The occurrence of diffuse axonal injury in the brain: associated with the accumulation and clearance of myelin debris
Liang Wen, Jun Xu, Tianxiang Zhan, Hao Wang, Xin Huang, Wenchao Liu, Xiaofeng Yang, Renya Zhan
Department of Neurosurgery, First Af fi liated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, China
The occurrence of diffuse axonal injury in the brain: associated with the accumulation and clearance of myelin debris
Liang Wen, Jun Xu, Tianxiang Zhan, Hao Wang, Xin Huang, Wenchao Liu, Xiaofeng Yang, Renya Zhan
Department of Neurosurgery, First Af fi liated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, China
The accumulation of myelin debris may be a major contributor to the in fl ammatory response after diffuse axonal injury. In this study, we examined the accumulation and clearance of myelin debris in a rat model of diffuse axonal injury. Oil Red O staining was performed on sections from the cerebral cortex, hippocampus and brain stem to identify the myelin debris. Seven days after diffuse axonal injury, many Oil Red O-stained particles were observed in the cerebral cortex, hippocampus and brain stem. In the cerebral cortex and hippocampus, the amount of myelin debris peaked at 14 days after injury, and decreased signi fi cantly at 28 days. In the brain stem, the amount of myelin debris peaked at 7 days after injury, and decreased signi fi cantly at 14 and 28 days. In the cortex and hippocampus, some myelin debris could still be observed at 28 days after diffuse axonal injury. Our fi ndings suggest that myelin debris may persist in the rat central nervous system after diffuse axonal injury, which would hinder recovery.
nerve regeneration; neurodegeneration; diffuse axonal injury; myelin debris; neuroinflammation; traumatic brain injury; head trauma; central nervous system; inflammation; axon; prognosis; NSFC grants; neural regeneration
Funding:This work was supported by the National Natural Science Foundation of China, No. 81200955, 81271357.
Wen L, Xu J, Zhan TX, Wang H, Huang X, Liu WC, Yang XF, Zhan RY. The occurrence of diffuse axonal injury in the brain: associated with the accumulation and clearance of myelin debris. Neural Regen Res. 2014;9(21):1902-1906.
Introduction
Diffuse axonal injury, characterized by widespread swelling and deformation of axons, often occurs in patients with severe traumatic brain injury and is associated with high mortality and morbidity rates. Among diffuse axonal injury patients, 43% do not survive, 9% suffer from a persistent vegetative state or major handicap, and less than 50% are able to lead independent lives (Chelly et al., 2011). Although diffuse axonal injury has devas tating consequences, research on diffuse axonal injury in the central nervous system is limited, and the mecha nisms underlying diffuse axonal injury are still unclear.
In diffuse axonal injury, axonal degeneration results from the stretching and shearing caused by mechanical force during the sudden movement of the brain tissue during the traumatic event. The axon lesion is, therefore, traditionally considered to occur immediately after the trauma. However, more recent studies revealed that much of the axonal degeneration develops progressively after traumatic brain injury (Park et al., 2008; Che et al., 2009). During traumatic brain injury, the stretching and shearing of axons lead to the damage to the axonal cytoskeleton and impairment of axoplasmic transport. These changes eventually cause the swelling and tearing of the axons (Povlishock et al., 1995). Effort has been made to identify possible therapeutic targets for axonal damage. However, so far no effective treatment has been established (Langham et al., 2000; Pillai et al, 2003). There is clearly a need for a better understanding of the pathogenesis of diffuse axonal injury so that new therapeutics can be developed.
In recent years, neuroinflammation has become a focus of diffuse axonal injury research (Schmidt et al., 2005; Lenz et al., 2007; Lin and Wen, 2013). In fl ammation and degeneration of brain white matter may continue several years after traumatic brain injury (Johnson et al., 2013a; Smith et al., 2013). Myelin debris accumulated after axonal damage during diffuse axonal injury is an important mediator of in fl ammation (Fancy et al., 2010). Unlike the peripheral nervous system, an effective clearance mechanism for myelin debris is not present in the central nervous system. However, very few studies have been performed to investigate the accumulation and clearance of the myelin debris in different brain regions after diffuse axonal injury. It is hypothesized that the myelin debris accumulated during diffuse axonal injury may persist at the damage site, resulting in chronic infl ammation. In this study, we investigated the accumulation and clearance of Oil Red O-stained myelin debris at different time points following head injury in a rat model of diffuse axonal injury.
