enhanced green fluorescent protein(EGFP)were generated at the Viral Vector Core Facility,Canadian Stroke Network,Ottawa Hospital Research Institute at the University of Ottawa using the AdEasy adenoviral system(Q-Biogene,Carlsbad,CA,USA) according to manufacturer′s instructions.The adenovirus encoding luciferase was generated as previously described[17].Primary mouse hepatocytes or HepG2 cells were incubated with adenovirus in a minimal amount of serum-free DMEM for 1 hour,culture media was added,and the cells were used for experimentation after 36 hours.
Immunoblot analysis
Primary cells were incubated with serum-free Hepatozyme medium,while HepG2 cells were incubated in serum-free DMEM±heparin(100 U/mL) for 4 hours.The cells were harvested in sample loading buffer(SLB:10 mM Tris,pH 8.0,8 M urea,2%SDS, 10%glycerol,5%β-mercaptoethanol and bromophenol blue).The secreted hHL,apoA-I,or apoE proteins were adsorbed onto hydrated fumed silica(Cab-O-Sil) as previously described[31],and eluted in SLB.The cellassociated and secreted proteins were resolved by sodium dodecyl sulphate polyacrylamide gel electrophoresis(SDS-PAGE),and transferred onto nitrocellulose membrane for immunoblot analysis with appropriate antibodies against HL,apoA-I,and apoE. Densitometric analysis was performed using Quantity One software from BioRad.
Metabolic labeling of proteins
Cells were pretreated with Met/Cys-free medium for 30 min and then labeled with[35S]Met/Cys(100 μCi/ mL)in Met/Cys-free DMEM for indicated times. Pretreatment and labeling media were supplemented with 10%FBS and±heparin(100 U/mL).The35S-labeled proteins(apoA-I,apoE,hHL and hHLmt)were immunoprecipitated from the cells and media and resolved by SDS-PAGE.Radioactivity associated with the35S-labeled proteins was quantified by scintillation counting and normalized to cell protein levels.
Subcellular fractionation
Primary hepatocytes were harvested 36 hours post hHL-or hHLmt-adenovirus transfection in homogenization buffer(25 mM Tris-HCl,250 mM sucrose, 1 mM EDTA),homogenized by ball bearing homogenizer,and fractionated as previously described[32].The microsomal pellet was subjected to Nycodenz gradient ultracentrifugation as previously described[32]to further separate the microsome into ER and Golgi fractions. After centrifugation,14 fractions were collected and an aliquot of each fraction was resolved by SDSPAGE.The presence of hHL,hHLmt,apoE,calnexin and giantin was analyzed by immunoblotting as described above.
Statistical analyses
Values were expressed as mean±SD.The significance of differences among control and hHL-expressing cells was analyzed using Student′s t-test.The significance of differences among control,hHL,and hHLmt was analyzed by ANOVA followed by Dunnett′s posthoc tests.P<0.05 was considered significant.
RESULTS
Expression of hHL inLipc-null hepatocytes decreased apoA-I production and secretion.
The Lipc-null mice displayed increased plasma apoA-I,and the fractional catabolic rate of apoA-I in Lipc-null mice was normal[9,10].These results suggested that the increased apoA-I in Lipc-null mice may be related to altered apoA-I production.Thus,we examined apoA-I secretion from primary hepatocytes isolated from Lipc-null mice.Immunoblot analysis of conditioned media from the Lipc-null cells showed that the accumulation of apoA-I,but not apoE,was markedly higher as compared to control hepatocytes isolated from C57BL/6 mice(Fig 1A).Metabolic labeling experiments confirmed that the incorporation of35S-labeled amino acids into medium apoA-I was significantly increased(albeit by only 20%)from Lipc-null cells as compared to that from C57BL/6 cells(Fig. 1B,right panels).The levels of cell-associated35S-apoA-I were similar between Lipc-null and C57BL/6 cells(Fig.1B,left panels).Concentrations of cellassociated and secreted35S-labeled apoE(another hepatic secretory protein)were comparable between the two cell types(Fig.1C).These results provide the first indication that HL expression may have an impact on hepatic apoA-I secretion.
We next examined whether or not restoration of hHL expression in Lipc-null hepatocytes would negatively impact apoA-I secretion.Expression of hHL was achieved using hHL-encoding adenovirus at increasing viral dosage.Immunoblot analysis showed robust hHL expression and secretion in a gene dose-dependent manner(Fig.2A).As expected,the expressed hHL was,to a large extent,cell surface associated and could be released into media following treatment with heparin(Fig 2B).Increased expression of hHL in Lipc-null hepatocytes resulted in gradually diminished accumulation of apoA-I in the media,as compared to cells transfected with control adenovirus(Fig.3A, left panel).The level of cell-associated apoA-I was slightly decreased in hHL-transfected cells as compared to cells transfected with control adenovirus (Fig.3A,right panel).The effect of hHL expression on cell-associated and secreted apoE was minimal (Fig.3B).Metabolic labeling experiments confirmed that expression of hHL negatively impacted apoA-I (but not apoE)synthesis and secretion.Thus,incorporation of35S-labeled amino acids into medium (Fig.3C,left panels)and cell-associated(Fig. 3C,right panels)apoA-I was decreased by 50%upon hHL expression.The effect of hHL expression on apoA-I was specific because incorporation of35S-label into cell-associated or medium apoE from hHL expressing cells was similar to control(Fig.3D).Decreased levels of cell and secreted apoA-I from hHL-adenovirus infected hepatocytes was also observed under varied cell culture conditions,such as presence/absence of heparin in the media,and different virus infection times(from18 to 48 h)(data not shown).These data together strongly suggest that expression of hHL in hepatocytes specifically compromised the synthesis and secretion of apoA-I(not apoE).
