Investigation on leukocyte profile of periparturient cows with or without postpartum reproductive disease
Rafiqul Islam, Harendra Kumar, Binsila B. Krishnan
Immuno-Reproduction Laboratory, Animal Reproduction Division, Indian Veterinary Research Institute, Izatnagar, Bareilly-243122, U.P., India
Investigation on leukocyte profile of periparturient cows with or without postpartum reproductive disease
Rafiqul Islam*, Harendra Kumar, Binsila B. Krishnan
Immuno-Reproduction Laboratory, Animal Reproduction Division, Indian Veterinary Research Institute, Izatnagar, Bareilly-243122, U.P., India
ARTICLE INFO
Article history:
Received 13 November 2013
Received in revised form 23 November 2013
Accepted 23 November 2013
Available online 20 January 2014
Periparturient cow
Objective: To determine the leukocyte profile in periparturient cows and their relation with the development of postpartum reproductive diseases (PRD). Methods: Forty one advanced pregnant cows at 240 days of gestation were selected and critically observed daily during the periparturient period. Blood sampling was done for each experimental animal on 15 days prepartum (-15 d), calving day (0 d), 15 days (15 d) and 30 days (30 d) post partum (pp) for determination of total leukocyte (TLC) and differential leukocyte count (DLC) and thorough gynaecological examination was performed on 0 d, 15 d, 30 d and 45 d pp for diagnosis of PRD like retained placenta (ROP), clinical metritis (CM), clinical endometritis (CE), cervicitis (CT) and delayed involution of uterus (DIU). Results: The percentage of cows suffered from various post partum reproductive diseases was 41.46% (17/41). In normal cows, a significant increase in TLC from -15 d to 0 d followed by significant decline has been observed at 15 d and 30 d. The cows suffering from ROP (25.52±2.49), CM (26.57±3.17) and CE (25.09±2.95) showed significantly (P<0.01) higher neutrophil% than normal cows (15.05±1.25) on day 15 pp. The normal cows showed significant higher lymphocyte% during the periparturient period than the cows with PRD on post partum days (15 d and 30 d). The neutrophil% and neutrophil: lymphocyte ratio increased from -15 d to the 0 d in all the groups of cows followed by a general decrease during the post partum days. Conclusions: The neutrophil% and neutrophil: lymphocyte ratio was higher in the cows with PRD during periparturient period. Thus, the increased neutrophil percentage and neutrophil: lymphocyte ratio observed at day15 postpartum is an indication of PRD in cows. The significant rise in TLC on the day of calving than prepartum and post partum days only in normal cows is physiological. It is also clear that cows suffering from ROP most likely to suffer from other post partum reproductive diseases.
1. Introduction
The dairy cows are highly prone to various reproductive diseases during postpartum period. The immunosuppression experienced by the healthy periparturient cows has been well characterized during the transition period. This period is defined as 3 weeks prior to and the 3 weeks following parturition, represents a time of physiological stress for the dairy cow [1]. Infectious diseases are more prevalent during peripartum period, because of an impaired immune status before and immediately after parturition[2]. Dilatation of the cervix after the onset of parturition[3] allows the entry of bacteria into the uterus, causing infection in 90% of cows by 21 days post-partum [4-6]. Postpartum reproductive diseases (PRD) such as retained placenta, acute metritis and clinical and subclinical endometritis have been reported to have a negative effect on reproductive performance[7]. These diseases may delay the complete regeneration of endometrium, and disrupt the resumption of cyclic ovarian function resulting in postponement of the first insemination (AI), increasing the number of AIs per conception, and thus prolonging the calving interval and decreasing the calving rate[8,9] and thereby decreasing the economy of the dairy farm.
Peripheral blood mononuclear cells and PMN cells haveimportant roles in the body’s defense mechanism. During the transition period in cows, the leukogram, and functional capacity of bovine PMN cells have been altered[10]. However, the altered leukogram and their function return to normal level within three to four weeks postpartum [11-13]. In the recent past, impressive works have been conducted on the leukocyte profile and PMN function in peripartum cattle[11-14]. Despite the encouraging results obtained during the past two decades, there is a substantial space for further research concerning subjects like immune status in the animals anticipating parturition, during and after parturition. Information on circulatory leukocytes of periparturient animals in relation to occurrence of post partum reproductive diseases in cattle is meagre. It warrants the need of the present study to know the changes in leukogram in peripheral blood of the periparturient cows that are going to develop post partum reproductive diseases which may help in understanding the course of development of the diseases.
