Optimal therapeutic dose and time window of picroside II in cerebral ischemic injury
Guangyi Liu, Li Zhao, Tingting Wang, Meizeng Zhang, Haitao Pei
Institute of Cerebrovascular Diseases, Af fi liated Hospital of Qingdao University, Qingdao, Shandong Province, China
Optimal therapeutic dose and time window of picroside II in cerebral ischemic injury
Guangyi Liu, Li Zhao, Tingting Wang, Meizeng Zhang, Haitao Pei
Institute of Cerebrovascular Diseases, Af fi liated Hospital of Qingdao University, Qingdao, Shandong Province, China
A preliminary study from our research group showed that picroside II inhibited neuronal apoptosis in ischemic penumbra, reduced ischemic volume, and improved neurobehavioral function in rats with cerebral ischemia. The aim of the present study was to validate the neuroprotective effects of picroside II and optimize its therapeutic time window and dose in a rat model of cerebral ischemia. We found that picroside II inhibited cell apoptosis and reduced the expression of neuron-speci fi c enolase, a marker of neuronal damage, in rats after cerebral ischemic injury. The optimal treatment time after ischemic injury and dose were determined, respectively, as follows: (1) 2.0 hours and 10 mg/kg according to the results of toluidine blue staining; (2) 1.5 hours and 10 mg/kg according to early apoptotic ratio by fl ow cytometry; (3) 2.0 hours and 10 mg/kg according to immunohistochemical and western blot analysis; and (4) 1.5 hours and 10 mg/kg according to reverse transcription polymerase chain reaction. The present fi ndings suggest that an intraperitoneal injection of 10 mg/kg picroside II 1.5-2.0 hours after cerebral ischemic injury in rats is the optimal dose and time for therapeutic bene fi t.
nerve regeneration; picroside II; therapeutic dose; time window; brain ischemia; neuron-specific enolase; toluidine blue staining; flow cytometry; immunohistochemical assay; western blot; RT-PCR; rats; NSFC grant; neural regeneration
Funding:This study was financially supported by the National Natural Science Foundation of China, No. 81041092, 81274116.
Liu GY, Zhao L, Wang TT, Zhang MZ, Pei HT. Optimal therapeutic dose and time window of picroside II in cerebral ischemic injury. Neural Regen Res. 2014;9(15):1437-1445.
Introduction
Neuron-speci fi c enolase (NSE), an acidic protease involved in the glycolytic pathway, is a marker enzyme of neurons and exists speci fi cally in neurons and neuroendocrine cells (Vos et al., 2004). Normally, there is little NSE in body fl uids; the highest concentration is in brain tissue, accounting for about 1.5-3.0% of the total soluble protein (Hein Née Maier et al., 2008). A growing body of evidence indicates that the content of NSE varies in different parts of the nervous system (Marquardt et al., 2009; Oksanen et al., 2009), and that NSE plays a neuroprotective role. NSE is essential in maintaining the excitability of neuronal membranes, and it is also involved in the formation of the membrane structure during the development of the central nervous system (Selakovic et al., 2005). It has been suggested that NSE is related to the regulation of the stress response, and is involved in the repair of brain cells (Selakovic et al., 2005). When neuronal injury or necrosis occurs after ischemia or hypoxia, NSE is quickly released by the neurons into the cell gap and then into the cerebrospinal fluid, or through the blood-brain barrier into peripheral blood, increasing the level of NSE in cerebrospinal fluid and serum (van Munster et al., 2009). Therefore, NSE is a specific and objective indicator by which to observe neuronal injury and necrosis in the brain (Lima et al., 2004). Recent studies have found that NSE correlates highly with the diagnosis and prognosis of ischemic brain injury, as well as the degree of injury and infarct volume (Anand et al., 2005; Jauch et al., 2006; Wunderlich et al., 2006; Brea et al., 2009; González-García Sienkiewicz-Jarosz et al., 2009; Whiteley et al., 2009; Saenger et al., 2010; Bharosay et al., 2012; Singh et al., 2013).
Increasing evidence indicates that picroside II has antioxidant, anti-inflammatory and anti-apoptotic effects (Guo et al., 2011; Meng et al., 2012). In preliminary studies, we explored the therapeutic dose and time window of picroside II in the treatment of cerebral ischemia/reperfusion injury from tests of neurobehavioral function, infarct volume and immunohistochemical staining in rats. The results suggested that picroside II has its strongest protective effect against cerebral ischemia at a dose of 20 mg/kg, 1.5 hours after ischemic injury (Pei et al., 2012). In the present experiment, we employ the orthogonal design principle to identify the optimal therapeutic dose and time window of picroside II using a variety of biological methods, in a broader attempt to qualitatively and quantitatively measure levels of NSE and neuronal apoptosis in brain tissue after cerebral ischemia.
Materials and Methods
Animals
A total of 255 adult healthy male Wistar rats of speci fi c patho-gen-free grade, weighing 230-250 g, were supplied by the Experiment Animal Center of Qingdao Drug Inspection Institute, Qingdao, Shandong Province, China (license No. SCXK (Lu) 20100100). All animals were acclimatized for 7 days to temperature (23 ± 2°C) and humidity-controlled housing with natural illumination and free access to food and water. The experiment was approved by the Ethics Committee of Qingdao University Medical College in China (approval No. QUMC 2011-09). The local legislation for ethics of animal experimentation and guidelines for the care and use of laboratory animals were followed for all animal procedures.
Experimental grouping
Animals were fasted for 12 hours before surgery. Twenty (5 × 4) rats were randomly selected as the control group, and the remaining 235 rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (3 mL/kg) and fi xed in the supine position to establish forebrain ischemic models by bilateral common carotid artery occlusion (Márquez-Martín et al., 2012). Core body temperature was maintained at 36-37°C using a rectal probe and homeothermic blanket control unit during and after surgery. Twenty-three rats that had died or not awoken within 2 hours of surgery were excluded, while the 212 successful models in which the cerebral blood fl ow curve (PeriFlux 5000, Swedish Perimed Medical Co. Ltd) dropped to 30% were included in the experiment. Control rats underwent identical surgical and experimental procedures except the common carotid artery was not occluded.