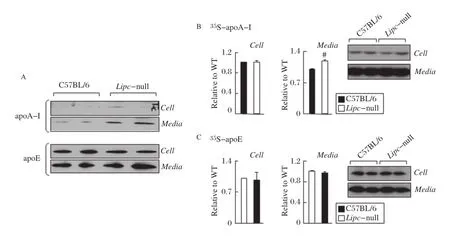
Fig.1 Lipc-null primary hepatocytes secrete increased levels of apoA-I relative to wildtype cells.A:Immunoblot analysis of apoA-I and apoE in cells or secreted into media(collected from 4 hours serum-free Hepatozyme media)from Lipc-null or wildtype(C57BL/6)primary hepatocytes.B and C:Lipc-null or wildtype hepatocytes were labeled with[35S]met/cys for 4 hours in DMEM containing 10%FBS and 100 U/mL heparin.ApoA-I(B)and apoE(C)were recovered from cells(left panels)and media(right panels),resolved by SDS-PAGE,and visualized by fluorography(insets).The intensity of35S-apoA-I and35S-apoE was quantified by scanning densitometry,and the results were expressed relative to wildtype,set to 1.Error bars indicate mean±SD of three independent experiments.Statistical significance:#P<0.01(Student′s t-test of KO vs. WT).

Fig.2Human HL is robustly expressed and secreted fromLipc-null primary hepatocytes 36 hours post-infection with hHL-encoding adenovirus.A:Immunoblot analysis of cell-associated and secreted hHL(collected from 4 hours serum-free Hepatozyme media containing 100 U/mL heparin)from Lipc-null primary hepatocytes infected with up to 5 pfu/cell of hHL-encoding adenovirus.Cell-associated levels of apoE were used as a loading control.B:The Lipc-null primary hepatocytes were treated similarly as in A except infected,or not(no inf),with 20 pfu/cell of hHL-adenovirus and the conditioned media either contained,or did not contain,100 U/mL heparin.Cell-associated levels of apoE were used as a loading control.
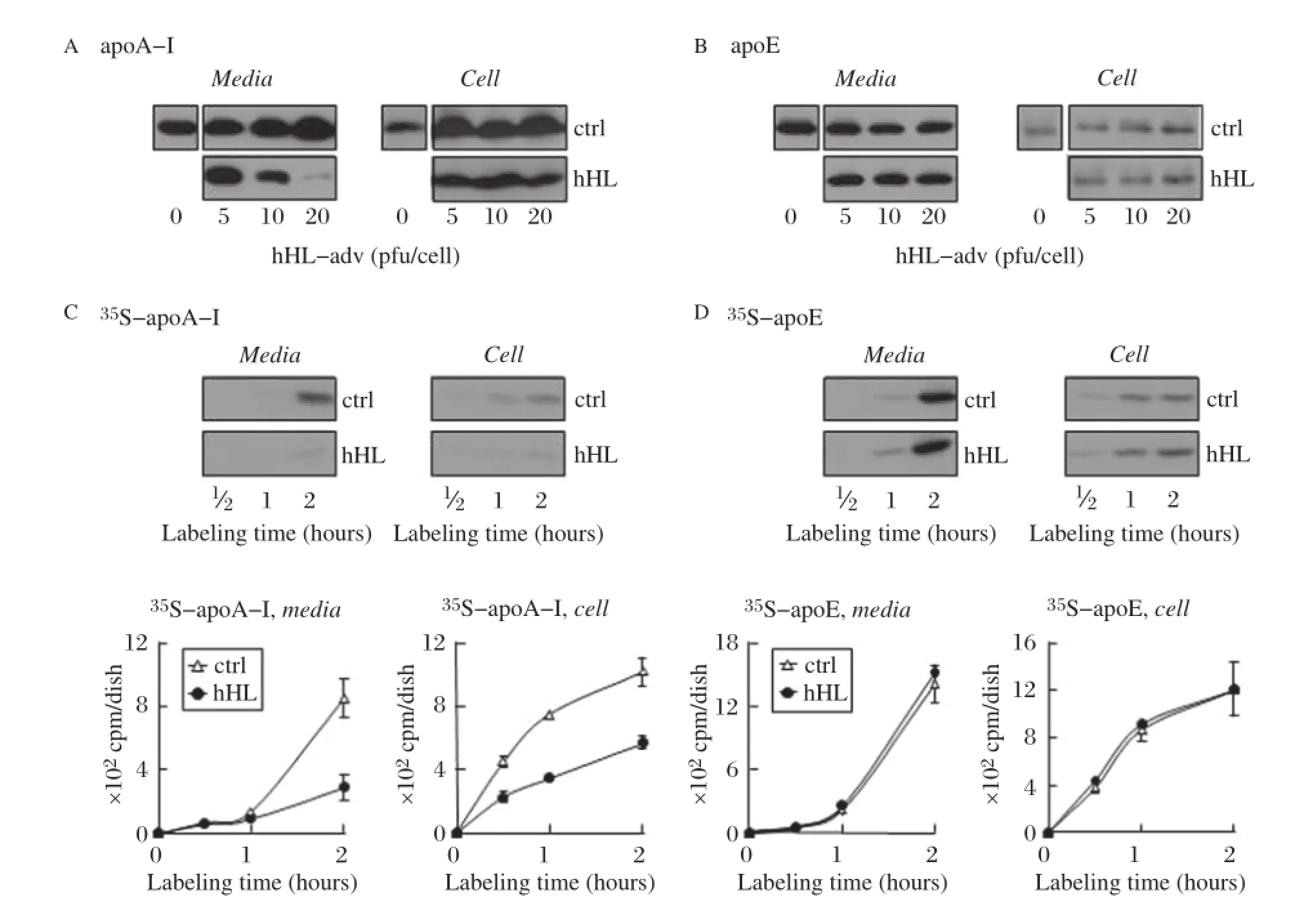
Fig.3Expression of hHL decreases the synthesis and secretion of apoA-I fromLipc-null primary hepatocytes transiently expressing hHL.A and B:Lipc-null primary hepatocytes infected with up to 20 pfu/cell of either control-or hHL-encoding adenovirus were incubated for 4 hours with serum-free Hepatozyme medium(containing 100 U/mL heparin).ApoA-I(A)and apoE(B)from media(left panels)and cells(right panels)were detected by immunoblot analysis.C and D:Lipc-null primary hepatocytes infected with 5 pfu/cell of either control-,or hHL-encoding adenovirus were labeled with[35S]met/cys for up to 2 hours in media containing 10%FBS and 100 U/mL heparin.The35S-apoA-I(C)and35S-apoE(D) were recovered from the media(left panels)and cells(right panels)by immunoprecipitation,resolved by SDS-PAGE,visualized by fluorography(upper panels),and quantified by scintillation counting(bottom panels).Error bars indicate mean±SD from two independent experiments.
