Neuroprotective effect of pretreatment with ganoderma lucidum in cerebral ischemia/reperfusion injury in rat hippocampus
Wangxin Zhang, Qiuling Zhang, Wen Deng, Yalu Li, Guoqing Xing, Xianjun Shi, Yifeng Du
1 Department of Neurology, Shandong Provincial Hospital, Shandong University, Jinan, Shandong Province, China
2 Department of Medical Psychology, Taishan Medical University, Taian, Shandong Province, China
Neuroprotective effect of pretreatment with ganoderma lucidum in cerebral ischemia/reperfusion injury in rat hippocampus
Wangxin Zhang1,2, Qiuling Zhang2, Wen Deng2, Yalu Li2, Guoqing Xing2, Xianjun Shi2, Yifeng Du1
1 Department of Neurology, Shandong Provincial Hospital, Shandong University, Jinan, Shandong Province, China
2 Department of Medical Psychology, Taishan Medical University, Taian, Shandong Province, China
Ganoderma lucidum is a traditional Chinese medicine, which has been shown to have both anti-oxidative and anti-in fl ammatory effects, and noticeably decreases both the infarct area and neuronal apoptosis of the ischemic cortex. This study aimed to investigate the protective effects and mechanisms of pretreatment with ganoderma lucidum (by intragastric administration) in cerebral ischemia/reperfusion injury in rats. Our results showed that pretreatment with ganoderma lucidum for 3 and 7 days reduced neuronal loss in the hippocampus, diminished the content of malondialdehyde in the hippocampus and serum, decreased the levels of tumor necrosis factor-α and interleukin-8 in the hippocampus, and increased the activity of superoxide dismutase in the hippocampus and serum.ese results suggest that pretreatment with ganoderma lucidum was protective against cerebral ischemia/reperfusion injury through its anti-oxidative and anti-in fl ammatory actions.
nerve regeneration; cerebral ischemia/reperfusion; ganoderma lucidum; anti-oxidative; anti-inflammatory; superoxide dismutase; malondialdehyde; interleukin-8; tumor necrosis factor-α; apoptosis; hippocampus; neural regeneration
Funding:This work was supported by the Natural Science Foundation of Taishan Medical University in China, No. 2007.ZR-087.
Zhang WX, Zhang QL, Deng W, Li YL, Xing GQ, Shi XJ, Du YF. Neuroprotective effect of pretreatment with ganoderma lucidum in cerebral ischemia/reperfusion injury in rat hippocampus. Neural Regen Res. 2014;9(15):1446-1452.
Introduction
Cerebral ischemic disease is among the leading causes of senile dementia and death worldwide (Brouns and De Deyn, 2009). During ischemia, reduced glucose and oxygen transport to the brain causes cellular bioenergetic failure, which may lead to oxidative stress, in fl ammation, blood-brain barrier dysfunction, and eventually neuronal cell death, particularly in the hippocampus (Atlas et al., 2013). Evidence suggests that post-ischemic oxidative stress and inflammation are major events in the pathophysiology of ischemic damage (Chan, 1996; Lakhan et al., 2009). Excessive generation of free radicals and reactive oxygen species in the human brain results in lipid peroxidation of the cell membrane, protein denaturation, DNA damage, and oxidative injury to tissues (Ikeda and Long, 1990).e production of proin fl ammatory cytokines, such as tumor necrosis factor-α, interleukin-8 and interleukin-6, participates in tissue remodeling after injury and contributes to inflammation of the central nervous system (Wang et al., 2007, 2014; Terao et al., 2008; He et al., 2013).
Ganoderma lucidum is a white rot fungus used as a traditional remedy in the treatment of human diseases, such as hepatitis, liver disorders, hypercholesterolemia, arthritis, bronchitis, and tumorigenic diseases (Yuan et al., 2007; Zhou et al., 2012; Pan et al., 2014). The major active ingredients of ganoderma lucidum are polysaccharides, ergosterol, unsaturated fatty acids, and triterpenoids (Zhou et al., 2012; Pan et al., 2013a, 2014). Previous studies have shown that ganoderma lucidum-polysaccharides are anti-oxidative, hypoglycemic, anti-in fl ammatory, and have anti-tumor and immunomodulatory activities (Lin and Zhang, 2004; Li et al., 2011; Zhao et al., 2012). Oral administration of ganoderma lucidum has been shown to signi fi cantly reduce both cerebral infarct area and neuronal apoptosis in the ischemic cortex (Zhao et al., 2012). Recent pharmacological studies suggest that ganoderma lucidum stimulates the production of cytokines and exerts immunomodulatory effects (Ma et al., 2008). Administration of ganoderma lucidum to db/db mice also increases both serum and liver activity of antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase (Pan et al., 2013a). Therefore, we hypothesized that ganoderma lucidum protects hippocampal neurons against cerebral ischemia/reperfusion injury because of both its antioxidant and anti-in fl ammatory activ-ities. To test this hypothesis, we investigated the e ff ect of the pretreatment with ganoderma lucidum on cerebral ischemia/ reperfusion injury.