2. Materials and methods
2.1. Animals
The experimental animals used in this study were selected from Vrindavani Cattle herd maintained at Cattle and Buffalo Farm, Livestock production and Management section, Indian Veterinary Research Institute, Izatnagar, India. A total of 41 healthy advanced pregnant cows between 2ndto 4thparity those had no reproductive disorder during the previous pregnancy/calving were selected randomly at 240 days of pregnancy.
2.2. Management of animals
The climate of Indian Veterinary Research Institute including the Cattle and Buffalo Farm is Tropical. The environmental temperature during summer varied from 30 ℃to 45 ℃ and during winter 10 ℃ to 25 ℃. The winter exists in the farm area from the month of November to February. The animals were managed under intensive system in the cattle sheds. During the day time they were allowed to remain in the paddock connected to the cattle shed. The feeding and watering of the cows were done in the paddock. All the animals were maintained under uniform feeding and managemental conditions throughout the year.
2.3. Gynaecological examination of the animals
2.3.1. Prepartum
The detail of pregnancy diagnosis, selection, regular observation for external signs for the approaching parturition and fixing of prepartum sampling days (15 d prior to calving; -15 d) of experimental cows has already been mentioned by Islam[15] and Islam et al. [16,17]. The ranges of the prepartum collection day fell between 10 (- 10 d) to 22 days (- 22 d) prior to calving.
2.3.2. Calving
The experimental cows were also monitored /observed on the day of calving (0 d) for nature of parturition (normal/ abnormal), expulsion of placenta (retained fetal membranes) and lochia[15-17].
2.3.3. Postpartum
During the post-partum period, the individual cow was critically monitored for the diagnosis of post partum disorders like retained placenta (ROP), clinical metritis (CM), clinical endometritis (CE), Cervicitis (CT), delayed uterine involution (DIU) and also for ovarian activity at 15, 30 and 45 days post-partum by trans-rectal palpation as has already been mentioned elsewhere[15-17].
2.4. Sampling
Blood sample was collected from jugular vein at -15 d (15 days before calving), 0 d (day of calving), 15 d (15 days after calving) and 30 d (30 days after calving) during peripartum period. Heparinised blood was used for the study of total leukocyte count (TLC) and differential leukocyte count.
2.5. Total leukocyte count (TLC)
Total leukocyte count (TLC) was made in each blood samples by conventional method using Neubauer counting chamber after dilution of whole blood at the rate of 1: 20 with WBC diluting fluid (S D Fine Chemicals, Mumbai). The blood was drawn by sucking into a white cell pipette containing white bead to the mark 0.5. Excessive blood sticking to the outer side of the pipette was wiped off. WBC Diluting fluid (S D Fine Chemicals, Mumbai) was drawn into diluting pipette up to mark 11. The pipette was kept =horizontal on the palm of the hand and mixed thoroughly by rotation with the other hand. After mixing for 1 minute, 3-4 drops of fluid was discarded from the stem of the pipette. A small drop of mixed fluid was then placed between cover slip and the ruled chamber and allowed to settle for some time and counted the number of WBC under high power (40×) objective of the microscope in four large Squares for WBC counting (sixteen small squares per large square) located at four corners.
Calculation:
Total leukocyte count (T.L.C)=X × 50/ μL of blood
X=Total number of cells counted in all four squares
50=Multiplication factor
Multiplication factor=20/(1×1×0.1×4) =50
Where, 20=dilution; 1x1=area of one large square; 0.1= depth between the cover slip and counting chamber; 4=No. of large squares in which counting is done.