Expression of HSPG-binding deficient hHLmt inLipc-null hepatocytes also resulted in decreased apoA-I production and secretion.
We have previously shown that expression of the HSPG-binding deficient hHLmt in C57BL/6 mice resulted in markedly decreased plasma apoA-I and HDL-associated phospholipids and cholesterol in vivo[17].To gain insight into hHLmt expression on apoA-I secretion,we transfected Lipc-null hepatocytes with hHLmt-encoding adenovirus,and compared its effect with that of hHL.Similar to what was observed for hHL-adenovirus(Fig.2A),transfection with hHLmt-adenovirus resulted in robust expression in Lipc-null hepatocytes(Fig.4A).The expressed hHLmt,as expected,exhibited decreased binding to HSPG.Thus,unlike what was observed for hHL, heparin treatment of hHLmt-expressing hepatocytes did not show marked increase in the released hHLmt protein into the media(Fig.4B).Secretion of hHLmt into the media(even in the presence of heparin),however,was less efficient from the transfected hepatocytes as compared to hHL(Fig.4B).Metabolic labeling experiments showed that the level of secreted35S-hHLmt was~50%lower as compared to that of35S-hHL under heparin treatment conditions(Fig.4C,left panel),even though cell-associated35S-hHLmt was similar to that of35S-hHL(Fig.4C,right panel). The levels of cell-associated and secreted35S-apoE were comparable in hHL-and hHLmt-expressing cells (Fig 4D).The decreased rate of35S-hHL secretion is probably attributable to impaired ER exit of the chimera protein(see below).
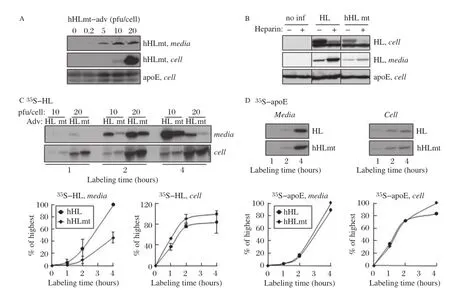
Fig.4The hHmt chimera exhibits reduced secretion relative to hHL fromLipc-null primary hepatocytes infected with either hHL-or hHLmt-adenovirus.A:Lipc-null primary hepatocytes infected with up to 20 pfu/cell of hHLmt-encoding adenovirus were incubated for 4 hours with serum-free Hepatozyme medium containing 100 U/mL heparin.The hHLmt protein was detected from cell and media by immunoblot analysis.Cellassociated levels of apoE were used as a loading control.B:The Lipc-null primary hepatocytes were treated similarly as described in A except infected,or not(no inf),with either hHL-or hHLmt-adenovirus(20 pfu/cell),and the conditioned media either contained,or did not contain,100 U/mL heparin.Cellassociated levels of apoE were used as a loading control.C and D:Lipc-null primary hepatocytes infected with 10 and 20 pfu/cell of either hHL(HL)-or hHLmt(mt)-encoding adenovirus were labeled with[35S]met/cys for up to 4 hours in media supplemented with 10%FBS and 100 U/mL heparin.The35SHL(C)and35S-apoE(D)were recovered from the media(left panels)and cells(right panels)by immunoprecipitation,resolved by SDS-PAGE,visualized by fluorography(upper panels),and quantified by scintillation counting(bottom panels).The radioactivity was expressed relative to the highest point in each plot,set to 100%.Error bars indicate mean±SD from two independent experiments.Notably,the observed rates of synthesis and secretion show that the synthesis of hHL or hHLmt reached a plateau after 2-hour labeling while the secretion of hHL and hHLmt continued to increase for up to 4-hour.This may support what has previously been described that the rate limiting step of the production of HL is the formation of functional dimers[49].In particular,a large amount of inactive HL monomers are synthesized,processed into dimers,and then secreted[19]).
The effect of hHLmt expression on the levels of cellassociated and secreted apoA-I was similar to that observed for hHL.Thus,as the level of hHLmt expression increased,the cell-associated and secreted levels of apoA-I decreased relative to cells transfected with the control adenovirus(Fig.5A).Semi-quantitative analysis by scanning densitometry showed that expression of either hHL or hHLmt exhibited a similar reduction in the levels of apoA-I(Fig.5A,bottom panels).[It is noteworthy that transfection of the cells with the control adenovirus(i.e.vector that did not encode HL)resulted in altered baseline expression of apoA-I;thus there was a~1.5-fold increase in secreted apoA-I and~3-fold increase in cell-associated apoA-I upon transfection with the control adenovirus.] Metabolic labeling experiments showed that incorporation of35S-label into cell and medium apoA-I was decreased(by 50%)in hHLmt-expressing hepatocytes as compared to control virus transfected cells (Fig.5B).Similar phenomena were observed in another cell culture model.HepG2 cells transfected with increasing doses of hHL and hHLmt adenovirus exhibited reduced levels of secreted apoA-I(Fig 5C).Altogether,these results suggest that expressionof either hHL or hHLmt negatively impacted the production and secretion of apoA-I from hepatocytes.