Materials and Methods
Animals
Thirty-two healthy, aged, and speci fi c-pathogen-free Wistar rats (male and female), 220 ± 10 g, were purchased from the Jining Lukang Co., Ltd. (Jining, Shandong Province, China) (license No. SCXK (Lu) 2008-0015) and housed in cages (4 rats per cage). All rats were allowed free access to food and water, and were maintained in the animal facility with filtered air under a 12-hour light/dark cycle at 23 ± 2°C and at a humidity of 45-55%. All procedures were approved by the Ethics Committee on Animal Experiments of Taishan Medical University and carried out in agreement with the Chinese Community guidelines for the Care and Use of Laboratory Animals. Rats were equally and randomly divided into four groups as follows: sham surgery, model, 3 or 7 days of pretreatment.
Preparation and administration of ganoderma lucidum
The ganoderma lucidum fungus mixture (water-soluble) was provided by Shandong Si Wei Co., Ltd. (Heze, Shandong Province, China) (license No. Z200220083). The preparation of ganoderma lucidum fungus mixture involved the inoculation of a pure culture of ganoderma lucidum mycelia into a solid culture medium (composed of bagasse and defatted rice bran) and cultured until just before the formation of the fruit body (for 3-4 months). The air-dried ganoderma lucidum fruit bodies were extracted with hot water and sterilized by fi ltration, as described previously (Gao et al., 2002; Kubo et al., 2005; Zhou et al., 2010). Ganoderma lucidum was administrated to rats at 20 mL/kg per dayviagastric gavage (the polysaccharides is 2 mg/mL) (Hu et al., 2003). Rats of the 3-and 7-day pretreatment groups were administrated for their respective treatment exposure before the modeling. Rats in both the model and sham groups were administrated water at 20 mL/kg for 7 days.
Focal cerebral ischemia/reperfusion rat model
Animals in both the model and pretreatment groups were deprived of food for 12 hours before the surgical procedure. The transient focal cerebral ischemia model was induced by middle cerebral artery occlusion, as described previously (Longa et al., 1989). Briefly, rats were anesthetized intraperitoneally with chloral hydrate at 400 mg/kg. The right common carotid artery was exposed, carefully isolated from the vagus nerve, and ligated on the proximal side through a right paramedian incision. The external carotid artery, the occipital artery, and the pterygopalatine artery were ligated similarly. Ischemia was induced by advancing a nylon monofi lament (0.26 mm) with its tip rounded into the interior carotid arteryviathe external carotid artery. After placement, the intraluminal suture was secured with a 4-0 silk suture tied around the external carotid artery. Reperfusion was produced when the intraluminal suture was withdrawn 1.5 hours after middle cerebral artery occlusion. Physiological parameters were monitored at baseline, during middle cerebral artery occlusion, and at reperfusion. Rectal temperature was maintained at 37°C with a heating lamp. Animals in the sham group were subjected to all the surgical procedures for ischemia/reperfusion except the occlusion.
The step-down test
The step-down test is widely used to measure passive avoidance for learning and memory (Longa et al., 1989). The procedure consisted of a training session and a test session 24 hours after training. Memory was measured 24 hours after ischemia. The apparatus (YLS-IA recorder for Multi-function autonomic activities in mice, Shandong academy of medical science, Jinan, Shandong Province, China) was a 40 cm × 40 cm plastic box with a 4.0 cm high and 10.0 cm wide platform in the left corner of the training box apparatus. The base of the apparatus was made of 0.1 cm caliber stainless steel bars spaced (in parallel) 1.0 cm apart. In the training session, animals were gently placed on the platform to habituate for 3 minutes. If the animals stepped down from the platform, they would receive a continuous scrambled foot shock (0.4 mA, 2 seconds), which made them immediately step up to the platform (i.e., passive avoidance). The training procedure was carried out 30 minutes daily for 3 consecutive days. In test sessions, foot shock was not delivered after the animal step-down from the platform. The step-down latency and number of errors made in 10 minutes were recorded.
Nissl staining for the histopathological assessment of the hippocampus
Brain sections from sacrificed animals were exposed to the Nissl stain for the assessment of neuronal cell loss at the dorsal CA1 sub fi eld of the hippocampus, as previously described (Atlas et al., 2013). Animals were deeply anesthetized with pentobarbital (50 mg/kg, intraperitoneally) and then transcardially perfused with cold saline followed by 4% paraformaldehyde in PBS (0.1 mol/L; pH 7.4). After post- fi xationin situovernight, brains were removed, washed in PBS, cryoprotected with 30% sucrose in PBS, and frozen in powdered dry ice. Coronal sections (20 μm) were cut at the level of the dorsal hippocampus (3.3-4.0 mm posterior from the bregma) (Paxinos and Watson, 2005) with a cryostat. Every fourth section was collected and stained with cresyl violet. For Nissl staining, the sections were mounted on slides (Superfrost-plus, Fisher Scienti fi c, Pittsburgh, PA, USA), dehydrated and rehydrated in graded ethanols and xylenes, respectively and then incubated in 1% cresyl violet for 30 seconds. Sections were then decolorized in acetic acid, dehydrated, and coverslipped with Permount. Sections were observed with a binocular microscope (Olympus, Tokyo, Japan).