Therefore, TLC per ml of Blood=X×50×1 000
2.6. Differential leukocyte count (DLC)
Differential leukocyte count was assessed using Giemsa’s stain following standard haematological procedures. A small drop of blood (10 μL) was placed on the surface of the slide near one end. The blood was spread by means of another glass slide known as spreader which was held in right hand. The spreader was held just next to the drop of blood and then it was pulled back to edge of the drop. The blood was spread out behind the spreader which was then pushed tothe left. The angle should be about 30 degree. The blood smear was allowed to dry on the glass in the open air. The blood film was fixed in Methyl Alcohol for five minutes. The stock solution was diluted as 1: 10 with distilled water. The fixed smears were put in diluted stain for one hour. After staining the slide was washed with slow running tap water and finally rinsed with distilled water. The slide was dried and examined under oil immersion lens.
2.7. Statistical analysis
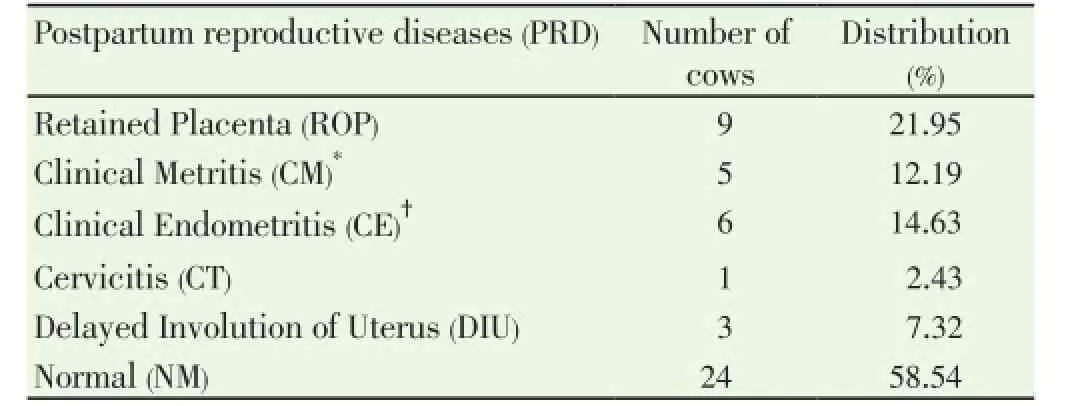
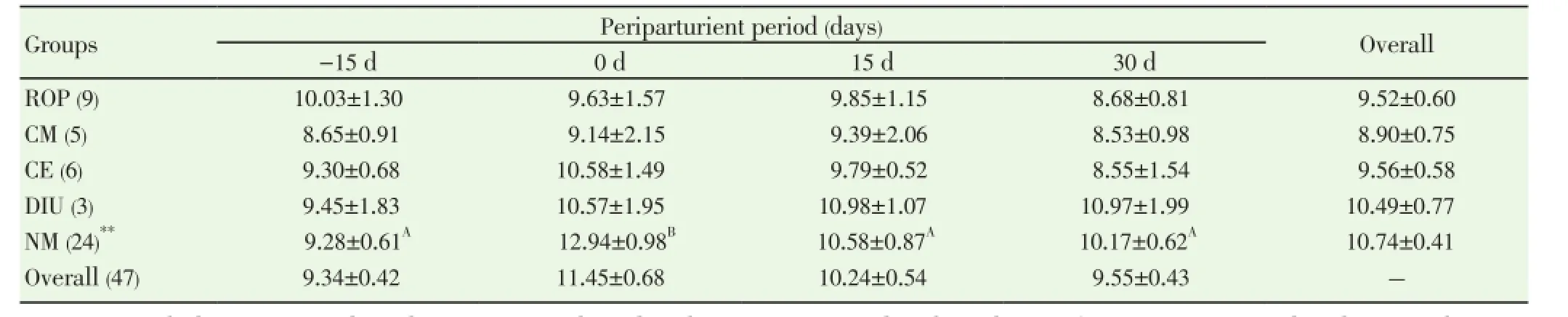
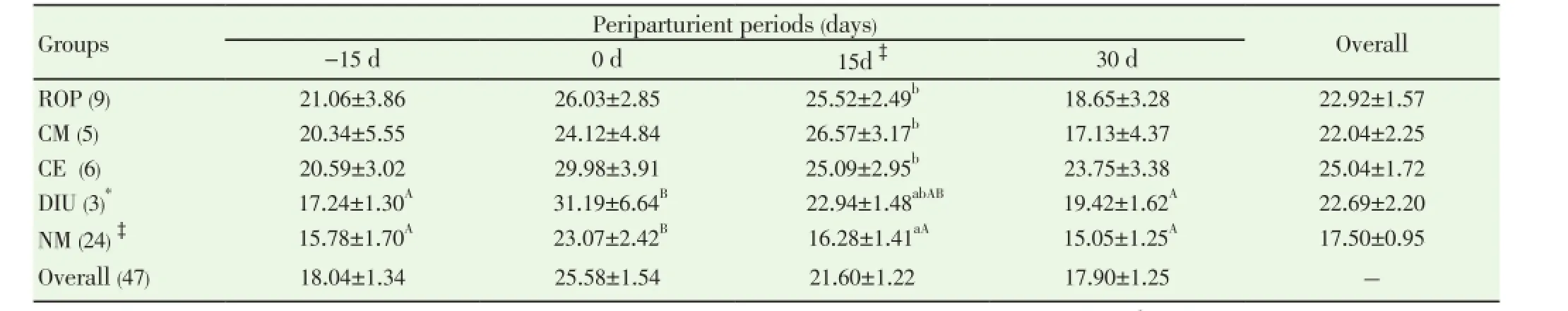
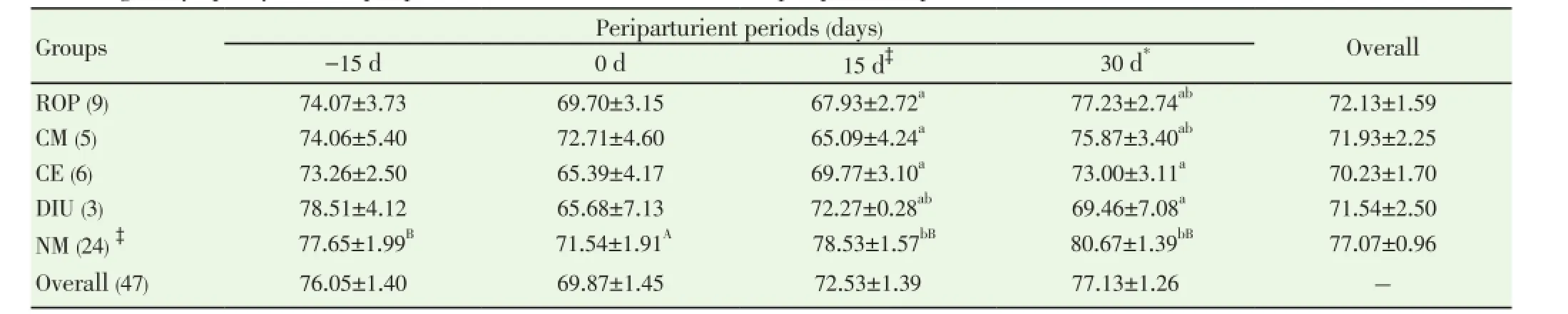
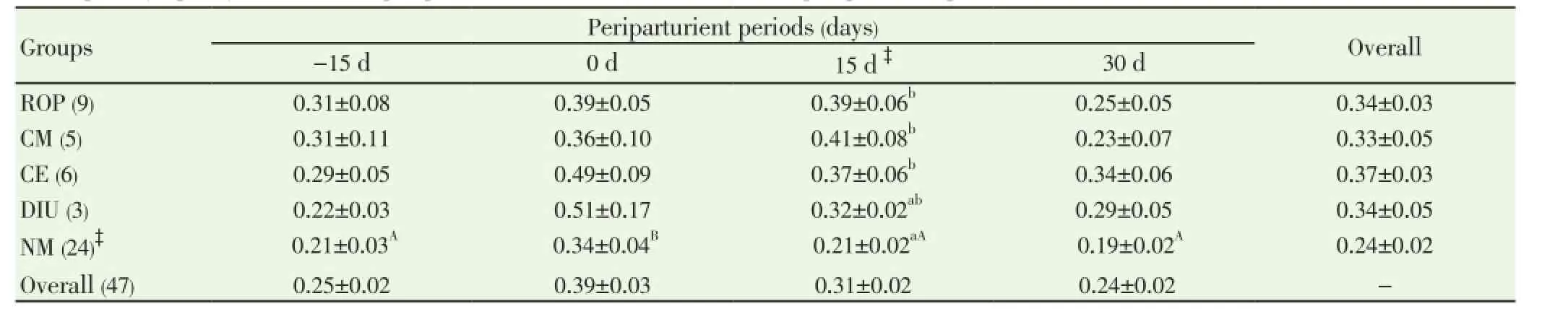
Data pertaining to total leukocyte count (TLC) and differential leukocyte count (DLC) from normal (n=24) and PRD cows (n=16) were analysed statistically by ANOVA using statistical software SPSS version 16.0. One cow suffering from only post partum cervicitis was not included for analysis for any parameter studied. The effects of postpartum reproductive diseases (Control, retained placenta, clinical metritis, clinical endometritis and delayed involution), and periods (days of sample collection -15 d, 0 d, 15 d and 30 d were observed. The cow was included in the analysis as a random effect. When an effect between groups and time was observed, post-hoc multiple comparisons were performed using Duncan’s multiple range test and cross checked with LSD. The level of significance was set at P<0.05 and considered a tendency to be significant if the P value is more than 0.05 but less than 0.10 (0.05 3.1. Distribution of postpartum reproductive diseases The percentage of cows suffered from various post partum reproductive disease was 41.46% (17/41). Out of the nine cows diagnosed for ROP, seven cows (77.78%) had suffered from other reproductive diseases. The cows accounted for delayed involution of uterus (7.32%) did not show any kind of reproductive abnormalities except the delay in the involution of uterus observed at 45 d (Table 1). Table 1Distribution of postpartum reproductive diseases. 