Current insights into gonadotropic pituitary function in the polycystic ovary syndrome
Cristianne Serafim da Silva Feuser, Jacklyne Silva Barbosa, Evelyn Barzotto da Silva, Sebasti?o Freitas de Medeiros,*
1Department of Obstetrics and Gynaecology, Faculty of Medicine, Federal University of Mato Grosso, Cuiabá - MT, Brazil
2Tropical Institute of Reproductive Medicine and Menopause, Cuiabá, MT, Brazil
Current insights into gonadotropic pituitary function in the polycystic ovary syndrome
Cristianne Serafim da Silva Feuser1, Jacklyne Silva Barbosa2, Evelyn Barzotto da Silva2, Sebasti?o Freitas de Medeiros1,2*
1Department of Obstetrics and Gynaecology, Faculty of Medicine, Federal University of Mato Grosso, Cuiabá - MT, Brazil
2Tropical Institute of Reproductive Medicine and Menopause, Cuiabá, MT, Brazil
ARTICLE INFO
Article history:
Received 16 December 2013
Received in revised form 18 December 2013
Accepted 18 December 2013
Available online 20 January 2014
Polycystic ovary syndrome
Objective: To assess the gonadotrope cell function in patients with polycystic ovary syndrome (PCOS). Methods: This study included 42 patients with polycystic ovary syndrome, aged 26.6± 5.9 years, and 13 eumenorrheic controls, aged 30.1± 4.6 years. Gonadotropins, sex steroids, sex hormone binding globulin (SHBG), cortisol, thyroid stimulating hormone (TSH), prolactin, free thyroxin, and insulin were measured. Gonadotrope cell function was assessed at baseline and at 30 and 60 min after gonadotropin releasing hormone (GnRH) injection. Results: Luteinizante hormone (LH), prolactin (PRL), total testosterone (T), androstenedione (A), and 17- hidroxyprogesterone (17-OHP4) levels were higher (P<0.05) and SHBG levels was lower (P<0.05) in PCOS patients. The LH response to GnRH stimulation was higher in PCOS patients than in controls (P<0.05). Positive correlations between progesterone (P4) and LH at baseline or the area under the curve (AUC) of LH was found only in PCOS patients. No association was found between estradiol (E2), insulin, T, and A concentrations with the LH increment in any group (P>0.05). Positive correlation between insulin and the AUC of LH was identified in controls. Conclusion: The current study confirms the hypersecretion of LH in PCOS patients after the newest diagnostic systematization. The gonadotrope response to GnRH stimulation in PCOS patients was associated with the baseline levels of LH, P4, insulin.
1. Introduction
The polycystic ovary syndrome (PCOS), which affects between 5% and 10% of women of reproductive age, is associated with increased risk for insulin resistance, hyperinsulinemia, central obesity, gestational diabetes, arterial hypertension, type 2 diabetes mellitus (T2DM), dyslipidemia and cardiovascular disease [1]. According to the latest consensuses, PCOS can be diagnosed when a woman presents oligovulation or anovulation, clinical or biochemical hyperandrogenism, polycystic ovary morphology, or at least two of these parameters. In addition, other causes of hyperandrogenism and menstrual disorders such as hyperprolactinemia, thyroid dysfunction, ovarian or adrenal androgen secreting tumors, Cushing syndrome, and late-onset congenital adrenal hyperplasia must be excluded [2,3].
The pathophysiology of PCOS remains not fully understood. The mechanisms proposed are primary hypothalamic abnormal gonadotrophic releasing hormone (GnRH) pulsatile secretion, excess of luteinizing hormone (LH), increased ovarian or adrenal androgen production, increased catabolism of cortisol, defects in insulin action or production,hyperleptinemia, and genetic polymorphisms [4]. A classic and central feature is an increased pulsatility of GnRH release with LH hypersecretion and increased LH/ follicle stimulation hormone (FSH) ratio [5]. Early studies have shown the existence of these pathophysiologic abnormalities in 35%-90% of PCOS patients [6,7]. High serum levels of LH, commonly seen in PCOS, can occur either by increased amplitude/ frequency of GnRH pulses, or increased gonadotrope sensitivity to a physiological GnRH stimulation [4,5]. At hypothalamic level there seems to be a decreased sensitivity of the GnRH pulse generator to the inhibitory action of the gonadal steroids, or changes in other local modulators of GnRH neurons [8].
After 1990, with the introduction and standardization of the new criteria for diagnosing PCOS, it became important to review the early laboratory and clinical data. So, the hypothalamic-pituitary function in PCOS patients diagnosed according to the new criteria of inclusion and exclusion should be reassessed. It is possible that studies performed before NIH/Rotterdam standardization may have included a more heterogeneous population and the results concerning the pituitary function could not be the same of those obtained after 1990 [2,3]. The current study was proposed to assess the gonadotrope function in PCOS patients diagnosed according the current established criteria, both at baseline condition and after GnRH stimulation. It was considered that the results of older studies may have been contaminated with patients in whom hyperandrogenic non-PCOS clinical conditions could modify the gonadotrope function.