Detection of oxidative stress in the hippocampus and serum
The level of malondialdehyde is used to measure the amount of lipid peroxidation, and this compound was determined spectrophotometrically, as previously described (Ohkawa etal., 1979). Brie fl y, 10 mg hippocampal tissues were homogenized with 0.1 mL sodium phosphate buffer (0.2 mol/L, pH 7.4). Acetic acid (1.5 mL, 20%, pH 3.5), thiobarbituric acid (1.5 mL, 0.8%), and sodium dodecyl sulfate (0.2 mL, 8.1%) were added to 0.1 mL of processed tissue sample and serum. The mixture was then heated at 100°C for 60 minutes, cooled with tap water and 5 mL of n-butanol plus pyridine (15:1, v/v) in 1 mL of distilled water, and then shaken vigorously. After centrifugation at 1,500 ×gfor 10 minutes, the organic layer was removed and its absorbance was measured at 532 nm using a spectrophotometer (Third Instrument Factory, Shanghai, China). Superoxide dismutase activity in hippocampal homogenates and serum was measured by the inhibition of nitroblue tetrazolium (Assay kit from Beyotime Institute of Biotechnology, China) reduction caused by the xanthine-XO system as the superoxide generator (Zhou and Prognon, 2006). Briefly, superoxide dismutase activity was assessed during the ethanol phase of the lysate after 1.0 mL ethanol/chloroform mixture (5/3, v/v) was added to the same volume of sample and then centrifuged. One unit of superoxide dismutase was de fi ned as the amount of enzyme that caused 50% inhibition of the nitroblue tetrazolium reduction rate. A calibration curve was derived with puri fi ed superoxide dismutase as the standard to calculate the activity of superoxide dismutase present in the samples.
Immunohistochemistry for tumor necrosis factor-αand interleukin-8 in the CA1 region of the hippocampus
Animals were transcardially perfused with a saline solution containing heparin (10 U/mL) followed by 4% paraformaldehyde dissolved in 0.1 mol/L phosphate buffer. The hippocampus was removed from the cranium, paraf fi n-embedded, and sectioned at a thickness of 4 μm for histology. Immunohistochemistry was performed using the Histostainplus kit (Beijing Zhongshan Biotechnology, Beijing, China). Brie fl y, brain sections were incubated in a peroxidase quenching solution (3% hydrogen peroxide in absolute methanol), rinsed twice with PBS and then incubated with serum blocking solution for 20 minutes. Sections were then incubated with the monoclonal antibodies, mouse anti-rat tumor necrosis factor-α (1:100; Boster, Wuhan, Hubei Province, China) or interleukin-8 (1:100; Boster), overnight at 4°C. After primary antibody incubation, the samples were rinsed with 0.3% skim milk in PBS containing 0.05% Triton X-100, then incubated with biotinylated goat anti-mouse IgG diluted in PBS containing 0.3% skim milk, followed by the enzyme conjugate diluted in PBS containing 0.3% skim milk. The bound antiserum was visualized by incubating the slides with 3,3′-diaminobenzidine. Finally, the sections were dehydrated and covered by a coverslip, and were then viewed, photographed, and analyzed by Image analysis software Image-proplus (Media Cybernetics, Bethesda, MD, USA). Photomicrographs were taken, and the absorbance was calculated. Omission of the primary or secondary antibody served as the negative control (Grif fi ths et al., 1991).
Statistical analysis
All data were expressed as mean ± SD and were analyzed by one-way analysis of variance followed by Dunnett’sposthoctest. All analyses were performed with SPSS 17.0 (SPSS Chicago, IL, USA). Signi fi cance was reached at values ofP< 0.05.
Results
Pretreatment with ganoderma lucidum improved learning and memory in rats with cerebral ischemia/reperfusion injury
Results in the step-down test showed that compared with the sham surgery group, the mean latency was signi fi cantly lower and the number of errors was signi fi cantly higher in rats of the model group (P< 0.05; Figure 1). However, the 3- and 7-day pretreatment with ganoderma lucidum significantly prolonged the mean latency and decreased the number of errors in the step-down test compared with the model group (P< 0.05; Figure 1). Furthermore, both pretreatments did not a ff ect rat behavior (Figure 1).