Materials and Methods
Animals
A total of 30 male clean Sprague-Dawley rats, purchased from the Experimental Animal Research Center of Zhe-jiang Academy of Medical Sciences, China (SCXK (Zhe) 2008-0033), were used in this study. According to Marmarou’s method, rats of approximately 2 months old, weighing between 300 and 350 g, were subjected to head trauma (Marmarou et al., 1994). All experimental protocols were approved by the Institutional Animal Care and Use Committee and were in accordance with the Guide for the Care and Use of Laboratory Animals issued by the U.S. National Institutes of Health.
Reagents
To prepare 0.15% Oil Red O staining solution, 0.15 g Oil Red O (Sigma, St. Louis, MO, USA) was dissolved in 100 mL isopropanol. The solution was heated until Oil Red O was completely dissolved. The 0.15% Oil Red O staining solution was then fi ltered and stored in a sealed bottle until use. To prepare 10% formaldehyde-calcium fi xative, 1 g CaCl2and 10 mL formaldehyde was added to 90 mL distilled water. Hematoxylin staining solution was purchased from Fude Biotechnology (Hangzhou, Zhejiang Province, China). All other chemical reagents including chloral hydrate, paraformaldehyde, 100% ethanol, isopropanol and glycerogelatin were purchased from Huadong Medical Technology Co., Ltd. (Hangzhou, Zhejiang Province, China).
Rat models of diffuse axonal injury
Twenty-five rats were used to produce the diffuse axonal injury model (diffuse axonal injury group); five rats were used as the control (control group). The experimental diffuse axonal injury rat model was produced according to a method previously developed and characterized by Marmarou et al. (1994), but with less impact strength. The device used for diffuse axonal injury model production included a 2.8-cm-diameter, 2-m-long steel tube, a 2-cm-diameter, 288-g cylindrical weight, a 2.5-cm-wide, 2-mm-thick steel plate, and a sponge cushion pad. Brie fl y, the rats were anesthetized by intraperitoneal injection of 10% chloral hydrate, and placed on a sponge cushion pad in a prone position. The rats were intubated and mechanically ventilated during the entire procedure. The rat’s head was protected by a steel helmet. Diffuse axonal injury was created by dropping the 288-g weight from a predetermined height (2 m) onto the steel plate. The brain peak acceleration reached 576 g. The rats in the control group were subjected to the same experimental procedure except that no impact was delivered.
Brain section preparation and Oil Red O staining
Five randomly selected diffuse axonal injury rats were processed at 1, 7, 14 and 28 days after diffuse axonal injury model production (Marmarou et al., 1994). Five rats in the control group were processed for brain dissection after the control experimental procedure. All rats were anesthetized by intraperitoneal injection of 10% chloral hydrate and perfused with 4% paraformaldehyde. The rat brains were snap frozen with liquid nitrogen and stored at -80°C before use. The brain samples were embedded in optimal cutting temperature medium, and coronal sections (10 μm thick) of the dorsal neocortex, dentate gyrus of the hippocampus, and mid-brain and medulla oblongata regions were made. The section temperature was maintained at -16-20°C. For each rat, fi ve sections were randomly selected from each site, randomly labeled, and stored at -80°C until further processing.
The frozen sections were first fixed in 10% formalde-hydecalcium fixative for 10 minutes and washed with distilled water once. For Oil Red O staining, the sections were then incubated for at least 30 seconds twice with 0.15% Oil Red O, followed by extensive washing with 60% dimethylcarbinol, and rinsed in H2O. After that, nuclei were counterstained with hematoxylin for 3 minutes, and then washed with distilled water for 1 minute. Sections were coverslipped with a glycerogelatin-based aqueous mounting medium (Vallieres et al., 2006).
Image analysis and statistics
Images of three randomly selected fields from each slide were taken under a light microscope (Olympus EX71, Tokyo, Japan) at 200 × magni fi cation to estimate the Oil Red O-stained staining in the neocortex, hippocampal dentate gyrus, and midbrain regions. The total number of Oil Red O-stained particles in each image was determined with Image-Pro Plus 16.0 software (Version 6.0.0.260; Media Cybernetics Inc., Silver Spring, MD, USA).