2. Materials and methods
The study, approved by the Research Ethics Committee of Federal University of Mato Grosso, was conducted at the Júlio Müller University Hospital and Tropical Institute of Medicine Reproductive and Menopause, Cuiabá, MT, Brazil, between 2010 and 2012. Written informed consent was obtained from each patient. The sample size of 45 patients was calculated considering LH levels of 4.6 mIU/mL, standard deviation of 2.4 mIU/mL, variance of 5.7 mIU/mL [9] and an imprecision of 5%. Calculation was performed using the equation n = 2 (Zα + Zβ)2Var x /(d)2, where Zα=1.96; Zβ=0.84, Var: LH variance, d = 2 mIU as the presumed difference between groups [10]. Three patients were lost before the GnRH test. Forty two patients, aged 26.6 ± 5.9 years, with PCOS diagnosed according to the NIH/Rotterdam criteria, completed the study. Considering the comprehensive difficulty to include normal controls, 13 healthy normal cycling women, aged 30.1 ± 4.9 years (P=0.030), were submitted to the same investigation procedures for comparison.
Anthropometric parameters were measured as described elsewhere [11,12]. Blood samples for hormone measurement were collected between the third and fifth days of a spontaneous menstrual flow or, in case of amenorrhea, at any day, regardless of time elapsed since the last menstrual period; in this case, progesterone (P4) level was measured to certify that the sample was collected in the follicular phase and the results were validated whenever P4 levels were ≤ 2.0 ng/mL (≤ 6.3 nmol/L) [12].
The hormones were measured by well-established and validated assays. P4 levels were measured using chemiluminescence (Advia Centaur, Siemens Healthcare Diagnostics, UK) with a sensitivity of 0.67 nmol/L (0.21 ng/mL), coefficients of intra- and interassay were between 2.6%-3.9% and 3.7%-12.4%, respectively. Concentrations of LH, FSH, thyroid stimulating hormone (TSH), estradiol (E2), total testosterone (T), prolactin (PRL), sex hormone binding globulin (SHBG) and free thyrosine were measured by electrochemiluminescence (ECLIA) (Elecsys 2010, Roche Diagnostics GmbH, Mannheim, Germany). Androstenedione (A), cortisol and insulin were measured using chemiluminescence (Siemens Medical Solutions Diagnostics, CA, USA), and 17-hydroxyprogesterone (17-OHP4) was measured by radioimmunoassay (Coat-a-Count, Diagnostic Products Corp., Los Angeles, CA). Intra-assay and interassay coefficients of variation for all hormones were published elsewhere [12].
The gonadotropin releasing hormone (GnRH) test was performed between 8:00 and 9:00 am after 12 hours fasting. Blood samples for LH, FSH, E2 and P4 measurement were taken before, and 30, and 60 min after the injection of 100 μg of synthetic GnRH (RelefactR, Aventis Pharma BV, Hoevelaken, The Netherlands). The early and delayed gonadotrope responsiveness to GnRH stimulation were assessed using the maximum increase (Δ) in LH and FSH levels, defined by the difference between the baseline and the maximum values, divided by the baseline values. In addition, the areas under the curves (AUC) of LH and FSH, calculated by the trapezoidal rule, with inclusion of the baseline values were also considered. The pituitary sensitivity was defined simply by the baseline concentrations of LH and FSH as a baseline physiological response to the normal hypothalamic pulsatile GnRH stimulation. The pituitary reserve was defined as the maximum increment occurred in LH and FSH concentrations after the in bolus GnRH stimulation. The pituitary capacity was defined by the AUC considering the total amount of gonadotropins secreted by the gonadotrope at baseline conditions plus the gonadotropins released after the GnRH stimulation.
The variables with normal distribution are presented as mean (x ) and standard deviation (SD); variables with non-parametric distribution are presented by median and 95% confidence interval (CI). The student t test was used to compare variables with normal distribution and the Mann-Whitney test for nonparametric comparison. Nominal variables were examined using the chi-square test. Relationships between variables with normal distribution were assessed using the Pearson’s correlation coefficient (r), and those with non-parametric distributions were comparedusing the Spearman’s rank correlation coefficient (rho). AUC was calculated using the trapezoidal role, P values < 0.05 were considered statistically significant.