Pretreatment with ganoderma lucidum reduced ischemia-induced neuronal loss in the hippocampus
Nissl staining showed that in the sham surgery group, CA1 pyramidal neurons exhibited a typical shape and regular surface structure, and were clearly visible and orderly arranged (Figure 2). In the model rats, pyramidal neurons were disarranged and exhibited shrinkage, a dark staining appearance with small cytoplasm, or neuronal loss (Figure 2). Cell junctions became loose and the intercellular spaces were widened. Pretreatment with ganoderma lucidum, particularly for 7 days, greatly reduced ischemia-induced neuronal loss in the hippocampus (Figure 2).
Pretreatment with ganoderma lucidum decreased malondialdehyde contents and increased superoxide dismutase levels in the hippocampus and serum in rats with cerebral ischemia/reperfusion injury
Compared with the sham surgery group, malondialdehyde and superoxide dismutase levels were signi fi cantly increased and decreased, respectively in the hippocampus and serum of the model group (P< 0.05; Figure 3). Pretreatment with ganoderma lucidum for 3 or 7 days signi fi cantly decreased and increased the levels of malondialdehyde and superoxide dismutase, respectively in the hippocampus and serum compared with the model group (P< 0.05; Figure 3). The malondialdehyde content and superoxide dismutase level in the hippocampus tissue and serum in rats with cerebral ischemia/reperfusion injury was similar at each pretreatment timepoint (Figure 3).
Pretreatment with ganoderma lucidum suppressed the expression of tumor necrosis factor-α and interleukin-8 in the hippocampus of rats with cerebral ischemia/reperfusion injury
Immunohistochemistry revealed that tumor necrosis factor-α and interleukin-8 were expressed at very low levels in the sham surgery group (Figure 4). The immunoreactivityof these two cytokines was significantly higher in the hippocampal CA1 region of the model group compared with the sham surgery group (P< 0.05; Figure 4). Pretreatment with ganoderma lucidum significantly reduced the immunoreactivity of both cytokines in the hippocampus (P< 0.05; Figure 4). The immunoreactivity of both cytokines in the hippocampus of rats with cerebral ischemia/reperfusion injury was similar at each pretreatment time point (Figure 4).
Discussion
Despite numerous therapeutic trials, stroke is still the leading cause of death in the world. The present treatment for stroke is to perfuse with recombinant tissue plasminogen activator (Lakhan et al., 2009). However, a narrow therapeutic time window and risk of hemorrhage has hindered the success of this treatment (Hickenbottom and Barsan, 2000). Therefore, a useful and safe-to-use protective agent is particularly important in treating and alleviating the unfavorable outcomes of stroke. The present study demonstrated that pretreatment with ganoderma lucidum was protective against cerebral ischemia/reperfusion injury through its anti-oxidative and anti-in fl ammatory actions.
To determine the aspects of neurobehavioral protection, the animals were subjected to the step-down test, which is widely used for evaluating passive avoidance memory in rats. In this study, pretreatment with ganoderma lucidum for 3 and 7 days increased the latency time and decreased the error number compared with the control group. Therefore, these results suggested that ganoderma lucidum could improve memory retention. Ganoderma lucidum has been shown to improve learning and memory in senescence-accelerated mice prone 8, and thus neuroactive components that may exist in ganoderma lucidum extracts may cross the blood-brain barrier to promote neuronal function (Wang et al., 2004; Zhou et al., 2012). The hippocampus plays a critical role in several fundamental memory operations (Eichenbaum, 2001). Oral administration of ganoderma lucidum-polysaccharides significantly reduces the cerebral infarct area, neurological functional deficits, and neuronal apoptosis in ischemic cortex (Zhou et al., 2010). To con fi rm the protective potential of ganoderma lucidum, neuronal injury was analyzed by Nissl staining. The present study showed that in addition to marked improvements in memory, rats pretreated with ganoderma lucidum also exhibited less neural death in the hippocampal CA1 region compared with model rats. This result further con fi rmed the protective effect of this compound against ischemia.
The brain is particularly vulnerable to oxidative stress injury because of its high consumption of oxygen, abundant polyunsaturated fatty acids, and low levels of endogenous antioxidants (Madamanchi et al., 2005; Schreibelt et al., 2007). Free radicals may attack protein and polyunsaturated phospholipids in membranes, including plasma membranes and cellular organelles, leading to the disruption of these organelles. Therefore, inducing anti-oxidative effects is considered to be a promising treatment for ischemic stroke (Hall and Murdoch, 1990; Powers and Jackson, 2008). Superoxide dismutase is the primary protective enzyme against tissue damage caused by reactive oxygen species. This enzyme catalyzes the dismutation of superoxide anion to hydrogen peroxide and prevents the formation of the hydroxyl radical (Huang et al., 2012). Superoxide dismutase activity in serum has been shown to be reduced in stroke patients, and increased antioxidant activity may be bene fi cial in the acute treatment of cerebral ischemia (Spranger et al., 1997). Our study showed that the reduction in superoxide dismutase activity after cerebral ischemia/reperfusion injury was prevented by administration of ganoderma lucidum. Brain malondialdehyde is one of the most sensitive indicators of lipid peroxidation (Cini et al., 1994). In the present study, malondialdehyde was significantly elevated in the model group, suggesting the involvement of lipid oxidation in cerebral injury. However, ganoderma lucidum significantly reduced the level of malondialdehyde. Overall, these results indicate an antioxidant effect of ganoderma lucidum. Therefore, this compound may induce a protective mechanism by increasing the endogenous defensive capacity of the brain to combat oxidative stress induced by ischemia/reperfusion.