3.2. Total leukocyte count (TLC) The total leukocyte count (×106/mL) in cows suffering from various reproductive diseases during periparturient period from -15 d (pre partum) to 30 d (post partum) is presented in Table 2. The TLC was 10.03±1.30, 8.65±0.91, 9.30±0.68, 9.45±1.83 and 9.28±0.61 for ROP, CM, CE, DIU and Normal cows, respectively at -15 d. The corresponding values were 8.68±0.81, 8.53±0.98, 8.55±1.54, 10.97±1.99 and 10.17±0.62, respectively at 30 d. In normal cows, a significant (P<0.05) increase in TLC from -15 d (9.28±0.61) to 0 d (12.94±0.98) followed by significant decline has been observed at 15 d (10.58±0.87) and 30 d (10.17±0.62). 3.3. Differential leukocyte count (DLC) 3.3.1. Neutrophil (%) Percentage of neutrophil was found to be higher in cows suffering from PRD during the periparturient period from 15 days pre-partum through 30 days post-partum (Table 3). The neutrophil percentage was 21.06±3.86, 20.34±5.55, 20.59±3.02, 17.24±1.30 and 15.78±1.70 on -15 d in ROP, CM, CE, DIU and normal group of cows, respectively. The cows suffering from ROP (25.52±2.49), CM (26.57±3.17) and CE (25.09±2.95) showed significantly (P<0.01) higher value than normal cows (16.28±1.41) on day 15 pp. On day 30 pp, cows with clinical endometritis (23.75±3.38) showed higher value than in other group of cows and the difference was non-significant between the groups. The neutrophil percentage increased from -15 d to the 0 d in all the groups of cows followed by a general decrease during the post partum days. In DIU and normal cows, the percentage increased significantly on 0 d and decreased significantly from 0 d to the post partum days in normal cows. 3.3.2. Lymphocyte (%) The Lymphocyte percentage did not show any clear cut picture on day -15 and calving day. However, the normal cows showed significant higher value during the periparturient period than the cows with PRD on 15 d (P<0.01) and 30 d (P<0.1) (Table 4). In general, the values decreased from prepartum to calving day in all groups of cows followed by increased during the post partum days. The value decreased significantly (P<0.01) from -15 d to 0 d followed by a significant increase at 15 d