Orthogonal experimental design
A total of 212 successful rat models were divided randomly into a model group (5 × 4) and a treatment group (16 × 4 × 3). The treatment group was then subdivided according to the principle of orthogonal experimental design of [L16(45)] consisting of two impact factors with four impact levels (Table 1). Impact factor A is the therapeutic time window, with four levels: 1.0, 1.5, 2.0, 2.5 hours after ischemia. Impact factor B is the therapeutic drug dose, also with four levels: 5, 10, 20 and 40 mg/kg body weight (Table 1). The orthogonal experimental test was repeated three times.
Drug administration
Picroside II (molecular formula: C23H28O13, molecular weight: 512.48, CAS No: 39012-20-9, purity > 98%) was supplied by Tianjin Kuiqing Medical Technology Co., Ltd., Tianjin, China. Each rat was weighed and the corresponding amount of picroside II powder was diluted in 1 mL isotonic saline solution to obtain the assigned dose, and injected intraperitoneally at the time determined by the orthogonal design [L16(45)] (Table 1). Rats in the control and model groups were intraperitoneally injected with the same volume of saline after 2 hours of cerebral ischemia.
Sample preparation
Paraffin sectioning
Twenty-four hours after injection, rats from the control group (n= 5), model group (n= 5) and treatment groups (three subgroups;n= 16 per subgroup) were randomly chosen and anesthetized by intraperitoneal injection of 10% chloral hydrate (3 mL/kg). The rats were perfused with 200 mL normal saline and 200 mL 4% paraformaldehyde solution successivelyviathe heart, and whole brains were removed and post- fi xed for 2 hours in 4% formaldehyde solution before soaking in distilled water for 4 hours. Brains were embedded in paraf fi n, and coronal sections (5 μm thickness) were cut continually from the posterior of the optic chiasm using a microtome (Leica CM2027, Germany). The sections were dehydrated through a conventional ethanol gradient, rendered transparent using xylene, and then adhered onto poly-L-lysine-coated slides and stored at 4°C.
Flow cytometry suspension
Rats were randomly selected and anesthetized as described above, then perfused through the heart with 200 mL normal saline. Whole brains were removed and 200 mg brain tissue from the ischemic area was quickly collected into a 1.5 mL Eppendorf tube with 0.5 mL precooled PBS (0.01 mol/L). These tissue was shredded and moved into a glass tube with 2 mL EDTA-free trypsin (2.5%) to incubate for 15 minutes at 37°C, and then pipetted gently and fi ltered into a 1.5 mL tube (on ice) through a 200 mesh fi lter. The fi ltrate was centrifuged at 800 r/min for 5 minutes at 4°C. With the supernatant discarded, cell concentration was adjusted to 1 × 106/100 μL with 1 × Annexin binding buffer and stored at 4°C.
Obvervational indexes
Toluidine blue staining
Paraffin sections were dewaxed in dimethyl benzene and washed for 30 seconds, three times, in PBS, dyed for 1 hour in 1% toluidine blue at 56°C, washed in distilled water, placed in 70% alcohol for 1 minute, and separated in 95% alcohol. Sections were dehydrated with anhydrous alcohol, placed in xylene, and mounted with neutral gum. Five randomly-chosen, non-overlapping visual fi elds in the parietal cortex were observed at 400 × magnification under a light microscope (Olympus IX141, Tokyo, Japan) and the number of denatured cells in each visual fi eld was counted; neuronal Nissl bodies were stained dark blue, karyoplasm pale blue, and the background appeared pale under the microscope. The degree of pathological damage was expressed as the denatured cell index (the number of denatured cells/total cells).
Immunohistochemical analysis

Figure 1 Neuronal morphology and expression of neuron-speci fi c enolase (NSE) in the parietal cortex of rats with cerebral ischemia after picroside II treatment.
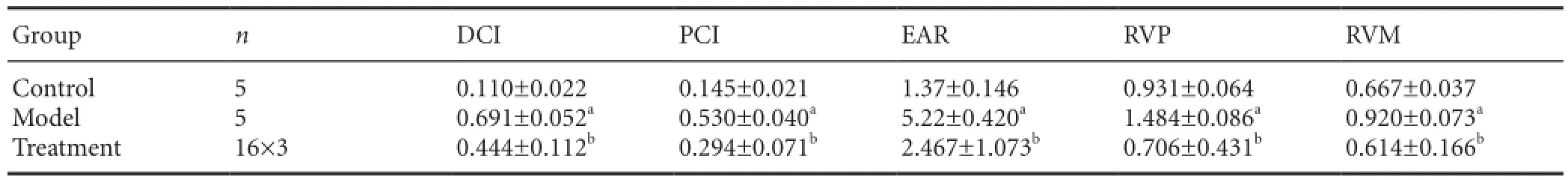
Table 3 Comparison of denatured cell index (DCI), positive cell index (PCI), early apoptosis ratio (EAR), relative gray value ratio of protein (RVP), and relative gray value ratio of mRNA (RVM)
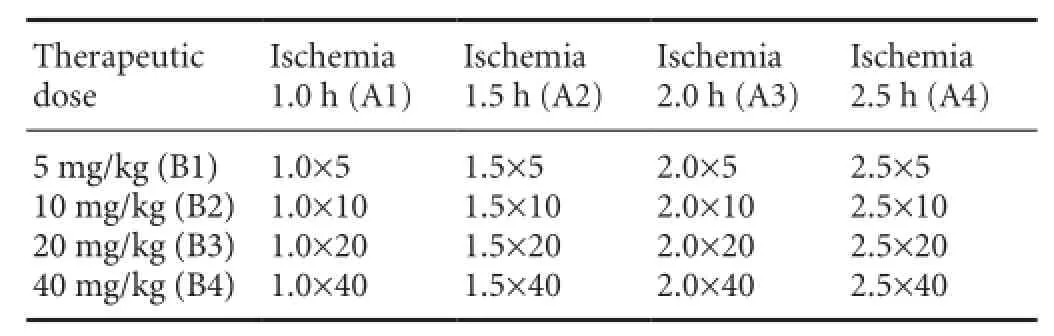
Table 1 Orthogonal experimental design of [L16(45)]
All antibodies and kits were provided by Wuhan Boster Biotechnology Co., Ltd. (Wuhan, Hubei Province, China). Paraffin sections were dewaxed in dimethylbenzene and washed in PBS for 30 seconds, three times, then incubated with rabbit anti-rat NSE primary polyclonal antibody (1:100) for 2 hours at 37°C and with horseradish peroxidase goat anti-rabbit antibody (1:200) for 30 minutes at 37°C using a streptavidin-biotin complex kit, according to the manufacturer’s instructions. Staining was visualized using DAB for 30 seconds and the sections were counterstained with hematoxylin for 5 seconds at room temperature. Immunopositive cells appeared as brown particles under a light microscope (Olympus IX141). Negative control sections were incubated with 0.01 mol/L PBS instead of NSE primary antibody and no positive reaction was found. Five randomly-chosen, non-overlapping visual fi elds in the parietal cortex were observed in each section at 400 × magni fi cation under a light microscope to calculate the positive cells. Positive cell index (number of positive cells/total cells in the visual fi eld) was used to determine NSE expression.