Inflammation is an important pathological process in ischemia, particularly during the acute phase (Candelario-Jalil, 2009; Lakhan et al., 2009). Focal cerebral ischemia elicits a strong in fl ammatory response involving tumor necrosis factor-α, which induces the synthesis of subsequent proinflammatory cytokines, such as interleukin-6 and interleukin-8 (Cie?lak et al., 2013; Zhang et al., 2013). These proinflammatory molecules induce multiple inflammatory cascades and contribute to the progression of brain damage following ischemic insult. Ganoderma lucidum has been shown to suppress lipopolysaccharide-mediated expression of tumor necrosis factor-α in murine RAW 264.7 cells (Dudhgaonkar et al., 2009). Ganoderma lucidum-polysaccharides signi fi cantly reduces the levels of both serum interleukin-6 and tumor necrosis factor-α and increases the levels of serum interleukin-2, interleukin-4, and interleukin-10 in rats (Pan et al., 2013b). Other studies found that ganoderma lucidum suppresses oxidative stress-induced secretion of interleukin-8 from breast cancer cells. In the present study, immunoreactivity of tumor necrosis factor-α and interleukin-8 was signi fi cantly reduced in the hippocampal CA1 region by the pretreatment of ganoderma lucidum compared with the model group. These results suggest that ganoderma lucidum protects neuronal cells from inflammation-induced injury after ischemia.
In conclusion, results of the present study indicate that ganoderma lucidum produces a distinct protective effect against cerebral ischemia/reperfusion injury in rats. This protective effect may be due to both its anti-oxidative and anti-in fl ammatory properties. Overall, ganoderma lucidum may be a potentially safe traditional Chinese medicine treatment for stroke patients.
Author contributions:Zhang WX, Zhang QL and Du YF conceived and designed the experiments. Zhang WX, Deng W, Li YL, Xing GQ and Shi XJ performed the experiments. ZhangWX and Zhang QL provided reagents/materials/analysis tools. Zhang WX, Zhang QL and Du YF wrote the manuscript. All authors approved the final version of the paper.
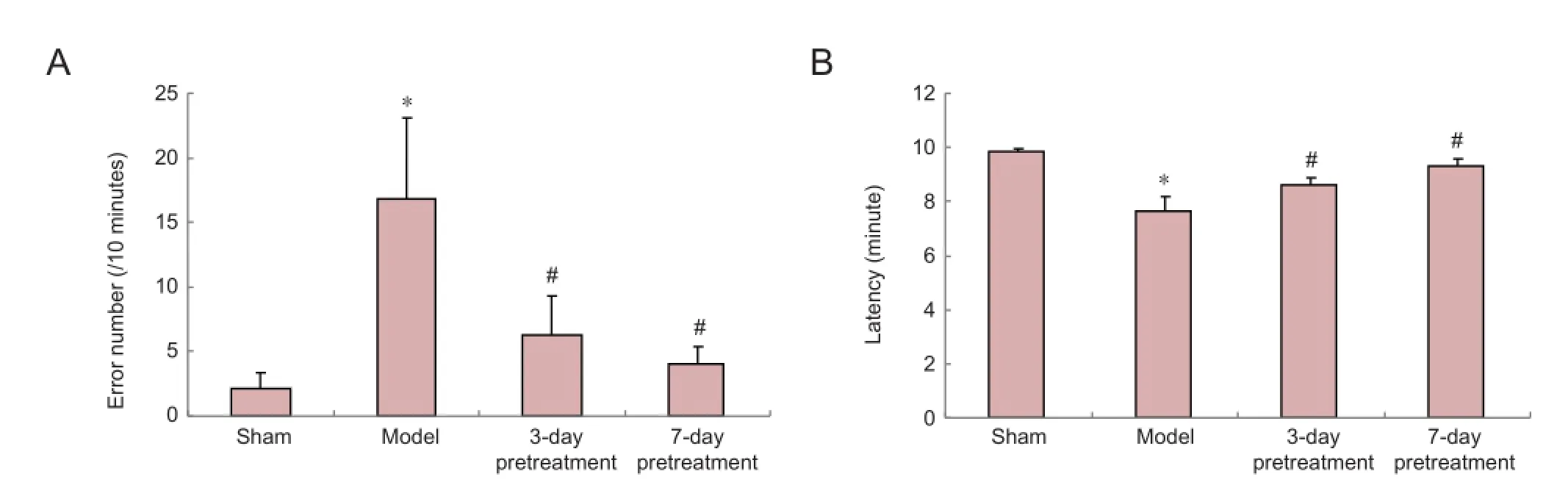
Figure 1 Pretreatment with ganoderma lucidum improves learning and memory after cerebral ischemia/reperfusion injury.