Fig.5Transient expression of hHL or hHLmt decreases the cell-associated and secreted levels of apoA-I.A:Lipc-null primary hepatocytes infected with up to 20 pfu/cell of control-,hHL-or hHLmt-encoding adenovirus were incubated with serum-free Hepatozyme media for 4 h. ApoA-I in the media(left panels)and cells(right panels)was detected by immunoblot analysis(top panels).The bands were quantified by densitometry and expressed relative to non-transfected(0)control,set to 1(bottom panels).Error bars indicate mean±SD of three independent experiments. Statistical significance:*P<0.05(One-way ANOVA analysis of hHLmt or hHL vs.control-adenovirus).Control adenoviruses used encoded either EGFP or luciferase.B:As described inFig 3Cexcept the cells were infected with hHLmt-encoding adenovirus instead of hHL-adenovirus.C:HepG2 cells infected with up to 20 pfu/cell of hHL(HL)-or hHLmt(mt)-adenovirus were incubated with serum-free DMEM containing 100 U/mL heparin for 4 hours.ApoA-I and HL in the media or cells were detected by immunoblot analysis.Results are representative of two independent experiments.
Intracellular hHL probably exerts its effect within the ER.
In an attempt to define mechanism(s)whereby hHL expression negatively exerts an effect on apoA-I secretion,we determined intracellular distribution of apoA-I within HL-expressing and control cells.Subcellular fractionation revealed that there was an increased association of apoA-I with the microsomal fraction in hHL-or hHLmt-expressing cells relative to non-transfected control cells(Fig.6A).The levels of microsome-associated apoE were unaltered by hHL expression as compared to non-transfected control cells (Fig.6B).Further fractionation of microsomes into Golgi and ER,by Nycodenz gradient ultracentrifugation, resulted in loss of the apoA-I signal(data not shown). Hence,whether or not apoA-I was retained within the ER was unascertained.Distribution of apoE within the ER/Golgi secretory pathway,however,was unchanged between hHL and hHLmt-expressing cells(Fig.6C, middle two panels).The expressed hHL was distributed normally throughout the ER/Golgi secretory pathway. However,the hHLmt chimera was mainly confined to the ER(Fig.6C,top two panels),suggesting retarded ER exit of the HSPG-binding deficient hHLmt chimera. It has been reported previously the HSPG-binding is necessary for efficient ER/Golgi trafficking of LPL[33]. Giantin and calnexin were used as markers for the frac-tionated Golgi and ER,respectively(Fig.4C,bottom two panels).Because both hHL and hHLmt expression exerted similar negative effect on apoA-I secretion,and because hHLmt protein showed predominant residence in the ER,it is tempting to speculate that hHL exerts its effect on apoA-I secretion within the ER.
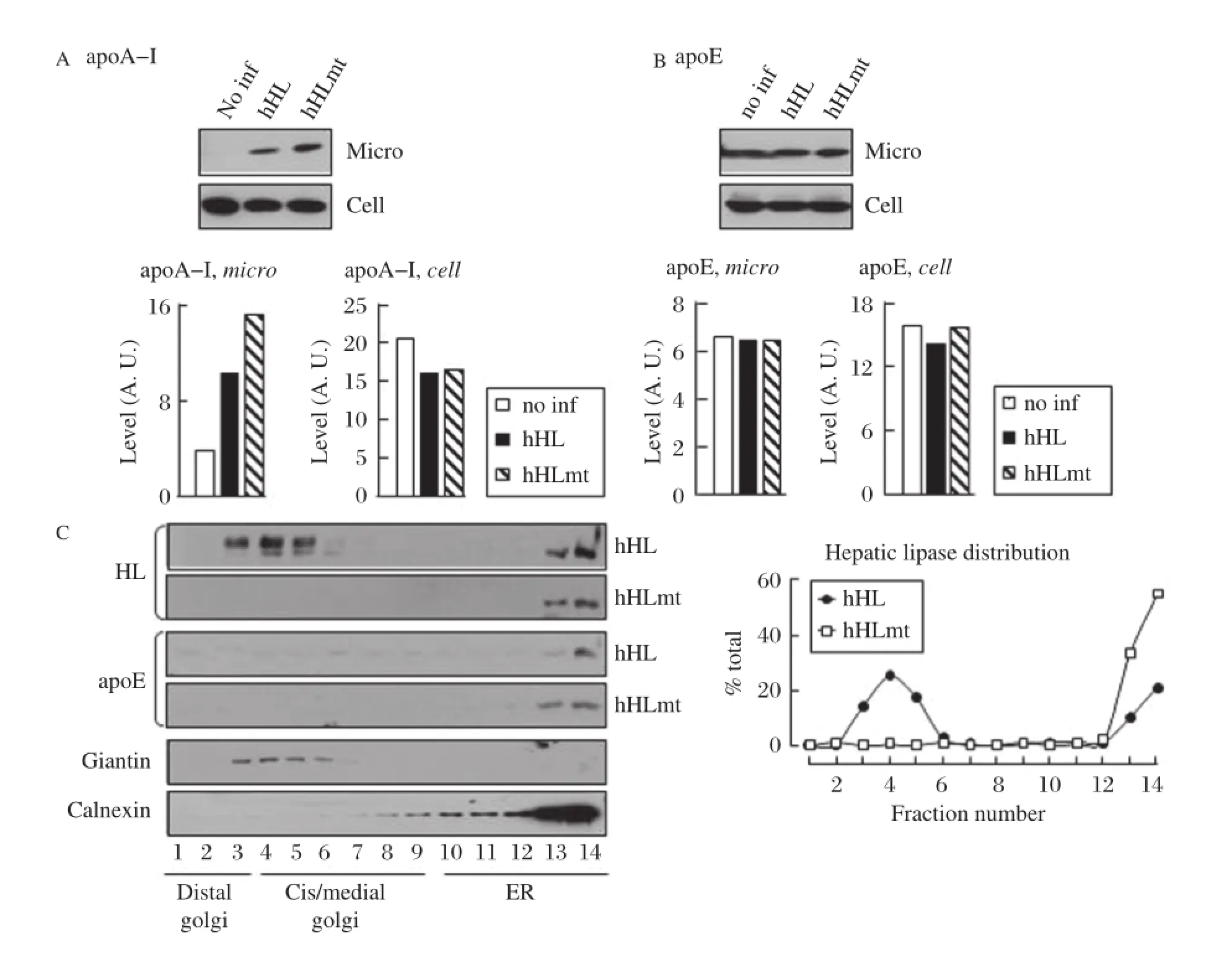
Fig.6hHL-and hHLmt-expressing cells exhibit increased levels of microsome-associated apoA-I relative to non-transfected control,and hHL and hHLmt are differentially distributed intracellularly.A and B:Lipc-null primary hepatocytes infected,or not(no inf), with 5 pfu/cell of either hHL-or hHLmt-adenovirus were homogenized and the microsomal fraction was isolated by sequential centrifugation steps. ApoA-I(A)and apoE(B)were detected in the microsomal fraction(left panels)and cell homogenate(right panels)by immunoblot analysis(top panels) and quantified by densitometry(bottom panels).Results are representative of two independent experiments.C:After hHL or hHLmt transfection(25 pfu/cell),the microsome was fractionated into Golgi and ER fractions by Nycodenz gradient.The steady-state distribution of HL(upper 2 panels),apoE (middle 2 panels),and giantin and calnexin(bottom 2 panels)were determined by immunoblot analysis.Results are representative of at least three independent experiments.The distribution of hHL or hHLmt within the secretory pathway was determined by plotting the intensity of each band as a fraction of total.