Statistical analysis
All data were presented as the mean ± SEM. All data were analyzed with SPSS 17.0 software (SPSS, Chicago, IL, USA). Statistical differences between the control and other groups were compared by one-way analysis of variance. Intragroup and intergroup differences were examined by the least significant difference method.P< 0.05 was considered statistically signi fi cant.
Results
Quantitative analysis of experimental animals
Three Sprague-Dawley rats in the diffuse axonal injury group died during production of the diffuse axonal injury model, and one rat died 21 days post injury. At least fi ve randomly selected diffuse axonal injury rats were processed at 1, 7 and 14 days, and the remaining six rats were processed at 28 days. All fi ve rats in the control group survived during the sham operation and were processed.
Morphology of the cerebral cortex, hippocampus and brain stem in rats with diffuse axonal injury
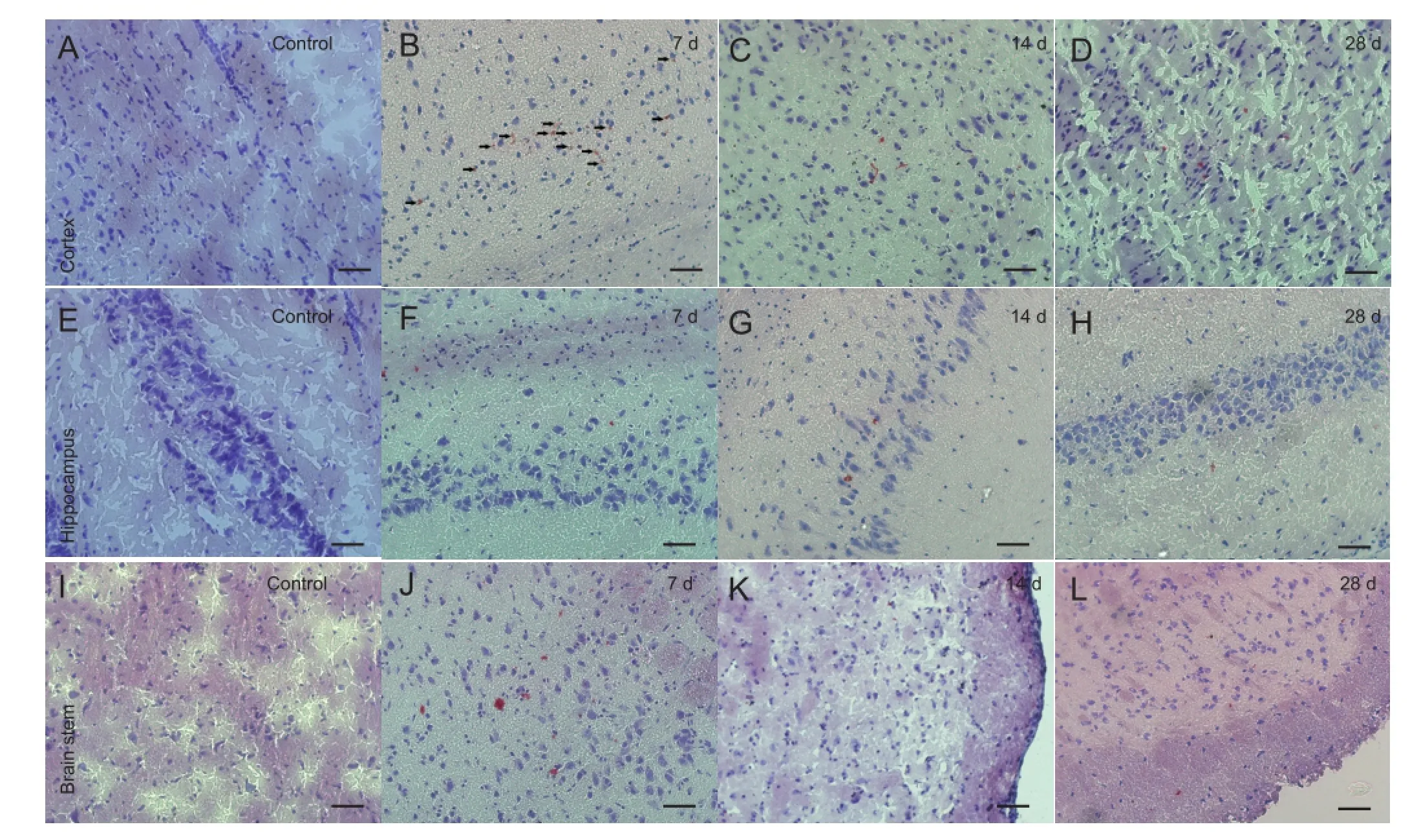
Figure 1 Morphology of the cerebral cortex, hippocampus and brain stem of rats with diffuse axonal injury and control rats (Oil Red O staining, × 200, bars: 50 μm).