Figure 2 Pretreatment with ganoderma lucidum greatly reduces ischemia-induced neuronal loss in the hippocampus (× 400).
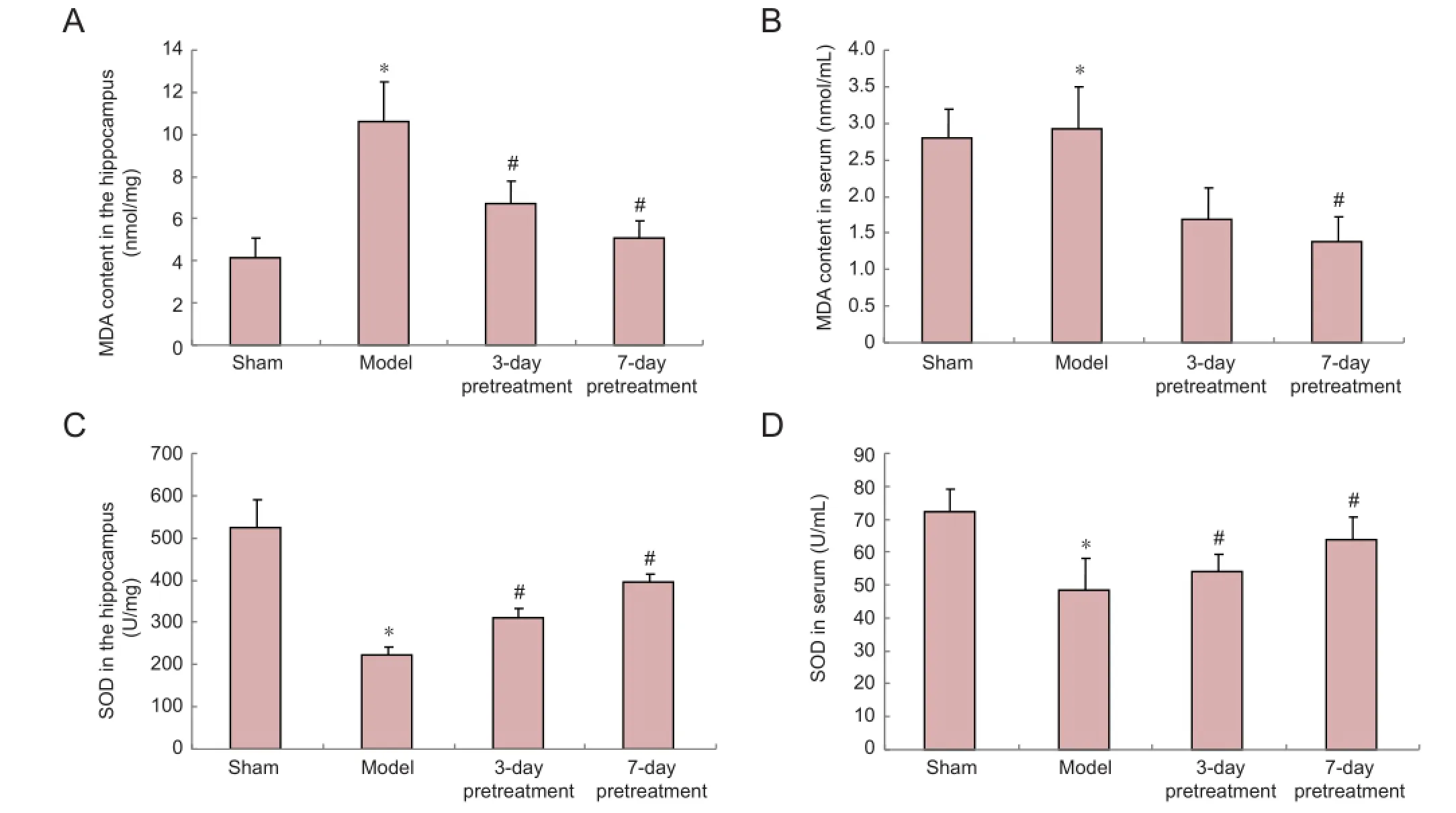
Figure 3 Pretreatment with ganoderma lucidum decreases malondialdehyde (MDA) content and increases superoxide dismutase (SOD) level in the hippocampus and serum of rats with cerebral ischemia/reperfusion injury.