DISCUSSION
Understanding how plasma HDL-cholesterol is regulated is of utmost importance because of its cardio-protective properties.Plasma HDL-cholesterol concentrations are positively correlated with apoA-I[34],and deficiency of apoA-I is associated with complete absence of HDL-cholesterol[35,36].Thus,the present study intended to define mechanisms that regulate hepatic apoA-I(and nascent HDL)production.Our data showed that in primary hepatocytes,isolated from Lipcnull mice,expression of hHL compromised apoA-I synthesis and secretion.The inhibitory effect of hHL was independent of its association with HSPG;thus, both normal hHL and the HSPG-binding deficient hHLmt exerted similar inhibition on apoA-I production. Determination of distribution of hHL and hHLmt within the ER/Golgi secretory compartments revealed that the hHLmt chimera has an extended retention within the ER,and kinetic studies showed impaired secretion of hHLmt comparedwiththatof hHL.Thus,itis likelythat hHL(and hHLmt as well)exerted its negative impact on apoA-I production within the ER.
Until the landmark studies with the liver-specific Abca1-deficient mice showing markedly decreased plasma HDL-cholesterol[23],HDL was thought to form extra-hepatically or extra-intestinally through acquiring lipids(i.e.cholesterol and phospholipids)from peripheral tissues by apoA-I.Indeed,acquisition of lipids by apoA-I,presumably lipid-poor apoA-I secreted from hepatocytes or enterocytes,was facilitated by ABCA1 on non-hepatic cells and lecithin:cholesterol acyl transferase.However,the liver-specific Abca1-deficient mice study[23]showed that the major organ for murine HDL production is the liver.Thus,HDL-cholesterol concentration in the liver-specific Abca1-deficient mice was only 17%of that in control mice[23].In HepG2 cells, approximately 20%of newly synthesized35S-apoA-I isolated from microsomes was associated with d<1.25 g/mL fractions[24],suggesting intracellular lipidation of apoA-I.Association of[3H]choline-labeled phospholipids and[3H]mevalonate-labeled sterol with apoA-I within ER and medial-Golgi was also observed in mouse primary hepatocytes[25].Analysis of apoA-I-containing particles by FPLC and 2-D PAGE showed that both mouse primary hepatocytes and HepG2 cells secreted nascent and mature HDL[37,38].Thus,hepatic HDLbiogenesis can be initiatedduringapoA-I traversing through the ER/Golgi compartments,resulting in secretion of nascent and/or mature HDL.
Studies with non-hepatic cells,such as macrophage, fibroblast and baby hamster kidney(BHK)cells,have shown that HDL biogenesis from exogenously supplied lipid-free apoA-I occurs at the plasma membrane as well as at endosomes[27,28,39].Inhibiting ABCA1 in human skin fibroblasts revealed the presence of a‘high-capacity binding site′(i.e.a lipid-rich entity on the plasma membrane unrelated to lipid raft domains) onto which apoA-I binds and is lipidated[39].In ABCA1 over-expressing BHK cells,exogenously added125I-apoA-I was found in association with plasma membranes as well as intracellular compartments(not involved in apoA-I degradation)[28].Incubation of these cells with apoA-I induced ABCA1 endocytosis[28]. These results support a model where apoA-I lipidation occurs through a retroendocytosis process involving endocytosis and re-secretion.It is unclear if the retroendocytosis process exists in hepatic cells.
Although mechanisms responsible for hepatic biogenesis of apoA-I/HDL remain incompletely defined, several lipid and protein factors affecting apoA-I/ HDL production have been identified.Diets rich in saturated fat and cholesterol increased hepatic production and secretion of apoA-I via mechanisms that occur prior to Golgi exit of newly synthesized apoA-I[40]. Rates of human apoA-I(hapoA-I)secretion,measured by[3H]leucine incorporation,were 40%greater from primary hepatocytes prepared from hapoA-I transgenic mice fed a high fat/high cholesterol diet relative to low fat/low cholesterol diet[40].The abundance of hapoA-I mRNA in these cells was similar,and inhibition of secretion using brefeldin A revealed an accumulation in cellular3H-hapoA-I[40].The phospholipid transfer protein(PLTP),which is known to mediate the transfer of phospholipids from TAG-rich lipoproteins to HDL in plasma,contributes to intracellular lipidation and secretion of apoA-I/nascent HDL[41].The level of apoA-I that accumulated in the media(as measured by ELISA)was reduced in Pltp-null cells.Kinetic analysis showed that the secretion efficiency of apoA-I was unaffected by Pltp inactivation,although liquid chromatography/mass spectrometry studies showed decreased choline-containing phospholipids in association with apoA-I from Pltp-null hepatocytes[41]. The cholesteryl ester transfer protein(CETP),which is a plasma protein that mediates the transfer of neutral lipids among lipoproteins,also possess(as yet unknown) intracellular functions that affect the levels of apoA-I[42]. Antisense oligodeoxynucleotides against CETP inHepG2 cells resulted in increased cell-associated and secreted apoA-I[42].Besides its role as a macrophage scavenger receptor in the progression of atherosclerosis, CD-36 seems to play a hepatic function as well[43]. Primary hepatocytes from cd36-null mice exhibited increased production and secretion of newly synthesized apoA-I[43].Although the mechanism by which CD-36 exerted an effect on apoA-I production was unclear, RT-PCR analysis revealed that the apoA-I gene expression was increased in cd36-null mice[43].Additionally, transcription factors that regulate apoA-I gene expression include HNF4α,PPARα,RXRα,LXRs,and ARP-1[44].