Flow cytometry
Apoptosis was determined in the samples by fl ow cytometry, using an Annexin V-FITC apoptosis detection kit (Nanjing Keygen Biotechnology Co., Ltd., Nanjing, Jiangsu Province, China) and FACSCalibur system (Becton Dickinson Co., Ltd., New Jersey, USA) with an excitation wavelength of 488 nm and emission at 535 nm and 575 nm. Early apoptotic ratio was analyzed using FlowJo 7.6 software (TreeStar, San Carlos, CA, USA).
Western blot analysis
Rats were randomly selected and anesthetized as described above. Ischemic brain tissue (200 mg) was harvested from the parietal cortex and placed into a 1.5 mL Eppendorf tube, and mixed with cell lysis buffer (No. P0013, Biyuntian Biotech Co., Ltd., Beijing, China) at a ratio of 1:4. The mixture was ground and homogenized ultrasonically at -4°C in an ice bath, and centrifuged (Eppendorf 5801, Hamburg, Germany) at 10,949 ×gfor 10 minutes at 4°C. The protein concentration in the supernatant was determined using the BCA-100 protein quantitation kit (Shenneng Biotech. Co., Ltd., China), and samples were stored at -20°C until protein separation. NSE proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (5% stacking gel at 120 V followed by 12% gel at 75 V) and transferred onto a polyvinylidene di fl uoride membrane (40 minutes at 360 mA). The membrane was rinsed with PBS and Tween-10, three times for 5 minutes each time, then incubated with rabbit anti-rat NSE primary monoclonal antibody (1:500; Ab53025, Abcam, Hong Kong, China) and horseradish peroxidase goat anti-rabbit antibody (1:10,000; ZB-2301, Beijing Golden Bridge Biotechnology Co., Ltd., Beijing, China) for 2 hours at 37°C. Proteins were visualized using enhanced chemiluminescence reagents (GE Healthcare Japan, Tokyo, Japan) and scanned in a Bio-Rad 2000 gel imaging system (Bio-Rad, Hercules, CA,USA). Gray values of the protein bands were analyzed using Quantity One software (Bio-Rad). Gray value (pixel intensity) was used to quantify protein content, and the value for each sample was normalized against that of β-actin (42 kDa) as an internal control. The relative value of target protein was calculated as follows: gray value of NSE/gray value of β-actin. The experiment was repeated three times.

Table 2 Primers of target gene neuron-speci fi c enolase (NSE) and GAPDH
RT-PCR
Rats were randomly selected and anesthetized as described above, 24 hours after injection. Ischemic brain tissue (200 mg) was harvested from the parietal cortex and placed into a 1.5 mL Eppendorf tube with 1 mL RNA-Solv Reagent (Omega Bio-Tek. Inc, Norcross, GA, USA), and the sample was minced and ground. The mixture was oscillated ultrasonically for 30 seconds, incubated for 5 minutes at room temperature, and centrifuged for 15 minutes (4°C, 12,000 ×g). The supernatant was transferred to a new Eppendorf tube containing 0.2 mL chloroform, shaken for 15 seconds, and placed on ice. After 10 minutes, the supernatant was collected into a fresh EP tube and centrifuged for 15 minutes (4°C, 12,000 ×g), and 0.5 mL isopropyl alcohol was added and gently mixed. The supernatant was placed on ice for 10 minutes, centrifuged (4°C, 12,000 ×g) for 15 minutes and discarded. The precipitate was washed using 1 mL of 75% alcohol, mixed and centrifuged (4°C, 7,500 ×g) for 5 minutes, and then the supernatant was carefully discarded. The precipitate was dried for 30 minutes under a fume hood, 30 μL of 0.1% diethylpyrocarbonate-treated water was added, and the sample was placed in a water bath at 57°C for 10 minutes. The purity and quantity of RNA were determined with an ultraviolet spectrophotometer (Bekaman DU640, Pasadena, CA, USA) and stored at -20°C.
Primer sequences for the target gene (NSE) and internal control (GAPDH) are listed inTable 2. PCR was performed under the following conditions: 95°C for 3 minutes, followed by 30 cycles at 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 40 seconds, and fi nally an extension step at 72°C for 3 minutes. The quantity of mRNA was expressed as the following ratio: gray value of NSE mRNA/gray value of GAPDH mRNA. The experiment was repeated three times.