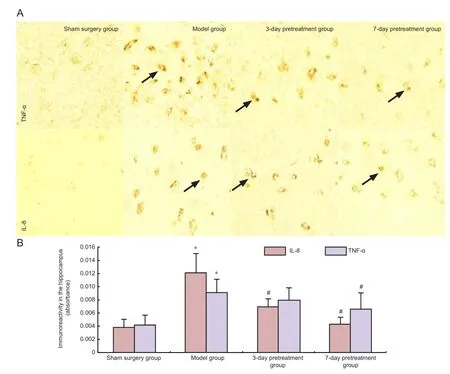
Figure 4 The effect of G. lucidum pretreatment on tumor necrosis factor-alpha (TNF-α) and interleukin-8 (IL-8) immunoreactivity in the CA1 of the rat hippocampus.
Con fl icts of interest:None declared.
Atlas iMA, Naderian H, Noureddini M, Fakharian E, Azami A (2013) Morphology of rat hippocampal ca1 neurons following modi fi ed two and four-vessels global ischemia models. Arch Trauma Res 2:124-128.
Brouns R, De Deyn PP (2009) The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 111:483-495.
Candelario-Jalil E (2009) Injury and repair mechanisms in ischemic stroke: considerations for the development of novel neurotherapeutics. Curr Opin Investig Drugs 10:644-654.
Chan PH (1996) Role of oxidants in ischemic brain damage. Stroke 27:1124-1129.
Cie?lak M, Wojtczak A, Cie?lak M (2013) Relationship between the induction of in fl ammatory processes and infectious diseases in patients with ischemic stroke. Acta Biochim Pol 60:345-359.
Cini M, Fariello RG, Bianchetti A, Moretti A (1994) Studies on lipid peroxidation in the rat brain. Neurochem Res 19:283-288.
Dudhgaonkar S, Thyagarajan A, Sliva D (2009) Suppression of the in fl ammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. Int Immunopharmacol 9:1272-1280.
Eichenbaum H (2001) The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res 127:199-207.
Gao Y, Zhou S, Wen J, Huang M, Xu A (2002) Mechanism of the antiulcerogenic effect of Ganoderma lucidum polysaccharides on indomethacin-induced lesions in the rat. Life Sci 72:731-745.
Griffiths CE, Barker JN, Kunkel S, Nickoloff BJ (1991) Modulation of leucocyte adhesion molecules, a T-cell chemotaxin (IL-8) and a regulatory cytokine (TNF-alpha) in allergic contact dermatitis (rhus dermatitis). Br J Dermatol 124:519-526.
Hall R, Murdoch J (1990) Brain protection: physiological and pharmacological considerations. Part II: the pharmacology of brain protection. Can J Anaesth 37:762-777.
He W, Chen W, Zhou Y, Tian Y, Liao F (2013) Xanthotoxol exerts neuroprotective effects via suppression of the in fl ammatory response in a rat model of focal cerebral ischemia. Cell Mol Neurobiol 33:715-722.
Hickenbottom SL, Barsan WG (2000) Acute ischemic stroke therapy. Neurol Clin 18:379-397.
Hu ZL, Wen SG, Yu RJ, Zhu Y (2003) Effects of Ganoderma lucidum fungus mixtureon immune enhancement in mice. Shandong Zhongyiyao Daxue Xuebao 27:683-687.
Huang TT, Zou Y, Corniola R (2012) Oxidative stress and adult neurogenesis--effects of radiation and superoxide dismutase de fi ciency. Semin Cell Dev Biol 23:738-744.
Ikeda Y, Long DM (1990) The molecular basis of brain injury and brain edema: the role of oxygen free radicals. Neurosurgery 27:1-11.
Kubo N, Myojin Y, Shimamoto F, Kashimoto N, Kyo E, Kamiya K, Watanabe H (2005) Protective effects of a water-soluble extract from cultured medium of Ganoderma lucidum (Rei-shi) mycelia and Agaricus blazei murill against X-irradiation in B6C3F1 mice:increased small intestinal crypt survival and prolongation of average time to animal death. Int J Mol Med 15:401-406.
Lakhan SE, Kirchgessner A, Hofer M (2009) In fl ammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7:97.
Li F, Zhang Y, Zhong Z (2011) Antihyperglycemic effect of ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int J Mol Sci 12:6135-6145.
Lin ZB, Zhang HN (2004) Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol Sin 25:1387-1395.
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91.
Ma C, Guan SH, Yang M, Liu X, Guo DA (2008) Differential protein expression in mouse splenic mononuclear cells treated with polysaccharides from spores of Ganoderma lucidum. Phytomedicine 15:268-276.
Madamanchi NR, Vendrov A, Runge MS (2005) Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25:29-38.
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351-358.
Pan D, Zhang D, Wu J, Chen C, Xu Z, Yang H, Zhou P (2013a) Antidiabetic, antihyperlipidemic and antioxidant activities of a novel proteoglycan from ganoderma lucidum fruiting bodies on db/db mice and the possible mechanism. PLoS One 8:e68332.