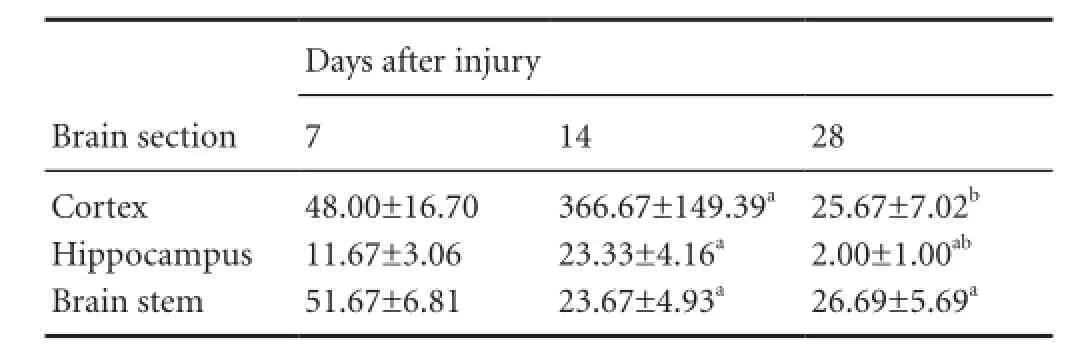
Table 1 Quanti fi cation (n/200-fold field of view) of Oil Red O-stained particles in brain sections
The accumulation and clearance of myelin debris in rats with diffuse axonal injury were monitored using Oil Red O staining. Oil Red O staining is a highly speci fi c staining method for myelin debris (Ma et al., 2002; Vallieres et al., 2006; Barrette et al., 2008). Oil Red O staining was not observed in the cortex, hippocampus or brain stem region among rats in the control group. In the diffuse axonal injury group, almost no Oil Red O staining was observed in the cortex, hippocampus or brain stem 1 day after injury. Seven days post injury, an apparent accumulation of Oil Red O staining was observed in the cortex, hippocampus and brain stem of diffuse axonal injury rats. Most of the Oil Red O-stained particles were distributed as clusters in the diffuse axonal injury brain tissue. Moreover, the Oil Red O-stained particles were still present in the cortex and brain stem 28 days post injury (Figure 1).
The amount of myelin debris was quanti fi ed by measuring the total number of Oil Red O-stained particles in the brain sections (Table 1). In the cortex, the accumulation of Oil Red O-stained particles was obvious 7 days post injury. The number of Oil Red O-stained particles increased dramatically to a peak at 14 days post injury. The number of Oil Red O-stained particles at 14 days was significantly higher than at 7 days post injury (P< 0.01). At 28 days post injury, the number of Oil Red O-stained particles was dramatically lower than at 14 days post injury. No signi fi cant difference in Oil Red O staining in the cortex was observed between 7 and 28 days after diffuse axonal injury (P> 0.05) (Table 1). A similar temporal pattern of Oil Red O staining was found in the hippocampus, with the number of stained particles peaking at 14 days after diffuse axonal injury. However, at 28 days post injury, the number of Oil Red O-stained particles was signi fi cantly lower than at 7 days (P< 0.01) (Table 1). In contrast, in the brain stem, the number of Oil Red O-stained particles peaked at 7 days after diffuse axonal injury, and decreased dramatically at 14 and 28 days post injury (P< 0.01) (Table 1). However, no significant difference was observed between 14 and 28 days (P> 0.05) (Table 1).