Statistical analysis
Data are expressed as mean ± SD. SPSS 17.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. One-way analysis of variance was used for the comparison of multiple sets of data, and differences were identi fi ed by the least sig-nificant differencepost-hoctest. Data was considered to be signi fi cant whenP< 0.05.
Results
Overall results
In the control group, neuronal Nissl bodies appeared as dark blue plaques, the karyoplasm was pale blue and the background was pale; in the model group, pyknosis was visible, with nuclei appearing fragmented or even absent; in the treatment group, neuronal morphology was improved compared with the model group (Figure 1).e denatured cell index and positive cell index (Figure 1; Table 3), early apoptotic ratio (Figure 2; Table 3), relative value of protein (Figure 3; Table 3) and relative value of mRNA (Figure 4; Table 3) were signi fi cantly higher in the model group than in the control group (P< 0.05). Aer treatment with picroside II, all indices were signi fi cantly lower than those of the model group (P< 0.05; Figures 1-4; Table 3).
Neuroprotective effect of picroside II in the treatment of cerebral ischemic injury and analysis of the optimal therapeutic dose and time window
Toluidine blue staining
With toluidine blue staining (Figure 1), a significant effect (P< 0.05) on the denatured cell index (Table 4) was observed with impact factor A (time after ischemic injury) but not B (drug dose) or C (time × dose interaction) (P> 0.05).is evidence indicates that the therapeutic time window (or cerebral ischemic time) has a signi fi cant in fl uence on the pathological changes in neurons after cerebral ischemic injury, whereas drug dose does not influence neuronal pathology and there are no interactions between therapeutic time window and drug dose. Signi fi cant di ff erences (P< 0.05) were found between the following groups: 1.0 h (A1) and 1.5 h (A2); 1.0 h (A1) and 2.0 h (A3); 1.0 h (A1) and 2.5 h (A4); 1.5 h (A2) and 2.5 h (A4); 2.0 h (A3) and 2.5 h (A4). No signi fi cant di ff erence was found between 1.5 h (A2) and 2.0 h (A3) (P> 0.05). There was a significant difference (P< 0.05) between the 10 mg/kg (B2) and 40 mg/kg (B4) dose groups, while no signi fi cant di ff erences were found between the other therapeutic doses (P> 0.05). According to the principle of minimizing medication dose and maximizing therapeutic time window, the best combination was revealed as A3B2 (2.0 h/10 mg),i.e., for optimal response, 10 mg/kg picroside II should be injected intraperitoneally at 2.0 hours of cerebral ischemia.
NSE immunohistochemistry
Analysis of NSE expression by immunohistochemistry (Figure 1) showed that di ff erent levels of impact factor A (time) had a statistically signi fi cant e ff ect on the NSE-immunopositive cell index (P< 0.05;Table 4), whereas factors B (dose) and C (interaction) had no statistically signi fi cant e ff ect (P>0.05), indicating that the time to treatment had a signi fi cant e ff ect on NSE expression following cerebral ischemia, whereas the injected dose and the time × dose interaction have no notable effect. Significant differences were found between the following times post-ischemic injury: 1.0 h (A1) and 2.5 h (A4); 1.5 h (A2) and 2.5 h (A4); 2.0 h (A3) and 2.5 h (A4) (P< 0.05). In addition, signi fi cant di ff erences (P< 0.05) were found between the following dose groups: 10 mg/kg (B2) and 40 mg/kg (B4); 20 mg/kg (B3) and 40 mg/kg (B4). According to our comprehensive evaluation, the optimal combination is A3B2 (2.0 h/10 mg),i.e., intraperitoneal injection of 10 mg/kg picroside II at 2.0 hours of cerebral ischemia.
Flow cytometry
Signi fi cant di ff erences in early apoptotic ratio were observed between various levels of impact factors A (time) and B (dose) (P< 0.05), but not in impact factor C (time-dose interaction;P> 0.05) in the fl ow cytometry study (Figure 2).is indicates that the therapeutic time window and the drug dose, but not their interaction, in fl uence the early apoptotic ratio after cerebral ischemia injury (Table 4). Significant di ff erences (P< 0.05) in treatment time were observed between the following groups: 1.0 h (A1) and 2.0 h (A3); 1.0 h (A1) and 2.5 h (A4); 1.5 h (A2) and 2.0 h (A3); 1.5 h (A2) and 2.5 h (A4); and 2.0 h (A3) and 2.5 h (A4). Furthermore, there were signi fi cant di ff erences (P< 0.05) in drug dose in the following groups: 5 mg/kg (B1) and 10 mg/kg (B2); 5 mg/kg (B1) and 40 mg/kg (B4); 10 mg/kg (B2) and 20 mg/ kg (B3); 10 mg/kg (B2) and 40 mg/kg (B4); 20 mg/kg (B3) and 40 mg/kg (B4).erefore, the best combination is A2B2 (1.5 h/10 mg),i.e., for optimal therapeutic bene fi t, picroside II should be injected intraperitoneally at a dose of 10 mg/kg and at 1.5 hours of cerebral ischemia.
Western blot analysis
According to quantitative analysis, NSE expression di ff ered across all groups (Figure 3). Expression of NSE in the treatment group was significantly lower than that in the model group. One-way analysis of variance showed that impact factor A (time), but not factor B (dose) or C (interaction), had a signi fi cant e ff ect on the expression of NSE (P< 0.05), highlighting the important e ff ect of treatment time on NSE expression, while drug dose and time × dose interaction had no notable e ff ect (Table 4). Least signi fi cant di ff erence analysis revealed signi fi cant di ff erences (P< 0.05) between the following treatment times: 1.0 h (A1) and 1.5 h (A2); 1.0 h (A1) and 2.0 h (A3); 1.5 h (A2) and 2.5 h (A4); 2.0 h (A3) and 2.5 h (A4). No notable di ff erences were found between any of the dose groups (P> 0.05).ese results indicate that the optimal combination is A3B2 (2.0 h/10 mg),i.e., an intraperitoneal injection of 10 mg/kg picroside II at 2.0 hours of cerebral ischemia.