in vivo studies have shown that Lipc-null mice exhibited increased plasma apoA-I[9,10],while the fractional catabolic rate of apoA-I was comparable to control[10].These findings suggest that HL may impair the secretion of apoA-I.Indeed,the present study shows that HL has a negative impact on the production and secretion of apoA-I/nascent HDL from the hepatocyte, and transfection studies with hHLmt shed some light on the mechanism underlying this phenomenon. Since hHLmt was mainly detected in the ER(and had similar phenotypes on apoA-I as hHL expression) it is tempting to speculate that the HL(temporarily) residing in the ER,during its maturation within the secretory pathway,affected the levels of microsomeassociated apoA-I leading to impaired apoA-I secretion.It is unclear how HL reduced cell-associated and secreted levels of apoA-I.Co-immunoprecipitation experiments with anti-HL and-apoA-I antibodies under non-denaturing conditions revealed that HDL displaced hHL from the cell surface of CHO cells stably expressing hHL,suggesting a protein-protein interaction between HDL and hHL[45].Thus,the possibility of apoA-I/HL interaction within the microsomal lumen does exist,and HL might exert an impact on apoA-I production through protein-protein interaction.It has been suggested that de novo synthesized apoA-I associates with phospholipids and cholesterol prior to its secretion as nascent HDL particles[25].It has also been reported that appropriate phospholipidation of apoA-I was required for apoA-I protein stability[41].Thus,HL expression could negatively impact the stability of newly synthesized nascent-HDL (apoA-I)particles within the ER resulting in apoA-I degradation.This would decrease cell-associated apoA-I levels,and ultimately,decrease secreted levels of apoA-I.Alternatively,HL expression might enhance the binding of apoA-I transcriptional repressors or impair the binding of positive regulators of apoA-I.
Our studies revealed a phenomenon that has not been previously described,which is that transfection of Lipc-null primary hepatocytes with control virus vector resulted in increased apoA-I in both cells and media as compared to non-transfected hepatocytes. Viral infection in vivo is expected to elicit host responses including lowering plasma apoA-I levels[46]. However,such a response of apoA-I expression to virus infection ex vivo/in vitro has not been reported previously.Primary hepatocytes incubated with a cytokine mixture(to stimulate the hepatic expression of inflammatory markers)induced apoA-I expression levels[47]showing that these changes in apoA-I were derived from transcriptional regulation of apoA-I in hepatic cells.
It is known that HL can reduce concentrations of apoB-containing lipoproteins and HDL in the plasma. Our recent work with transfected McA-RH7777 cells has shown that expression of hHL had an intracellular, but non-catalytic,role in reducing the lumenal lipid droplet and impairing secretion of lipid-rich VLDL particles[20].The present study uncovered an intracellular role of hHL in the production and/or secretion of liver derived apoA-I/HDL.The negative impact of HL expression on the production of these lipoprotein particles may be related to its unusual lengthy maturation time(hours rather than minutes)within the ER/ Golgi compartments[48].Prior to dimerization and gaining catalytic activity,the nascent HL might play an intracellular function in attenuating lumenal lipid droplet formation and apoA-I production.Once HL is dimerized,the catalytic active form of HL is rapidly secreted to play its extracellular function.Thus,in addition to its role in the catabolism of apoB-containing lipoproteins and HDL in the circulation,HL exerts a negative effect on the production and/or secretion of these particles,adding another level of complexity for hHL function in lipoprotein metabolism.
Acknowledgements
The authors wish to thank Dr.Yuwei Wang,Dr. Maroun Bou Khalil,Dr.Meenakshi Sundaram and Ms. Shumei Zhong for technical guidance and assistance, and Dr.Robin J.Parks for advice on adenovirus preparation.This work is supported by a grant-in-aid (#T6903)from the Heart and Stroke Foundation of Ontario.
[1] Gordon T,Castelli WP,Hjortland MC,Kannel WB, Dawber TR.High density lipoprotein as a protective factor against coronary heart disease.The Framingham Study.Am J Med 1977;62:707-14.
[2] Teslovich TM,Musunuru K,Smith AV,Edmondson AC, Stylianou IM,Koseki M,et al.Biological,clinical and population relevance of 95 loci for blood lipids.Nature 2010;466:707-13.
[3] Kathiresan S,Melander O,Guiducci C,Surti A,Burtt NP, Rieder MJ,et al.Six new loci associated with blood lowdensity lipoprotein cholesterol,high-density lipoprotein cholesterol or triglycerides in humans.Nat Genet 2008;40:189-97.
[4] Kathiresan S,Willer CJ,Peloso GM,Demissie S, Musunuru K,Schadt EE,et al.Common variants at 30 loci contribute to polygenic dyslipidemia.Nat Genet 2009;41:56-65.
[5] Santamarina-Fojo S,Gonzalez-Navarro H,Freeman L, Wagner E,Nong Z.Hepatic lipase,lipoprotein metabolism,and atherogenesis.Arterioscler Thromb Vasc Biol 2004;24:1750-4.
[6] Breckenridge WC,Little JA,Alaupovic P,Wang CS, Kuksis A,Kakis G,et al.Lipoprotein abnormalities associated with a familial deficiency of hepatic lipase. Atherosclerosis 1982;45:161-79.