Our results show that the accumulation and clearance of myelin debris differ between the various brain regions examined. The amount of myelin debris peaked at 14 and 7 days after diffuse axonal injury in the cortex and brain stem,respectively. At 28 days post injury, a large amount of myelin debris was still present in these two brain regions. In contrast, although the amount of myelin debris in the hippocampus continued to increase signi fi cantly until 14 days post injury, most of the myelin debris was cleared at 28 days.
Discussion
This study surveyed the accumulation and clearance of Oil Red O-stained myelin debris in the cortex, hippocampus and brain stem regions at 1, 7, 14 and 28 days after brain injury using a diffuse axonal injury rat model. As expected, the brain in the control group was free of myelin debris. There was no myelin debris accumulation in the brain 1 day post injury. Myelin debris accumulation in the cortex, hippocampus and brain stem was apparent, and the amount peaked at 7-14 days post injury. The amount of myelin debris was decreased by 28 days post injury. However, a significant amount of myelin debris was still seen in the cortex and brain stem 28 days post injury. This indicates that there is a delayed accumulation and incomplete clearance of myelin debris in diffuse axonal injury rats.
Diffuse axonal injury is one of the most common consequences of closed head injury, occurring in approximately half of all severe traumatic brain injury cases (Johnson et al., 2013b). The inflammatory response after traumatic brain injury is considered one of the major causes of secondary brain injury. Numerous studies have investigated the role of neuroin fl ammation in focal brain injury (Woodroofe et al., 1991; Csuka et al., 2000; Harting et al., 2008; Kadhim et al., 2008). However, little is known about inflammation in the context of diffuse axonal injury, although the topic is currently under investigation (Lin and Wen, 2013). Several factors, including myelin debris, have been proposed as potential inducers of chronic in fl ammation in the brain, which leads to progressive secondary neuronal damage and the inhibition of damage repair. Therefore, comprehensive longterm studies using animal models are required to clarify the effects of diffuse axonal injury in the central nervous system. Our study is one of the fi rst long-term studies on myelin debris in the diffuse axonal injury rat model. A major fi nding of our study is that myelin debris accumulates and persists in the brain for a protracted period of time after diffuse axonal injury.
Normal axon fibers are covered by a myelin sheath. As axonal damage progresses, demyelination often follows and myelin debris is produced. In the peripheral nervous system, axonal degeneration triggers an inflammatory response, which is followed by the activation of Schwann cells, in a process called Wallerian degeneration (Gaudet et al., 2011). Macrophages and other immune cells are then recruited to the site of damage by Schwann cells to clear the myelin debris after injury. The process is swift and ef fi cient; therefore, myelin debris accumulation often does not happen following peripheral neuronal injury (David et al., 2012; Dubovy et al., 2013). In contrast, the clearance of the myelin debris in the central nervous system is often limited because of the weak phagocytic capabilities of activated microglial cells. Although macrophage infiltration can occur following blood-brain barrier damage after brain injury, the number of in fi ltrated peripheral immune cells is too low to contribute signi fi cantly to myelin debris clearance. Kelley et al. (2007) reported that myelin debris can be observed under an electron microscope 7-28 days following diffuse axonal injury. Consistent with their observations, we found that a signi fi cant amount of myelin debris was present in the cortex, hippocampus and brain stem regions 7-28 days following diffuse axonal injury. In addition, we observed that myelin debris accumulation in the brain was not obvious immediately after injury.
Myelin debris is an important mediator of the in fl ammatory response in the nervous system. Its regulatory functions in in fl ammation have been well documented in peripheral nerve injury and multiple sclerosis (Gaudet et al., 2011; Zindler and Zipp, 2011; David et al., 2012; Rawji and Yong, 2013). Furthermore, as a byproduct of axonal degeneration, myelin debris is considered one of the inducers of secondary neuronal damage, which has been documented in multiple sclerosis (Fancy et al., 2010; Sun et al., 2010; Clarner et al., 2012). Therefore, it is possible that the accumulated myelin debris in diffuse axonal injury leads to chronic inflammatory responses in the central nervous system and subsequent damage to the brain. The persistent presence of the myelin debris and the long-term neuroin fl ammation in the cortex and brain stem might be important contributors to the poor prognosis in diffuse axonal injury patients. In addition, the presence of the myelin debris itself inhibits regeneration and myelin repair as well, leading to limited recovery. Myelin debris is known to inhibit oligodendroglial maturation and regeneration in the central nervous system (Fawcett et al., 2012; Plemel et al., 2013). A recent study showed that oligodendrocyte precursor cells attached to myelin debris are strongly inhibited from maturing (Plemel et al., 2013). The inhibition of oligodendrocyte precursor cell maturation may contribute to the failure of remyelination and neuronal recovery, because oligodendrocytes are actively involved in these processes. Moreover, other signaling molecules, such as MAG and Nogo, associated with the myelin debris may prevent regeneration in the central nervous system (Fawcett et al., 2012). Both the in fl ammatory response and the inhibition of myelin regeneration are disadvantageous for recovery from diffuse axonal injury and lead to poor outcome.