3. Results
The comparison of anthropometric characteristics between PCOS patients and non-PCOS women are shown in Table 1. The hormone concentrations found in PCOS and non-PCOS subjects are compared in Table 2. Baseline levels of FSH, E2, P4, TSH, free thyroxine, insulin, and cortisol were similar in both groups. Luteinizing hormone, LH:FSH ratio, PRL, T, A and 17-OHP4 were higher in PCOS patients (P<0.050). The baseline physiological gonadotrope secretion in response to GnRH pulsatile stimulation (sensitivity) was as follows: 7.5 mIU/mL of LH in PCOS patients and 4.1 mIU/mL in non-PCOS women (P=0.010); FSH levels of 5.2 mIU/mL in PCOS and 3.9 mIU/mL in normal women (P=0.400). The gonadotrope reserve, taken as the maximum increment in LH and FSH concentrations after the administration in bolus of GnRH, is depicted in Figure 1. At 30 min after the concentrations of LH increased 341%, (from 7.5 mIU/mL to 33.8 mIU/mL; 95% CI-24.2 to 43.3) in PCOS patients, and 220% (from 4.1 mIU/mL to 15. 0 mIU/mL; 95% CI - 11.9 to 18.1) in normal cycling women (P< 0.001). Even at 60 min after GnRH injection, there was no difference in the increment of FSH between PCOS and normal women: increase of 49% (5.2 to 7.9 mIU/mL) in PCOS and of 61% (3.9 mIU/mL to 7.1 mIU/mL) in normal women (P= 0.850) (Figure 1). The gonadotrope capacity for LH secretion, given by the AUC, was higher in PCOS patients (1 569 mUI/mL, 60 min; 95% CI: 1 151-1 987) than in non-PCOS women (679.5 mIU/mL, 60 min; 95% CI 528.2 to 830.8) (P<0.001). The gonadotrope capacity for FSH secretion was 391.5 mUI/mL, 60 min (95% CI: 304.2 to 478.8) in the PCOS group and 377.0 mUI/mL, 60 min (246.3 to 507.7; 95% CI) in non-PCOS women (P= 0.850).
Concerning the gonadotrope capacity it was demonstrated that the baseline levels of estradiol did not influence the gonadotrope response to GnRH neither in PCOS patients (r= -0.029, P= 0.859) nor in non-PCOS women (r= 0.183, P= 0.548) but the baseline levels of P4 were positively associated with the LH response in PCOS patients (r= 0.460, P<0.050) (Figure 2). The gonadotrope capacity in secreting LH was not influenced by the baseline levels of insulin in PCOS patients (r=-0.22, P=0.210), but insulin was positively correlated with the gonadotrope secretion of LH in normal women (r=0.74, P<0.010). There also was a negative correlation between BMI and the capacity of the gonadotrope to secrete LH in PCOS patients (r=-0.28, P=0.050). There were no relationship between the baseline concentrations of T, A, dehydroepiandrosterone sulfate (DHEAS) and free androgen index (FAI), and the capacity of the gonadotrope to secrete gonadotropins in both PCOS and non-PCOS subjects (P>0.050).
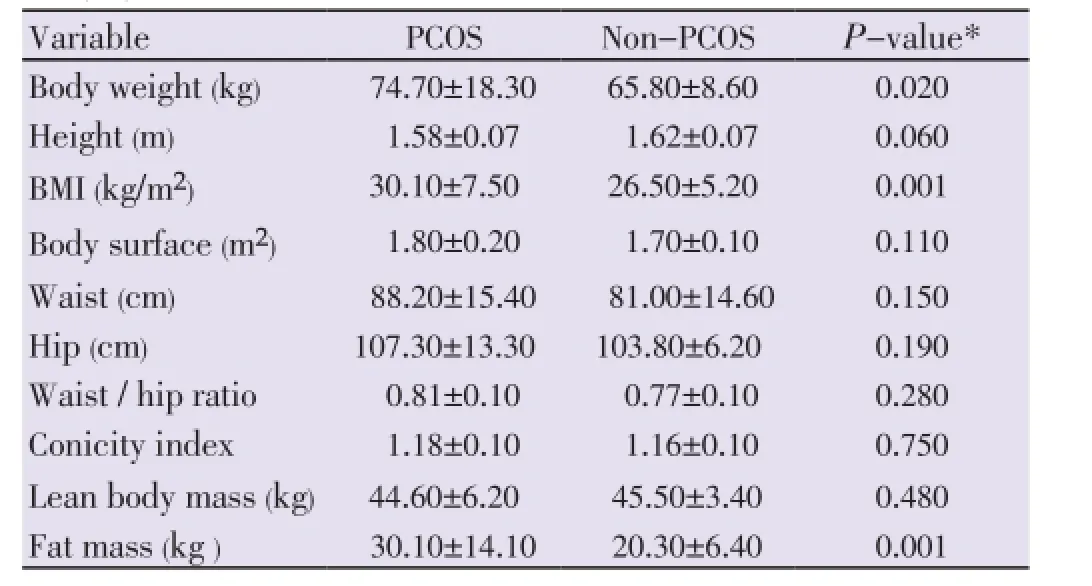
Table 1Anthropometric characteristics of patients with and without polycystic ovary syndrome.