[7] Hegele RA,Little JA,Vezina C,Maguire GF,Tu L, Wolever TS,et al.Hepatic lipase deficiency.Clinical, biochemical,and molecular genetic characteristics. Arterioscler Thromb 1993;13:720-8.
[8] Connelly PW,Maguire GF,Lee M,Little JA.Plasma lipoproteins in familial hepatic lipase deficiency. Arteriosclerosis 1990;10:40-8.
[9] Qiu S,Bergeron N,Kotite L,Krauss RM,Bensadoun A, Havel RJ.Metabolism of lipoproteins containing apolipoprotein B in hepatic lipase-deficient mice.J Lipid Res 1998;39:1661-8.
[10]Lambert G,Amar MJ,Martin P,Fruchart-Najib J,Foger B,Shamburek RD,et al.Hepatic lipase deficiency decreases the selective uptake of HDL-cholesteryl esters in vivo.J Lipid Res 2000;41:667-72.
[11]Applebaum-Bowden D,Kobayashi J,Kashyap VS, Brown DR,Berard A,Meyn S,et al.Hepatic lipase gene therapy in hepatic lipase-deficient mice.Adenovirusmediated replacement of a lipolytic enzyme to the vascular endothelium.J Clin Invest 1996;97:799-805.
[12]Dugi KA,Amar MJ,Haudenschild CC,Shamburek RD, Bensadoun A,Hoyt RF,Jr.,et al.In vivo evidence for both lipolytic and nonlipolytic function of hepatic lipase in the metabolism of HDL.Arterioscler Thromb Vasc Biol 2000;20:793-800.
[13]Brown RJ,Schultz JR,Ko KW,Hill JS,Ramsamy TA, White AL,et al.The amino acid sequences of the carboxyl termini of human and mouse hepatic lipase influence cell surface association.J Lipid Res 2003;44:1306-14.
[14]Peterson J,Bengtsson-Olivecrona G,Olivecrona T. Mouse preheparin plasma contains high levels of hepatic lipase with low affinity for heparin.Biochim Biophys Acta 1986;878:65-70.
[15]Davis RC,Wong H,Nikazy J,Wang K,Han Q,Schotz MC.Chimeras of hepatic lipase and lipoprotein lipase. Domain localization of enzyme-specific properties.J Biol Chem 1992;267:21499-504.
[16]Yu W,Hill JS.Mapping the heparin-binding domain of human hepatic lipase.Biochem Biophys Res Commun 2006;343:659-65.
[17]Brown RJ,Gauthier A,Parks RJ,McPherson R,Sparks DL,Schultz JR,et al.Severe hypoalphalipoproteinemia in mice expressing human hepatic lipase deficient in binding to heparan sulfate proteoglycan.J Biol Chem 2004;279:42403-9.
[18]Verhoeven AJ,Neve BP,Jansen H.Intracellular activation of rat hepatic lipase requires transport to the Golgi compartment and is associated with a decrease in sedimentation velocity.J Biol Chem 2000;275:9332-9.
[19]Ben-Zeev O,Doolittle MH.Maturation of hepatic lipase. Formation of functional enzyme in the endoplasmic reticulum is the rate-limiting step in its secretion.J Biol Chem 2004;279:6171-81.
[20]Bamji-Mirza M,Sundaram M,Zhong S,Yao EF,Parks RJ,Yao Z.Secretion of triacylglycerol-poor VLDL particles from McA-RH7777 cells expressing human hepatic lipase.J Lipid Res 2011;52:540-8.
[21]Erickson B,Selvan SP,Ko KW,Kelly K,Quiroga AD,Li L,et al.Endoplasmic reticulum-localized hepatic lipase decreases triacylglycerol storage and VLDL secretion. Biochim Biophys Acta 2013;1831:1113-23.
[22]Sundaram M,Zhong S,Bou Khalil M,Links PH,Zhao Y, Iqbal J,et al.Expression of apolipoprotein C-III in McARH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions.J Lipid Res 2010;51:150-61.
[23]Timmins JM,Lee JY,Boudyguina E,Kluckman KD, Brunham LR,Mulya A,et al.Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I.J Clin Invest 2005;115:1333-42.
[24]Chisholm JW,Burleson ER,Shelness GS,Parks JS. ApoA-I secretion from HepG2 cells:evidence for the secretion of both lipid-poor apoA-I and intracellularly assembled nascent HDL.J Lipid Res 2002;43:36-44.
[25]Maric J,Kiss RS,Franklin V,Marcel YL.Intracellular lipidation of newly synthesized apolipoprotein A-I in primary murine hepatocytes.J Biol Chem 2005;280:39942-9.
[26]Banerjee D,Redman CM.Biosynthesis of high density lipoprotein by chicken liver:conjugation of nascent lipids with apoprotein A1.J Cell Biol 1984;99:1917-26.
[27]Denis M,Landry YD,Zha X.ATP-binding cassette A1-mediated lipidation of apolipoprotein A-I occurs at the plasma membrane and not in the endocytic compartments. J Biol Chem 2008;283:16178-86.
[28]Hassan HH,Bailey D,Lee DY,Iatan I,Hafiane A,Ruel I, et al.Quantitative analysis of ABCA1-dependent compartmentalizationandtraffickingofapolipoproteinA-I:implicationsfordeterminingcellularkineticsofnascenthighdensity lipoprotein biogenesis.J Biol Chem 2008;283:11164-75.
[29]Subrahmanyan L,Kisilevsky R.Effects of culture substrates and normal hepatic sinusoidal cells on in vitro hepatocyte synthesis of Apo-SAA.Scand J Immunol 1988;27:251-60.
[30]Thomas SS,Plenkiewicz J,Ison ER,Bols M,Zou W, Szarek WA,et al.Influence of monosaccharide derivatives on liver cell glycosaminoglycan synthesis:3-deoxy-D-xylo-hexose(3-deoxy-D-galactose)and methyl (methyl 4-chloro-4-deoxy-beta-D-galactopyranosid)uronate.Biochim Biophys Acta 1995;1272:37-48.