In summary, the accumulation and clearance of myelin debris at different time points post diffuse axonal injury in different brain regions were evaluated. Our results show a delayed accumulation and incomplete clearance of myelin debris in the injured brain. As an important mediator of in fl ammation and inhibitor of regeneration, the long-term presence of myelin debris after diffuse axonal injury may lead to persistent secondary immune damage to the brain. However, there are several limitations of this study, such as the limited observation period. Further studies are required to examine the effects of the long-term presence of myelin debris after diffuse axonal injury, and whether it may lead to persistent secondary immune damage to the brain.
Author contributions:Wen L designed and performed research, and wrote the paper. Xu J, Liu WC and Zhan TX performed the research. Wang H and Huang X analyzed the data. Yang XF designed the research and provided technical or material support. Zhan RY provided technical or material support, and supervised the research. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Barrette B, Hebert MA, Filali M, Lafortune K, Vallieres N, Gowing G, Julien JP, Lacroix S (2008) Requirement of myeloid cells for axon regeneration. J Neurosci 28:9363-9376.
Chelly H, Chaari A, Daoud E, Dammak, H, Medhioub F, Mnif J, Hamida CB, Bahloul M, Bouaziz M (2011) Diffuse axonal injury in patients with head injuries: an epidemiologic and prognosis study of 124 cases. J Trauma 71:838-846.
Clarner T, Diederichs F, Berger K, Denecke B, Gan L, van der Valk P, Beyer C, Amor S, Kipp, M (2012) Myelin debris regulates in fl ammatory responses in an experimental demyelination animal model and multiple sclerosis lesions. Glia 60:1468-1480.
Csuka E, Hans VH, Ammann E, Trentz O, Kossmann T, Morganti-Kossmann MC (2000) Cell activation and in fl ammatory response following traumatic axonal injury in the rat. Neuroreport 11:2587-2590.
David S, Lopez-Vales R, Wee Yong V (2012) Harmful and beneficial effects of in fl ammation after spinal cord injury: potential therapeutic implications. Handb Clin Neurol 109:485-502.
Dubovy P, Jancalek R, Kubek T (2013) Role of in fl ammation and cytokines in peripheral nerve regeneration. Int Rev Neurobiol 108:173-206.
Fancy SP, Kotter MR, Harrington EP, Huang JK, Zhao C, Rowitch DH, Franklin RJ (2010) Overcoming remyelination failure in multiple sclerosis and other myelin disorders. Exp Neurol 225:18-23.
Fawcett JW, Schwab ME, Montani L, Brazda N, Muller HW (2012) Defeating inhibition of regeneration by scar and myelin components. Handb Clin Neurol 109:503-522.
Gaudet AD, Popovich PG, Ramer MS (2011) Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroin fl ammation 8:110.
Harting MT, Jimenez F, Adams SD, Mercer DW, Cox CS (2008) Acute, regional in fl ammatory response after traumatic brain injury: Implications for cellular therapy. Surgery 144:803-813.
Jiang J (2006) Concepts and pathological mechanisms of diffuse axonal injury. Zhonghua Shenjing Waike Zazhi 22:645-646.
Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W (2013a) In fl ammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136:28-42.
Johnson, VE, Stewart W, Smith DH (2013b) Axonal pathology in traumatic brain injury. Exp Neurol 246:35-43.
Kadhim HJ, Duchateau J, Sebire G (2008) Cytokines and brain injury: invited review. J Intensive Care Med 23:236-249.
Kelley BJ, Lifshitz J, Povlishock JT (2007) Neuroin fl ammatory responses after experimental diffuse traumatic brain injury. J Neuropathol Exp Neurol 66:989-1001.