Pan D, Zhang D, Wu J, Chen C, Xu Z, Yang H, Zhou P (2014) A novel proteoglycan from Ganoderma lucidum fruiting bodies protects kidney function and ameliorates diabetic nephropathy via its antioxidant activity in C57BL/6 db/db mice. Food Chem Toxicol 63:111-118.
Pan K, Jiang Q, Liu G, Miao X, Zhong D (2013b) Optimization extraction of Ganoderma lucidum polysaccharides and its immunity and antioxidant activities. Int J Biol Macromol 55:301-306.
Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates. London: Academic Press.
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243-1276.
Reynolds A, Laurie C, Lee Mosley R, Gendelman HE (2007) Oxidative Stress and the Pathogenesis of Neurodegenerative Disorders. Int Rev Neurobiol 82:297-325.
Schreibelt G, van Horssen J, van Rossum S, Dijkstra CD, Drukarch B, de Vries HE (2007) Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res Rev 56:322-330.
Spranger M, Krempien S, Schwab S, Donneberg S, Hacke W (1997) Superoxide dismutase activity in serum of patients with acute cerebral ischemic injury. Correlation with clinical course and infarct size. Stroke 28:2425-2428.
Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T, Granger DN (2008) In fl ammatory and injury responses to ischemic stroke in obese mice. Stroke 39:943-950.
Wang J, Wang P, Li S, Wang S, Li Y, Liang N, Wang M (2014) Mdivi-1 prevents apoptosis induced by ischemia-reperfusion injury in primary hippocampal cells via inhibition of reactive oxygen species-activated mitochondrial pathway. J Stroke Cerebrovasc Dis 23:1491-1499.
Wang MF, Chan YC, Wu CL, Wong YC, Hosoda K, Yamamoto S (2004) Effects of Ganoderma on aging and learning and memory ability in senescence accelerated mice. Int Congr Ser 1260:399-404.
Wang Q, Tang XN, Yenari MA (2007) The in fl ammatory response in stroke. J Neuroimmunol 184:53-68.
Yuan JP, Wang JH, Liu X (2007) Distribution of free and esteri fi ed ergosterols in the medicinal fungus Ganoderma lucidum. Appl Microbiol Biotechnol 77:159-165.
Zhang Y, Li YW, Wang YX, Zhang HT, Zhang XM, Liang Y, Zhang XS, Wang WS, Liu HG, Zhang Y, Zhang L, Zheng YH (2013) Remifentanil preconditioning alleviating brain damage of cerebral ischemia reperfusion rats by regulating the JNK signal pathway and TNF-α/ TNFR1 signal pathway. Mol Biol Rep 40:6997-7006.
Zhao W, Jiang X, Deng W, Lai Y, Wu M, Zhang Z (2012) Antioxidant activities of Ganoderma lucidum polysaccharides and their role on DNA damage in mice induced by cobalt-60 gamma-irradiation. Food Chem Toxicol 50:303-309.
Zhou JY, Prognon P (2006) Raw material enzymatic activity determination: a specific case for validation and comparison of analytical methods--the example of superoxide dismutase (SOD). J Pharm Biomed Anal 40:1143-1148.
Zhou Y, Qu ZQ, Zeng YS, Lin YK, Li Y, Chung P, Wong R, H?gg U (2012) Neuroprotective effect of preadministration with Ganoderma lucidum spore on rat hippocampus. Exp Toxicol Pathol 64:673-680.
Zhou ZY, Tang YP, Xiang J, Wua P, Jin HM, Wang Z, Mori M, Cai DF (2010) Neuroprotective effects of water-soluble Ganoderma lucidum polysaccharides on cerebral ischemic injury in rats. J Ethnopharmacol 131:154-164.
Copyedited by Mark F, Wysong S, Yu J, Qiu Y, Li CH, Song LP, Zhao M
Yifeng Du, M.D, Department of Neurology, Shandong Provincial Hospital, Shandong University, Jinan 250021, Shandong Province, China, duyifeng2013@163.com.
10.4103/1673-5374.139461
http://www.nrronline.org/
Accepted: 2014-05-05
 中國(guó)神經(jīng)再生研究(英文版)2014年15期
中國(guó)神經(jīng)再生研究(英文版)2014年15期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Changes in cortical activation patterns accompanying somatosensory recovery in a stroke patient: a functional magnetic resonance imaging study
- Changes in brain activation in stroke patients after mental practice and physical exercise: a functional MRI study
- Integration of animal behaviors under stresses with different time courses
- A feasible strategy for focal cerebral ischemiareperfusion injury: remote ischemic postconditioning
- Pretreatment with Danhong injection protects the brain against ischemia-reperfusion injury
- Optimal therapeutic dose and time window of picroside II in cerebral ischemic injury