and 30 d in normal cows. 3.3.3. Neutrophil: lymphocyte ratio The N: L ratio of the cows suffering various reproductive diseases was higher during the periparturient period from -15d through 30d than in normal cows. However, the difference in N: L ratio was only significantly (P<0.01) higher for cows suffering from ROP (0.39±0.06), CM (0.41±0.08) and CE (0.37±0.06) than in normal cows (0.21±0.02) on 15 d. The ratio (N: L) varied from 0.21±0.03 in normal to 0.31±0.08 in ROP cows at -15d. The corresponding ratio varied from 0.34±0.04 (normal) to 0.51±0.17 (DIU) at calving, 0.19±0.02 (normal) to 0.34±0.06 (CE) at 30 days post calving (Table 5). The N: L ratio showed an increase from -15d to calving day (0 d) in all the groups of cows, followed by a decrease during the post calving days. However, the ratio in normal cows increased significantly from -15 d (0.21±0.03) to the day of calving (0.34±0.04) followed by a significant decrease on the day 15 ( 0.21±0.02) and 30 post calving ( 0.19±0.02). Table 2Total leukocyte count (x106/mL) in cows with and without postpartum reproductive diseases. Table 3Percentage of neutrophil in the peripheral blood of cows with or without postpartum reproductive diseases. Table 4Percentage of lymphocyte in the peripheral blood of cows with or without postpartum reproductive diseases. Table 5Neutrophil: lymphocyte ratio in the peripheral blood of cows with or without postpartum reproductive diseases. 4.1. Distribution of postpartum reproductive diseases The occurrence of post partum reproductive diseases (PRD) recorded in present study was 41.46% and rest 58.54% cows did not reveal any kind of clinical reproductive diseases, which is in accordance with Gautam et al.[18] and LeBlanc et al.[7] and lower than Hammon et al.[14]. The various uterine diseases recorded by Hammon et al. [14] were puerperal metritis (21.7%), subclinical endometritis(51.8%) and endometritis (15.7%). Nevertheless, the lactation incidence of metritis is typically between 10% and 20%, of clinical endometritis or PVD approximately 15%, and of subclinical or cytological endometritis a further 15%[7]. Cows with retained placenta usually suffer from other post partum uterine diseases. Of nine ROP cows, 5 suffered from clinical metritis (CM) and another 2 cows developed clinical endometritis (CE). Uterine disease within a week of parturition (metritis) is present in up to 40% of