Kwon HG, Jang SH (2012) The usefulness of diffusion tensor imaging in detection of diffuse axonal injury in a patient with head trauma. Neural Regen Res 7:475-478.
Langham J, Goldfrad C, Teasdale G, Shaw D, Rowan K (2000) Calcium channel blockers for acute traumatic brain injury. Cochrane Database Syst Rev CD000565.
Lenz A, Franklin GA, Cheadle WG (2007) Systemic in fl ammation after trauma. Injury 38:1336-1345.
Lin Y, Wen L (2013) In fl ammatory response following diffuse axonal injury. Int J Med Sci 10:515-521.
Ma M, Wei T, Boring L, Charo IF, Ransohoff RM, Jakeman LB (2002) Monocyte recruitment and myelin removal are delayed following spinal cord injury in mice with CCR2 chemokine receptor deletion. J Neurosci Res 68:691-702.
Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K (1994) A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg 80:291-300.
Park E, Bell JD, Baker AJ (2008) Traumatic brain injury: can the consequences be stopped? CMAJ 178:1163-1170.
Pillai SV, Kolluri VR, Mohanty A, Chandramouli BA (2003) Evaluation of nimodipine in the treatment of severe diffuse head injury: a double-blind placebo-controlled trial. Neurol India 51:361-363.
Plemel JR, Manesh SB, Sparling JS, Tetzlaff W (2013) Myelin inhibits oligodendroglial maturation and regulates oligodendrocytic transcription factor expression. Glia 61:1471-1487.
Povlishock JT, Jenkins LW (1995) Are the pathobiological changes evoked by traumatic brain injury immediate and irreversible? Brain Pathol 5:415-426.
Rawji KS, Yong VW (2013) The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clin Dev Immunol 2013:948976.
Schmidt OI, Heyde CE, Ertel W, Stahel PF (2005) Closed head injury--an in fl ammatory disease? Brain Res Brain Res Rev 48:388-399.
Smith C, Gentleman SM, Leclercq PD, Murray LS, Grif fi n WS, Graham DI, Nicoll JA (2013) The neuroin fl ammatory response in humans after traumatic brain injury. Neuropathol Appl Neurobiol 39:654-666.
Sun X, Wang X, Chen T, Li T, Cao K, Lu A, Chen Y, Sun D, Luo J, Fan J, Young W, Ren Y (2010) Myelin activates FAK/Akt/NF-kappaB pathways and provokes CR3-dependent inflammatory response in murine system. PLoS One 5:e9380.
Vallieres N, Berard JL, David S, Lacroix S (2006) Systemic injections of lipopolysaccharide accelerates myelin phagocytosis during Wallerian degeneration in the injured mouse spinal cord. Glia 53:103-113.
Woodroofe MN, Sarna GS, Wadhwa M, Hayes GM, Loughlin AJ, Tinker A, Cuzner ML (1991) Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J Neuroimmunol 33:227-236.
Zindler E, Zipp F (2011) Neuronal injury in chronic CNS in fl ammation. Best Pract Res Clin Anaesthesiol 24:551-562.
Copyedited by Patel B, Maxwell R, Wang J, Qiu Y, Li CH, Song LP, Zhao M
Xiaofeng Yang, M.D., Department of Neurosurgery, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China, jediwen@163.com. Renya Zhan, M.D., Department of Neurosurgery, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China, zhanry1960@163.com.
10.4103/1673-5374.145358
http://www.nrronline.org/
Accepted: 2014-08-15
- 中國神經(jīng)再生研究(英文版)的其它文章
- Hot spots and future directions of research on the neuroprotective effects of nimodipine
- Recovery of cerebellar peduncle injury in a patient with a cerebellar tumor: validation by diffusion tensor tractography
- Amyloid precursor-like protein 2 C-terminal fragments upregulate S100A9 gene and protein expression in BV2 cells
- Inhibition of Sirtuin 2 exerts neuroprotection in aging rats with increased neonatal iron intake
- Reversible lesions in the brain parenchyma in Wilson’s disease con fi rmed by magnetic resonance imaging: earlier administration of chelating therapy can reduce the damage to the brain
- Adult neurogenesis in the four-striped mice (Rhabdomys pumilio)