RT-PCR
NSE mRNA transcription levels in rat brain tissue were di ff erent in each group, and the expression of NSE mRNA was lower aer treatment than in the model group (Figure 4). Oneway analysis of variance showed that factors A (time), B (dose) and C (time × dose interaction) had no statistically signi fi cant e ff ect on the expression of NSE mRNA (P> 0.05) (Table 4). Least signi fi cant di ff erence results revealed signi fi cant di ff erences (P< 0.05) between the following treatment time groups:1.0 h (A1) and 2.0 h (A3); 2.0 h (A3) and 2.5 h (A4). However, there were no significant differences between dose groups (P> 0.05).us, the optimal combination is A2B2,i.e. 10 mg/ kg picroside at 1.5 hours of cerebral ischemia.
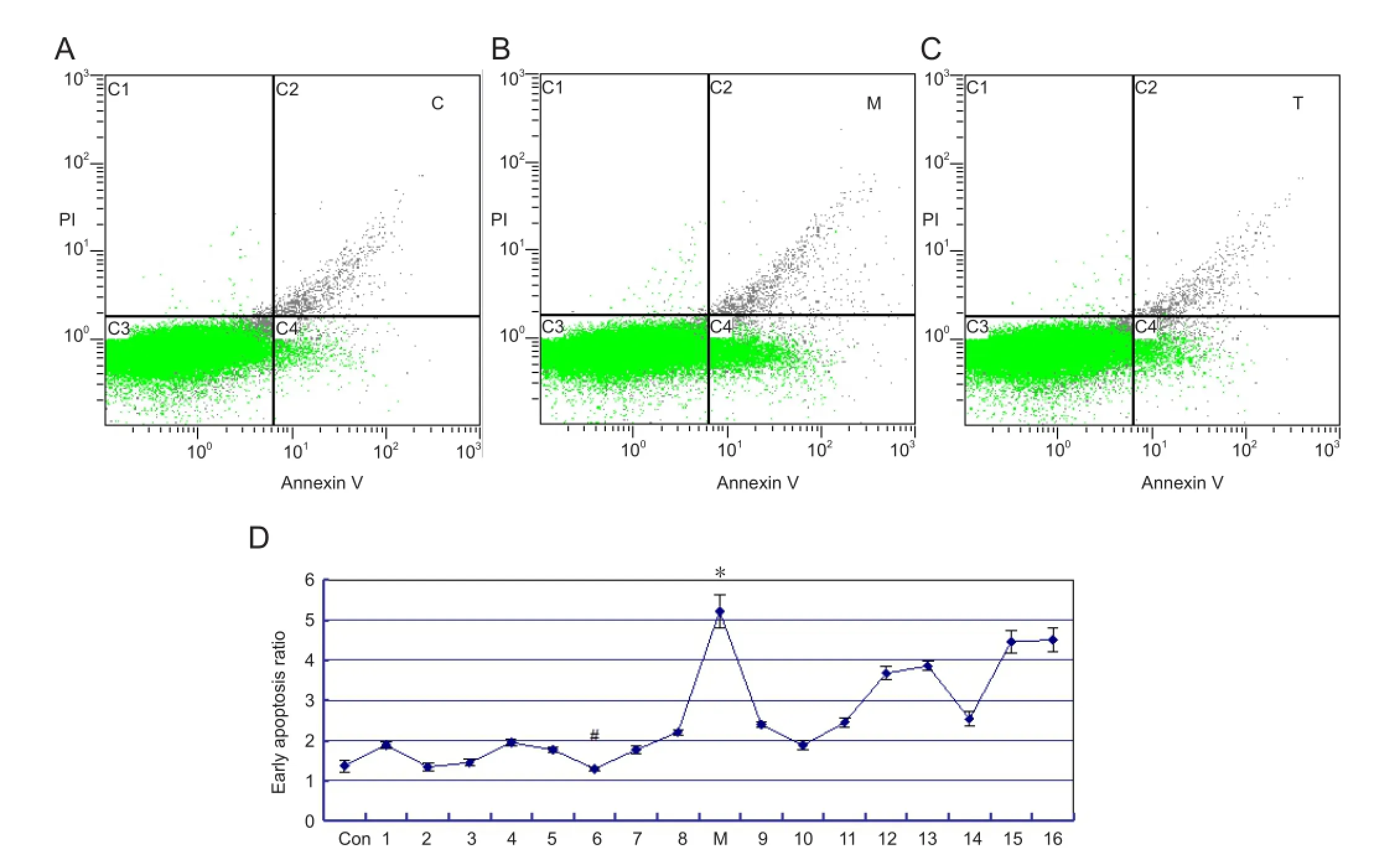
Figure 2 Neuronal apoptosis in the parietal cortex of rats with cerebral ischemia, with or without picroside II treatment ( fl ow cytometry).