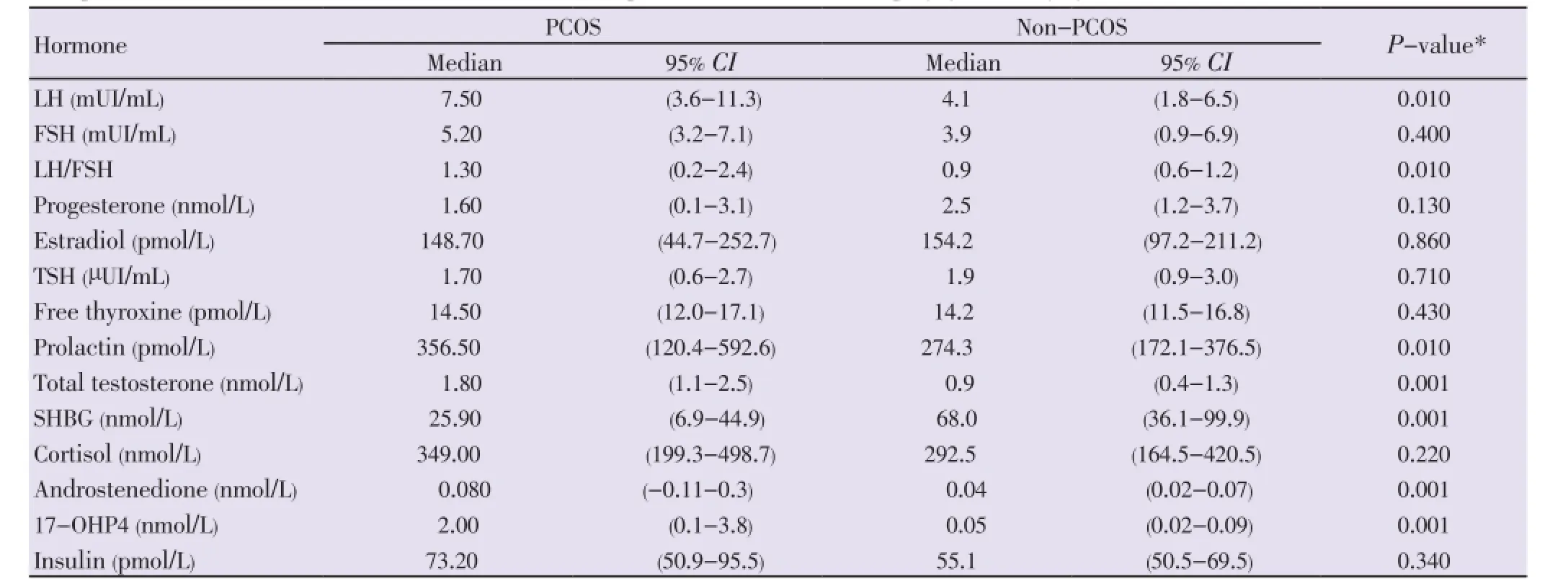
Table 2Comparison of baseline hormone concentrations between patients with and without polycystic ovary syndrome.
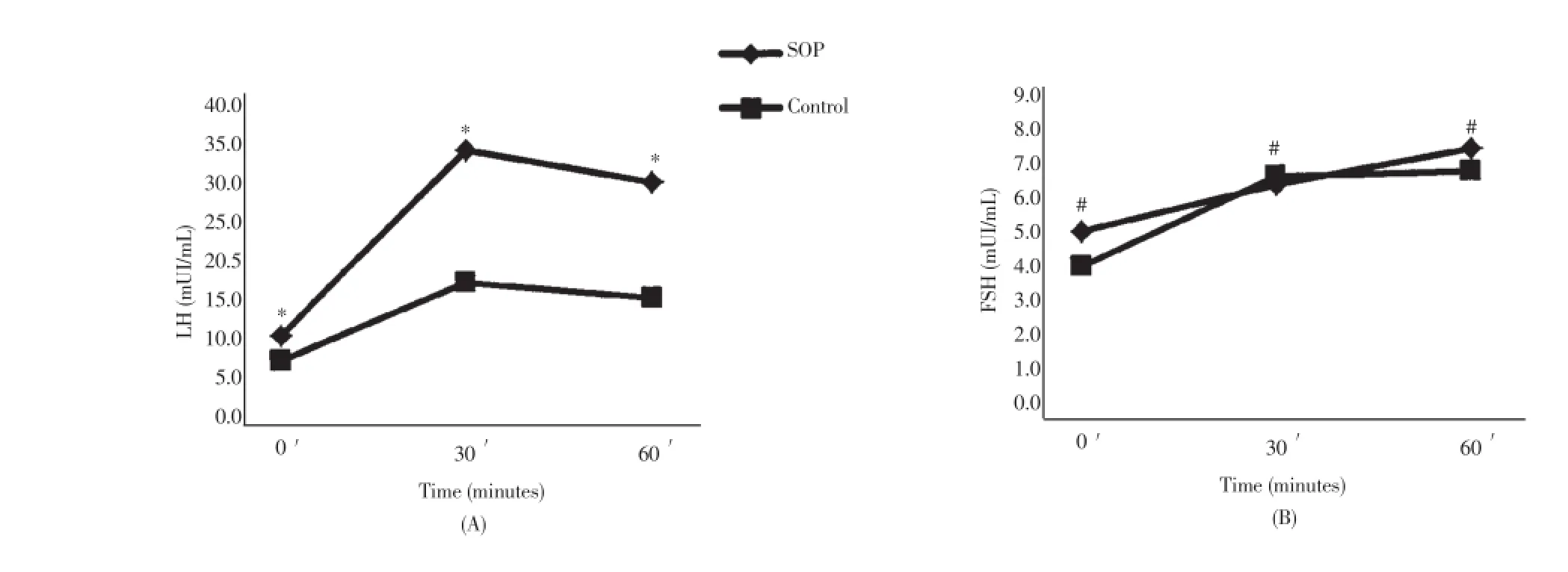
Figure 1. Pituitary response at baseline and at 30 and 60 min after GnRH administration.*P<0.01;#P>0.05.