[31]Vance DE,Weinstein DB,Steinberg D.Isolation and analysis of lipoproteins secreted by rat liver hepatocytes. Biochim Biophys Acta 1984;792:39-47.
[32]Tran K,Thorne-Tjomsland G,DeLong CJ,Cui Z,Shan J, Burton L,et al.Intracellular assembly of very low density lipoproteins containing apolipoprotein B100 in rat hepatoma McA-RH7777 cells.J Biol Chem 2002;277: 31187-200.
[33]Masuno H,Sakayama K,Okuda H.Effect of longterm treatment of 3T3-L1 adipocytes with chlorate on the synthesis,glycosylation,intracellular transport and secretion of lipoprotein lipase.Biochem J 1998;329(Pt3):461-8.
[34]Srivastava RA,Srivastava N.High density lipoprotein, apolipoprotein A-I,and coronary artery disease.Mol Cell Biochem 2000;209:131-44.
[35]Ikewaki K,Matsunaga A,Han H,Watanabe H,Endo A, Tohyama J,et al.A novel two nucleotide deletion in the apolipoprotein A-I gene,apoA-I Shinbashi,associated with high density lipoprotein deficiency,corneal opacities,planar xanthomas,and premature coronary artery disease.Atherosclerosis 2004;172:39-45.
[36]Pisciotta L,Miccoli R,Cantafora A,Calabresi L,Tarugi P,Alessandrini P,et al.Recurrent mutations of the apolipoprotein A-I gene in three kindreds with severe HDL deficiency.Atherosclerosis 2003;167:335-45.
[37]Kiss RS,McManus DC,Franklin V,Tan WL,McKenzie A,Chimini G,et al.The lipidation by hepatocytes of human apolipoprotein A-I occurs by both ABCA1-dependent and-independent pathways.J Biol Chem 2003;278:10119-27.
[38]Krimbou L,Marcil M,Genest J.New insights into the biogenesis of human high-density lipoproteins.Curr Opin Lipidol 2006;17:258-67.
[39]Hassan HH,Denis M,Lee DY,Iatan I,Nyholt D,Ruel I, et al.Identification of an ABCA1-dependent phospholipid-rich plasma membrane apolipoprotein A-I binding site for nascent HDL formation:implications for current models of HDL biogenesis.J Lipid Res 2007;48:2428-42.
[40]Azrolan N,Odaka H,Breslow JL,Fisher EA.Dietary fat elevates hepatic apoA-I production by increasing the fraction of apolipoprotein A-I mRNA in the translating pool.J Biol Chem 1995;270:19833-8.
[41]Siggins S,Bykov I,Hermansson M,Somerharju P, Lindros K,Miettinen TA,et al.Altered hepatic lipid status and apolipoprotein A-I metabolism in mice lacking phospholipid transfer protein.Atherosclerosis 2007; 190:114-23.
[42]Huang Z,Inazu A,Kawashiri MA,Nohara A,Higashikata T,Mabuchi H.Dual effects on HDL metabolism by cholesteryl ester transfer protein inhibition in HepG2 cells. Am J Physiol Endocrinol Metab 2003;284:E1210-9.
[43]Yue P,Chen Z,Nassir F,Bernal-Mizrachi C,Finck B, Azhar S,et al.Enhanced hepatic apoA-I secretion and peripheral efflux of cholesterol and phospholipid in CD36 null mice.PLoS One 2010;5:e9906.
[44]Mogilenko DA,Dizhe EB,Shavva VS,Lapikov IA, Orlov SV,Perevozchikov AP.Role of the nuclear receptors HNF4 alpha,PPAR alpha,and LXRs in the TNF alpha-mediated inhibition of human apolipoprotein A-I gene expression in HepG2 cells.Biochemistry 2009;48: 11950-60.
[45]Rouhani N,Young E,Chatterjee C,Sparks DL.HDL composition regulates displacement of cell surface-bound hepatic lipase.Lipids 2008;43:793-804.
[46]Khovidhunkit W,Kim MS,Memon RA,Shigenaga JK, Moser AH,Feingold KR,et al.Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host.J Lipid Res 2004;45:1169-96.
[47]Han CY,Chiba T,Campbell JS,Fausto N,Chaisson M, Orasanu G,et al.Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol 2006;26:1806-13.
[48]Doolittle MH,Ben-Zeev O,Bassilian S,Whitelegge JP, Peterfy M,Wong H.Hepatic lipase maturation:a partial proteome of interacting factors.J Lipid Res 2009;50: 1173-84.
[49]Doolittle MH,Peterfy M.Mechanisms of lipase maturation.Clin Lipidol 2010;5:71-85.
Received 20 November 2013,Revised 28 December 2013,Accepted 22 February 2014,Epub 20 March 2014
?Corresponding author:Prof.Zemin Yao,Department of Biochemistry, Microbiology&Immunology,University of Ottawa,Roger Guidon Hall,Rm 4210D,451 Smyth Road,Ottawa,Ontario,K1H 8M5,Canada.
Tel/Fax:613-562-5800x8665/613-562-5452,E-mail:zyao@uottawa.ca.
The authors reported no conflict of interests.
?2014 by the Journal of Biomedical Research.All rights reserved.
10.7555/JBR.28.20130184
 THE JOURNAL OF BIOMEDICAL RESEARCH2014年3期
THE JOURNAL OF BIOMEDICAL RESEARCH2014年3期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Analytical characteristics of a qPCR-based molecular diagnostic assay-conceptual considerations for laboratory personnel
- Drainage vs.non-drainage after cholecystectomy for acute cholecystitis:a retrospective study
- Position and complications of pedicle screw insertion with or without image-navigation techniques in the thoracolumbar spine: a meta-analysis of comparative studies
- Correlation of obstructive sleep apnea hypopnea syndrome with metabolic syndrome in snorers
- Class A scavenger receptor activation inhibits endoplasmic reticulum stress-induced autophagy in macrophage
- A genetic variant in pseudogene E2F3P1contributes to prognosis of hepatocellular carcinoma