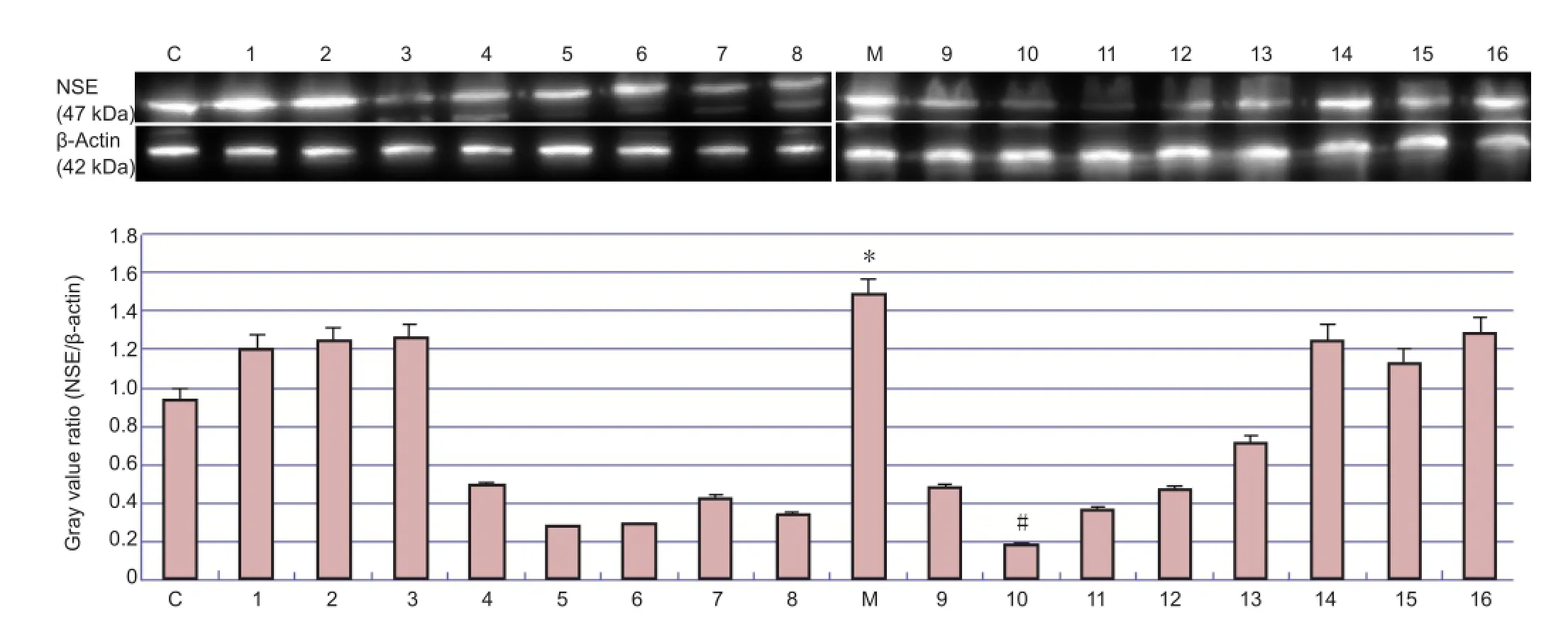
Figure 3 Western blot analysis of neuron speci fi c enolase (NSE) protein expression in parietal cortex of rats with cerebral ischemia, with or without picroside II administration.
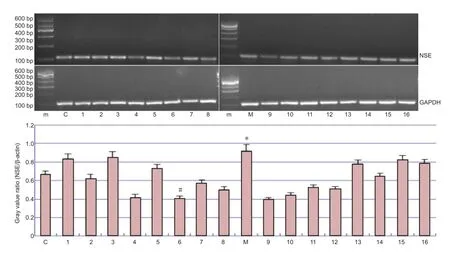
Figure 4 The mRNA expression of neuron speci fi c enolase (NSE) in the parietal cortex of rats with cerebral ischemia, with or without picroside II administration.

Table 4 One-way analysis of variance of denatured cell index (DCI), positive cell index (PCI), early apoptotic ratio (EAR), relative value of protein (RVP) and relative value of mRNA (RVM)
Discussion
The orthogonal design balances sampling across different factors, increasing the statistical representation of each group while reducing the number of necessary tests (Liu et al., 2010). In the present study, we applied the orthogonal layout to the entire experiment to ensure comprehensive comparisons using a smaller number of experiments.
As a key enzyme in the glycolytic pathway and widely distributed in various tissues, enolase catalyzes 2-phosphoglycerate into 2-phosphoenolpyruvate during glucose metabolism. Enolase has fi ve isozymes (αα, ββ, γγ, αβ and αγ). NSE is the γγ isozyme and exists in neurons and neuroendocrine cells (Wu et al., 2004). It is a biological macromolecule with molecular weight of 78 kDa and has stable physicochemical properties (Wu et al., 2004). Normally, the concentration of NSE in brain tissue, cerebrospinal fl uid or blood is low. When hypoxic-ischemic brain damage occurs, however, its expression in brain increases (Hou, 2003). It is different from lactate dehydrogenase, aldolase and creatine kinase, which cannot combine with actin in cells, and can be released readily from ischemic or necrotic cells with the increase of apoptotic neurons and disintegration of the myelin sheath. There are a large number of studies addressing the biological markers of brain injury (for example, Jickling et al., 2011; Whiteley et al., 2011), and many experiments have revealed that the content of NSE increases signi fi cantly after cerebral ischemia injury (Brouns et al., 2010; Kaca-Oryńska et al., 2010; Ji et al., 2012). In a recent clinical trial, it was found that the concentration of NSE in serum within the fi rst 72 hours of acute stroke was signi ficantly higher than that in a control group (Singh et al., 2013),showing that the concentration of NSE in serum is correlated with the severity of neurological injury after acute cerebral ischemia and has high predictive value in the prognosis of neurological function. It was also con fi rmed in patients with ischemic cerebrovascular disease that the rise of NSE in serum is associated with the degree of neuronal necrosis and re fl ects the extent of neuronal ischemic injury; high expression of NSE in serum can prompt ultra-early cerebral infarction (Fan et al., 2011; Huang et al., 2012). Furthermore, the elevation of NSE shows two peaks after cerebral ischemia, the fi rst appearing 7-18 hours after onset, the second in 2-4 days (Al-Rawi et al., 2009). Here, we found that the number of NSE-immunopositive cells, expression of NSE protein, and NSE mRNA transcription level all increased after modeling. NSE can therefore be used as a marker of ischemic brain injury and be valuable in clinical diagnosis.