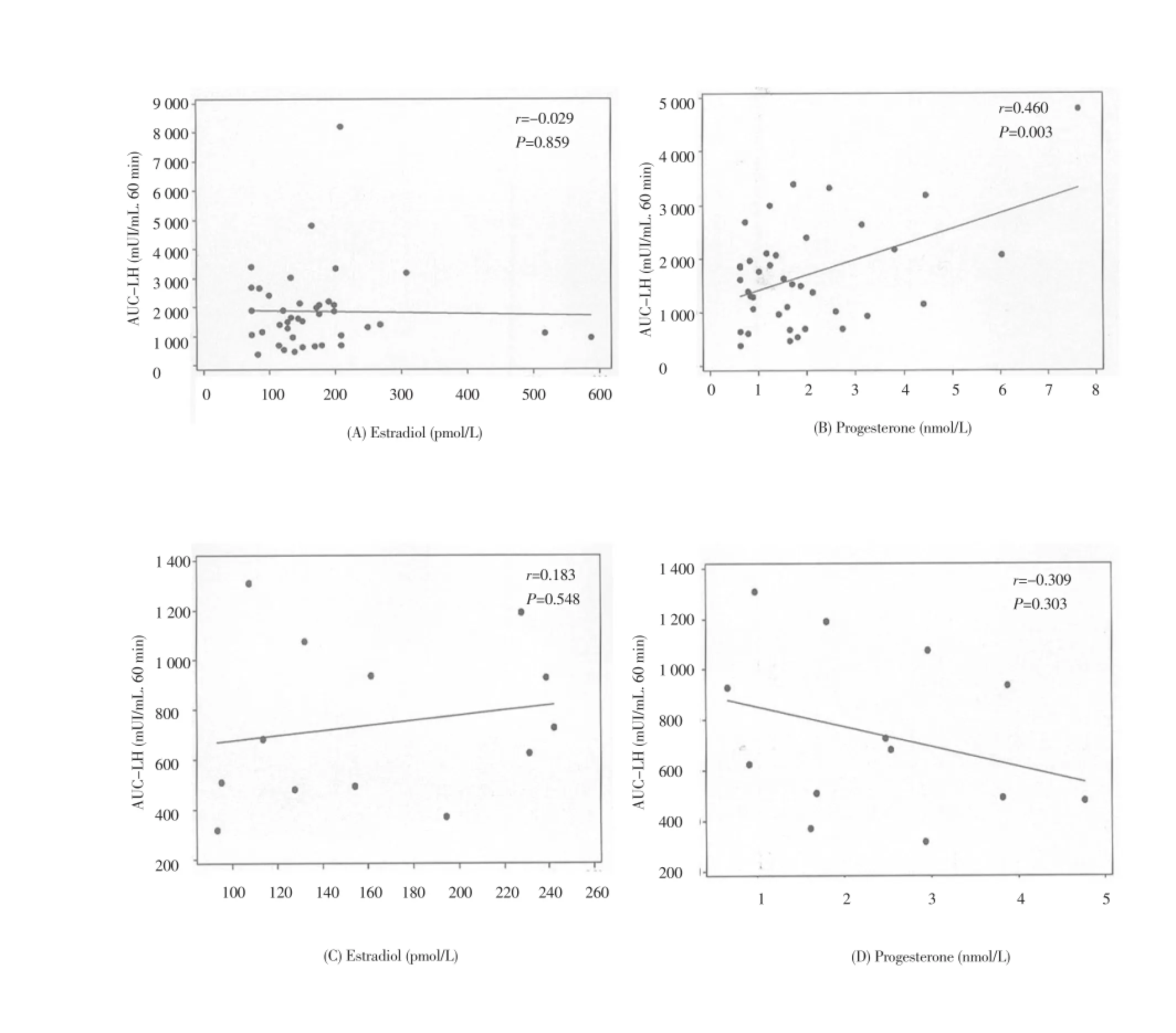
Figure 2. Correlation between estradiol and progesterone baseline levels and area under the curve of LH response to GnRH in PCOS and non-PCOS subjects.
4. Discussion
The current study reassessed the gonadotrope function in PCOS patients after the introduction of the new diagnostic criteria, and compared the findings between PCOS patients and normal cycling non-PCOS women. In addition, it compared the current findings with those studies published before NIH/Rotterdam diagnostic systematization. In the present study, PCOS and non-PCOS subjects presented different body weight, BMI, and fat mass, and equal body surface area, waist circumference, waist: hip ratio, and conicity index. As expected the baseline levels of LH, LH:FSH ratio, total testosterone, androstenedione, and 17-OHP4 were higher in PCOS patients. Cortisol, insulin, estradiol, and progesterone baseline concentrations were similar in PCOS and non-PCOS subjects. The gonadotrope secretion of LH in response to GnRH stimulation was higher in PCOS patients. In the PCOS patients the gonadotrope secretion of LH was positively associated with the progesterone baseline concentrations and negatively associated with the insulin concentrations and BMI.
Although a study similar to the current one became necessary after the PCOS diagnostic standardization, the present study may have some limitations. Firstly, PCOS and non-PCOS subjects were not matched for body weight and this factor may limit the comparison of the influence of BMI on LH secretion in both groups. Second, the definition of pituitary sensitivity, reserve and capacity is somewhat arbitrary and may not be universally adopted. Finally, the main concern with the imprecise androgen measurement in women was attenuated by the inclusion of non-PCOS controls for comparison. Even PCOS and non-PCOS patients presented different body weight, total fat mass and BMI, both groups had similar adipose tissue distribution: equal waist circumference, waist:rip ratio and conicity index. As the central adiposity is the major feature that could influence the pituitary function, the differences seen in gonadotropin secretion in PCOS and non-PCOS in the present study are reliable. It is well established that the baseline levels of LH in PCOS patients are correlated with GnRH pulses [6,8] but because it is less invasive and expensive to use the baseline levels of LH to assess the gonadotrope response and function than to collect multiple blood samples for registration of LH pulses, the present study used baseline concentrations of LH as surrogate of LH pulses to infer about hypothalamic/ pituitary function.
LH concentrations appear to be inversely correlated with obesity in studies performed before [13] and after [7,14] NIH/ Rotterdam diagnostic standardization, despite the elevated insulin concentrations reported in many of them. Currently, BMI has been negatively associated with the baseline levels of LH [15], LH pulse amplitude [16], and the pituitary response to GnRH [17]. The negative correlation between BMI, baseline levels of LH and AUC of LH response (gonadotrope capacity) in PCOS patients, observed in the present study, supports studies published before [13] and after NIH/Rotterdam [15]. In fact, it is knowledge that BMI attenuates LH pulse amplitude and secretion in PCOS patients, but not in normal ovulatory women by a direct effect, or as yet undefined factor, associated with obesity at the pituitary level [15]. Other factors, such as leptin, growth factors, and cytokines may also have autocrine/paracrine participation on gonadotrope response to GnRH in obese PCOS patients.
The gonadotrope sensitivity, expressed by the baseline secretion of gonadotropins in the current study, was significantly higher in the secretion of LH in PCOS patients. This observation is not novel and supports the results of other studies performed before the establishment of the current exclusion criteria for PCOS diagnosis [3,4]. Therefore, the current findings indicate either that other hyperandrogenic clinical conditions such as thyroid dysfunction, hyperprolactinemia, and non-classic adrenal hyperplasia do not modify significantly the gonadotrope function, when they are compared with PCOS. Other possibility is that the older studies were not contaminated with these conditions in on extension capable of modifying the gonadotrope function.
The existence of increased gonadotrope response to GnRH stimulation in PCOS observed in the present study also corroborates with the findings of studies published both before [18] and after [19] the introduction of the new diagnostic criteria. The higher reserve, and capacity, of the gonadotrope cells to release LH molecules in PCOS patients after GnRH acute stimulation compared with non-PCOS subjects, indicate differential expression of LHβ mRNA as a result of the increased GnRH pulse frequence and amplitude demonstrated in PCOS [20]. These findings have also been demonstrated in studies performed before [20] and after the PCOS diagnostic standardization [6,19].
Several gonadotrope modulators may affect differently the expression of FSHβ and LHβ subunits in mRNA according to the pattern of GnRH pulsation [5,6,21,22]. A negative correlation between insulin and the gonadotrope secretion of LH was demonstrated in the current study in PCOS patients. Other studies have attempted to clarify the effect of insulin on gonadotrope cell secretion. Insulin may enhance the transcription of LHβ gene or augment LH and FSH releasethat small increase in concentrations in the late follicular phase, and immediately before the estradiol surge, may facilitate the FSH ovulatory peak [30,33]. In addition, treatment of pre-estrogenized gonadotrope with P4 produces a significantly greater LH release in PCOS patients compared with normal subjects [33]. While a hypothalamic insensitivity to progesterone is implicated in the pathogenesis of PCOS [8], the role of P4 on the gonadotrope cell secretion seems to act biphasically. At low concentrations P4 enhances the LH response to GnRH and favours FSH surge at mid cycle [21,31,32], but its increase in blood affects gonadotrope function, decreasing both LH and FSH secretion [30].