Picroside II is one of the active ingredients of the traditional Chinese medicine Picrorhizae, the functions of which are to reduce heat, humidity, fever, dampness and steam, cool the blood, and purge bile (Jiangsu New Medical College, Dictionary of Chinese Traditional Drugs, 1996). Cell culture experiments con fi rmed that picroside II could reduce H2O2-induced injury in PC12 cells and improve cell survival (Li et al., 2002a), and its antioxidant effect has also been demonstrated (Li et al., 2007d). Our early animal experiments con fi rmed that picroside II could inhibit the expression of in fl ammatory cytokines, Toll-like receptor 4, nuclear factor κB, caspase-3, and tumor necrosis factor α, as well as the expression of in fl ammatory factors in the cerebral ischemic penumbra after middle cerebral artery occlusion and reperfusion, thus inhibiting neuronal apoptosis induced by ischemia (Guo et al., 2010; Li et al., 2010b, c, e, f). The present study indicates that both the reverse transcription level of NSE mRNA and its protein expression in the ischemia model group were significantly higher than in the control group, showing that NSE can be used as a marker to judge the extent of brain injury. Compared with the model group, Nissl body damage and apoptosis were lower after picroside II treatment, and the expression of NSE protein and reverse transcription of mRNA were also lower to varying degrees. Together, the above evidence shows that picroside II can protect the brain against different levels of ischemic injury. Intervention of picroside II at 1.5-2.0 hours of ischemia had a greater effect on the protection against brain injury than administration at other times, while there was no signi fi cant difference between different doses of picroside II. Therefore, although the optimal dose of picroside II needs further study, our results suggest that the effective therapeutic time window might be more important than the dose used.
Given the principle of obtaining the lowest therapeutic dose with the longest time window, the optimal therapeutic response in a rat model of cerebral ischemia is after an intraperitoneal injection of picroside II with 10 mg/kg body weight, 1.5-2.0 hours after injury.
Acknowledgments:We are grateful for Dr. Guo YL, Head of Institute of Cerebrovascular Diseases, Affiliated Hospital ofQingdao University, China for his professional guidance and his patience in supervisions.
Author contributions:Liu GY and Zhao L designed the study. Zhao L, Wang TT and Pei HT performed the study. Zhao L and Zhang MZ analyzed data. Zhao L wrote the paper. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Al-Rawi NH, Atiyah KM (2009) Salivary neuron specific enolase: an indicator for neuronal damage in patients with ischemic stroke and stroke-prone patients. Clin Chem Lab Med 47:1519-1524.
Anand N, Stead LG (2005) Neuron-specific enolase as a marker for acute ischemic stroke: a systematic review. Cerebrovasc Dis 20:213-219.
Bharosay A, Bharosay VV, Varma M, Saxena K, Sodani A, Saxena R (2012) Correlation of brain biomarker neuron speci fi c enolase (NSE) with degree of disability and neurological worsening in cerebrovascular stroke. Indian J Clin Biochem 27:186-190.
Brea D, Sobrino T, Blanco M, Cristobo I, Rodríguez-González R, Rodríguez-Ya?ez M, Moldes O, Agulla J, Leira R, Castillo J (2009) Temporal pro fi le and clinical signi fi cance of serum neuron-speci fi c enolase and S100 in ischemic and hemorrhagic stroke. Clin Chem Lab Med 47:1513-1518.
Brouns R, De Vil B, Cras P, De Surgeloose D, Mari?n P, De Deyn PP (2010) Neurobiochemical markers of brain damage in cerebrospinal fl uid of acute ischemic stroke patients. Clin Chem 56:451-458.
Fan YQ (2011) Clinical signi fi cance to detect 6-keto-PGF1α and NSE in Neonatal hypoxia-ischemic encephalopathy. Zhonghua Quanke Yixue 9:1952-1953.
González-García S, González-Quevedo A, Fernández-Concepción O, Pe?a-Sánchez M, Menéndez-Saínz C, Hernández-Díaz Z, Arteche-Prior M, Pando-Cabrera A, Fernández-Novales C (2012) Short-term prognostic value of serum neuron specific enolase and S100B in acute stroke patients. Clin Biochem 45:1302-1307.
Guo Y, Xu X, Li Q, Li Z, Du F (2010) Anti-in fl ammation e ff ects of picroside II in cerebral ischemic injury rats. Behav Brain Funct 6:43-53.
Guo YL, Shen W, Du F, Li Q, Li Z (2011)e interference of picroside II on the expressions of TLR4 and NFκB following cerebral ischemia reperfusion injury in rats. Chin J Integr Med 31:58-61.
Hein Née Maier K, K?hler A, Diem R, S?ttler MB, Demmer I, Lange P, B?hr M, Otto M (2008) Biological markers for axonal degeneration in CSF and blood of patients with the fi rst event indicative for multiple sclerosis. Neurosci Lett 436:72-76.
Hou L, Pu HP, Shao BQ (2003) Effects of ginkgo biloba extract on expressions of NSE S-100 mRNA in newborn rat brain with hypoxic-ischemic brain damage. Chin Phar Bull 19:100-102.
Huang WX, Li YQ, Liu JY, Huang JS (2012)e expression and clinical significance of neurone specific enolase in patients with ischemic cerebral vascular disease. Zhonghua Quanke Yixue 10:1225-1226.
Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR; NINDS rt-PA Stroke Study Group (2006) Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke 37:2508-2513.
Ji YB, Wu YM, Ji Z, Song W, Xu SY, Wang Y, Pan SY (2012) Interrupted intracarotid artery cold saline infusion as an alternative method for neuroprotection aer ischemic stroke. Neurosurg Focus 33:E10.
Jiangsu new medical college Chinese medicine dictionary editorial (1996) Traditional Chinese medicine dictionary (part II). Shanghai: Shanghai Science and Technology Press 1548-1550.
Jickling GC, Sharp FR (2011) Blood biomarkers of ischemic stroke. Neurotherapeutics 8:349-360.
Kaca-Oryńska M, Tomasiuk R, Friedman A (2010) Neuron-specific enolase and S 100B protein as predictors of outcome in ischemic stroke. Neurol Neurochir Pol 44:459-463.