No relationship was found between the androgen blood levels and baseline gonadotropin levels or gonadotrope response to GnRH in the present study. Even most of PCOS patients seem to have higher levels of total and free testosterone [1,2,4,26,34], the current knowledge concerning the relationship between testosterone levels and gonadotrope function in adult female is still limited. Furthermore, the majority of data come from animal studies and it seems that testosterone has both facilitatory and inhibitory effects on LH secretion and suppressive effect on the progesterone facilitator’s role to stimulate the LH secretion [8,35]. In vitro and in vivo studies have shown that testosterone increases FSHβ mRNA levels but decrease, or have no effect, on the alpha-subunit or LHβ mRNA expression [35,36]. In general, at least in animals, testosterone has both negative and positive action on the female gonadotrope via modulation of the GnRH receptor, and LH and FSH gene expression [37]. The infusion of testosterone in women with PCOS does not appear to influence the secretion of LH, or the gonadotrope response to GnRH stimulation, [37,38]. Finally, women with PCOS have identical responses to GnRH stimulation regardless of their androgen levels [36].
In summary, the current study demonstrated that the knowledge of the pituitary function in PCOS patients learned before the systematization of the current diagnostic criteria was not changed with the current findings. The present studies suggest that the clinical conditions, currently excluded to establish the diagnosis of PCOS, may have not contaminated the results published before the NIH recommendation and support that the androgen levels do not have a significant role in the gonadotrope secretion.
Conflict of interest statement
The authors declare no conflict of interest.
[1] Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yldiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004; 89(6): 2745-2749.
[2] Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif AGJ, Haseltine F (eds). Polycystic ovary syndrome. Baston: Blackwall Scientific; 1992, p. 377-384.
[3] The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81(1): 19-25.
[4] Tsilchorozidou T, Overton C, Conway GS. The pathophysiology of polycystic ovary syndrome. Clin Endocrinol 2004; 60(1): 1-17.
[5] McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med 2002; 20: 317-336.
[6] Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab 1997; 82(7): 2248-2256.
[7] Fulghesu AM, Cucinelli F, Pavone V, Murgia F, Guido M, Caruso A, et al. Changes in luteinizing hormone and insulin secretion in polycystic ovarian syndrome. Hum Reprod 1999; 14(3): 611-617.
[8] Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 1998; 83(2): 582-590.
[9] Dighe AS, Moy JM, Hayes FJ, Sluss PM. High-resolution reference ranges for estradiol, luteinizing hormone, an follicle-stimulating hormone in men and women using the AxSYM assay system. Clin Biochem 2005; 38: 175-179.
[10] Daly LE, Bourke GJ. Sample size determination. In: Interpretation and uses of medical statistics. 5th ed. Oxford: Blackwell Science; 2000, p. 286-289.
[11] Gil Junior AB, Rezende AP, do Carmo AV, Duarte El, de Medeiros MM, de Medeiros SF. Adrenal androgen participation in the polycystic ovary syndrome. Bras Ginecol Obstet 2010; 32(11): 541-548.
[12] de Medeiros SF, Gil-Junior AB, Barbosa JS, Isaias ED, Yamamoto MMW. New insights into steroidogenesis in normo- and hyperandrogenic polycystic ovary syndrome patients. Arq Bras Endocrinol Metab. 2013; 57: 437-444.
[13] Pasquali R, Casimirri F, Venturoli S, Paradisi R, Mattioli L, Capelli M, et al. Insulin resistance in patients with polycystic ovaries: its relationship to body weight and androgen levels. Acta Endocrinol 1983; 104(1): 110-116.
[14] Morales AJ, Laughlin GA, Butzow T, Maheshwari H, Baumann G, Yen SSC. Insulin, somatotropic, and luteinizing hormone axes in non-obese and obese women with polycyctic ovary syndrome: Common and distinct features. J Clin Endocrinol Metab 1996; 81(8): 2854-2864.
[15] Srouji SS, Pagán YL, D’Amato F, Dabela A, Jimenez Y, Supko JG, et al. Pharmacokinetic factors contribute to the inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome. J Clin Endocrinol Metab 2007; 92(4): 1347-1352.
[16] Arroyo A, Laughlin GA, Morales AJ, Yen SSC. Inapropiate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab 1997; 82(11): 3728-3733.
[17] Pagán YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE. Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab 2006; 91(4): 1309-1316.
[18] Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest 1976; 57(5): 1320-1329.
[19] Lewandowski KC, Cajdler-?uba A, Salata I, Bieńkiewicz M, Lewiński A. The utility of the gonadotrophin releasing hormone (GnRH) test in the diagnosis of polycystic ovary syndrome (PCOS). Endokrynol Pol 2011; 62(2): 120-128.
[20] Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinol 1989; 125(2): 917-924.