Li P, Matsunaga K, Yamakuni T, Ohizumi Y (2002a) Picrosides I and II, selective enhancers of the mitogen-activated protein kinase-dependent signaling pathway in the action of neuritogenic substances on PC12D cells. Life Sci 71:1821-1835.
Li Q, Guo YL, Li Z, Xu XY (2010b)e interference of picroside II on the expressions of Caspase-3 and PARP following cerebral ischemia reperfusion injury in rats. Chin Phar Bull 26:342-345.
Li Q, Li Z, Xu XY, Du F (2010c) Neuroprotective properties of picroside II in rat model of focal cerebral ischemia. Int J Mol Sci 11:4580-4590.
Li T, Liu JW, Zhang XD, Guo MC, Ji G (2007d) The neuroprotective e ff ect of picroside II from hu-huang-lian against oxidative stress. Am J Chin Med 35:681-691.
Li Z, Li Q, Guo YL, Qin LH, Luan LJ (2010e) Intervention e ff ect of picroside II in cerebral ischemic injury rats. Acta Anat Sin 41:9-12.
Li Z, Li Q, Shen W, Guo YL (2010f)e interference of picroside II on the expressions of NFκB and IκB following cerebral ischemia reperfusion injury in rats. Chin Phar Bull 26:52-55.
Lima JE, Takayanagui OM, Garcia LV, Leite JP (2004) Use of neuron-specific enolase for assessing the severity and outcome in patients with neurological disorders. Braz J Med Biol Res 37:19-26.
Liu RJ, Zhang XY, Wen CW, Tang J (2010)e study of orthogonal experiment design and analysis method. Shiyan Jishu yu Guanli 27:52-55.
Marquardt G, Setzer M, Szelenyi A, Seifert V, Gerlach R (2009) Prognostic relevance of serial S100B and NSE serum measurements in patients with spinal intradural lesions. Neurol Res 31:265-269.
Márquez-Martín A, Jiménez-Altayó F, Dantas AP, Caracuel L, Planas AM, Vila E (2012) Middle cerebral artery alterations in a rat chronic hypoperfusion model. J Appl Physiol 112:511-518.
Meng FJ, Hou ZW, Li Y, Yang Y, Yu B (2012)e protective e ff ect of picroside II against hypoxia/reoxygenation injury in neonatal rat cardiomyocytes. Pharm Biol 50:1226-1232.
Oksanen T, Tiainen M, Skrifvars MB, Varpula T, Kuitunen A, Castrén M, Pettil? V (2009) Predictive power of serum NSE and OHCA score regarding 6-month neurologic outcome aer of hospital ventricular fi brillation and therapeutic hypothermia. Resuscitation 80: 165-170.
Pei HT, Su X, Zhao L, Li H, Guo YL, Zhang M, Xin H (2012) Primary study for the therapeutic dose and time window of picroside II in treating cerebral ischemic injury in rats. Int J Mol Sci 13:2551-2562.
Saenger AK, Christenson RH (2010) Stroke biomarkers: progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin Chem 56:21-33.
Selakovic V, Raicevic R, Radenovic L (2005) The increase of neuron-speci fi c enolase in cerebrospinal fl uid and plasma as a marker of neuronal damage in patients with acute brain infarction. J Clin Neurosci 12:542-547.
Sienkiewicz-Jarosz H, Ga?ecka-Wolska M, Bidziński A, Turzyńska D, Sobolewska A, Lipska B, P?a?nik A, Ryglewicz D (2009) Predictive value of selected biochemical markers of brain damage for functional outcome in ischemic stroke patients. Neurol Neurochir Pol 43:126-133.
Singh HV, Pandey A, Shrivastava AK, Raizada A, Singh SK, Singh N (2013) Prognostic value of neuron speci fi c enolase and IL-10 in ischemic stroke and its correlation with degree of neurological deficit. Clin Chim Acta 419:136-138.
van Munster BC, Korse CM, de Rooij SE, Bonfrer JM, Zwinderman AH, Korevaar JC (2009) Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC Neurol 9:21.
Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C, van Geel W, de Reus H, Biert J, Verbeek MM (2004) Glial and neuronal proteins in serum predict outcome aer severe traumatic brain injury. Neurology 62:1303-1310.
Whiteley W (2011) Identifying blood biomarkers to improve the diagnosis of stroke. J R Coll Physicians Edinb 41:152-154.
Whiteley W, Chong WL, Sengupta A, Sandercock P (2009) Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke 40:e380-389.
Wu YC, Zhao YB, Lu CZ, Qiao J, Tan YJ (2004) Correlation between serum level of neuron-speci fi c enolase and long-term functional outcome aer acute cerebral infarction: prospective study. Hong Kong Med J 10:251-254.
Wunderlich MT, Lins H, Skalej M, Wallesch CW, Goertler M (2006) Neuron-speci fi c enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and longterm outcome in acute ischemic stroke. Clin Neurol Neurosurg 108:558-563.
Copyedited by Murphy S, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
Guangyi Liu, Institute of Cerebrovascular Diseases, Affiliated Hospital of Qingdao University, Qingdao 266003, Shandong Province, China, 15898877166@163.com.
10.4103/1673-5374.139460
http://www.nrronline.org/
Accepted: 2014-05-06
- 中國神經(jīng)再生研究(英文版)的其它文章
- Changes in cortical activation patterns accompanying somatosensory recovery in a stroke patient: a functional magnetic resonance imaging study
- Changes in brain activation in stroke patients after mental practice and physical exercise: a functional MRI study
- Integration of animal behaviors under stresses with different time courses
- A feasible strategy for focal cerebral ischemiareperfusion injury: remote ischemic postconditioning
- Pretreatment with Danhong injection protects the brain against ischemia-reperfusion injury
- Neuroprotective effect of pretreatment with ganoderma lucidum in cerebral ischemia/reperfusion injury in rat hippocampus