[21] Lasley BL, Wang CF, Yen SSC. The effects of estrogen and progesterone on the functional capacity of the gonadotrophs. J Clin Endocrinol Metab 1975; 41: 820-826.
[22] Adashi EY, Hsueh AJ, Yen SS. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinol 1981; 108: 1441-1449.
[23] Poretski L, Piper B. Insulin resistance, hypersecretion of LH and a dual defect hypothesis for the pathogenesis of PCOS. Obstet Gynecol 1994; 84: 613-621.
[24] Lawson MA, Jain S, Sun S, Patel K, Malcolm PJ, Chang RJ. Evidence for insulin suppression of baseline luteinising hormone in women with polycystic ovarian syndrome and normal women. J Clin Endocrinol Metab 2008; 93(6): 2089-2096.
[25] Pralong FP. Insulin and NPY pathways and the control of GnRH function and puberty onset. Mol Cell Endocrinol 2010; 324: 82-86. [26] Barnes RB, Rosenfield RL, Burstein S, Ehrmann D. Pituitaryovarian responses to nafarelin testing in the polycystic ovary syndrome. Eng J Med 1989; 320: 559-565.
[27] Lobo RA, Granger L, Goebelsmann U, Mishell DR Jr. Elevations in unbound serum estradiol as a possible mechanism for inappropriate gonadotropin secretion in women with PCO. J Clin Endocrinol Metab 1981; 52: 156-158.
[28] Shupnik MA, Gharib SD, Chin WW. Divergent effects of estradiol on gonadotropin gene transcription in pituitary fragments. Mol Endocrinol 1989; 3: 474-380.
[29] Hamernik DL, Clay CM, Turzillo A, Van Kirk EA, Moss GE. Estradiol increases amounts of messenger ribonucleic acid for gonadotropin-releasing hormone receptors in sheep. Biol Reprod 1995; 53: 179-185.
[30] Nillius SJ, Wide L. Progesterone-induced augmentation of pituitary gonadotrophin response to luteinizing hormone-releasing hormone in oestrogen-pre-treated amenorrhoeic women. Acta Endocrinol (Copenh).1976; 83(4): 684-691.
[31] Batista MC, Cartledge TP, Zellmer AW, Nieman LK, Merriam GR, Loriaux DL. Evidence for a critical role of progesterone in the regulation of the midcycle gonadotrophin surge and ovulation. J Clin Endocrinol Metab 1992; 74(3): 565-570.
[32] Hoff JD, Quigley ME, Yen SSC. Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab 1983; 57: 792-796.
[33] Shaw RW. Tests of the hypothalamic-pituitary-ovarian axis. Clin Obstet Gynaecol 1976; 3: 485-503.
[34] Carmina E, Rosato F, Janni A, Rizzo M, Longo RA. Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism. J Clin Endocrinol Metab 2006; 91(1): 2-6.
[35] Yasin M, Dalkin AC, Haisenleder DJ, Marshall JC. Testosterone is required for gonadotropin-releasing hormone stimulation of luteinizing hormone-beta messenger ribonucleic acid expression in female rats. Endocrinol 1996; 137(4): 1265-1271.
[36] Thackray VG, Mellon PL, Coss D. Hormones in synergy: Regulation of the pituitary gonadotropin. Mol Cell Endocrinol 2010; 314(12): 192.
[37] Dunaif A. Do androgens directly regulate gonadotropin secretion in the polycystic ovary syndrome? J Clin Endocrinol Metab 1986; 63(1): 215-221.
[38] Ropelato MG, Rudaz MG, Escobar ME, Bengolea SV, Calcagno ML, Veldhuis JD, et al. Acute effects of testosterone infusion on the serum luteinizing hormone profile in eumenorrheic and polycystic ovary syndrome adolescents. J Clin Endocrinol Metab 2009; 94(9):3602-3610.
ment heading
10.1016/S2305-0500(14)60004-X
*Corresponding author: Sebasti?o Freitas de Medeiros, Rua Almirante Henrique
Pinheiro Guedes, 195, Duque de Caxias, Cuiabá, MT, Zip code: 78043-306, Brazil.
Tel: (55) (65) 3322 2017
Fax: (55) (65) 3623 0071
E-mail: de.medeiros@terra.com.br
GnRH
LH
Pituitary function
 Asian Pacific Journal of Reproduction2014年1期
Asian Pacific Journal of Reproduction2014年1期
- Asian Pacific Journal of Reproduction的其它文章
- Tuberculous orchitis mimicking a testicular tumor: A diagnostic dilemma
- Klinefelter syndrome and its association with male infertility
- Investigation on leukocyte profile of periparturient cows with or without postpartum reproductive disease
- Tranexamic acid reduces blood loss during and after cesarean section: A double blinded, randomized, controlled trial
- Maternal outcome in multiple versus singleton pregnancies in Northern Tanzania: A registry-based case control study
- Soluble fms-like tyrosine kinase-1 and vascular endothelial growth factor: Novel markers for unexplained early recurrent pregnancy loss