dairy cows[9]. The presence of mucopurulent or purulent cervicovaginal discharge on vaginoscopy between 15 and 60 d postpartum was defined as clinically relevant endometritis that had a prevalence of 25.9% in Japanese dairy herd [18]. It is confirmed that great proportion of dairy cows develop varying degree of clinically relevant endometritis (metritis and endometritis) during the post partum days. Further cows suffered from ROP are most likely to develop other uterine diseases postpartum. 4.2. Total leukocyte count (TLC) The increase in TLC (×106/mL) on the day of calving in normal cows is in concurrence with the earlier reports[12,19]. The leukocytosis observed on the day of calving in our study might be due to the antepartum rise in cortisol[15,19]. The post partum decrease in TLC in all group of cows except DIU are in line with Mateus et al.[12] and Kim et al.[13] and is in close agreement with the Moreira da Silva et al.[11]. However, the total leukocytes count returns to normal or higher level over the next three to four weeks postpartum is in accordance with the earlier reports[11-13]. In contrast to our results, Kim et al.[13] reported higher TLC in endometritic than in normal cows during the periparturient period. In the present study, the TLC was not found to be differed significantly between the groups in any time point. Hence, no inference could be drawn in relation to occurrence of PRD. Leukopenia developed shortly after i.u. inoculation of Escherichia coli (3h pi) in mare as reported by Mette et al.[20] supports the lower TLC observed in cows with PRD in the present study. 4.3. Differential leukocyte count Of the leukocyte, neutrophil and lymphocyte are the major and important population of cells for innate immunity to resolve infection and safeguard from various diseases. Neutriphil is the first cell to arrive in the location of infection and protect the animals from various infectious diseases by phagocytosis and killing mechanism with the production of various agents. Neutrophil being the most important cells for the innate immunity, its profile in the animals with different reproductive health condition may give important indication about the development and presence of the disease condition. Our objective was to explore the possibilities for prediction at prepartum or calving before the actual development of the post partum reproductive disease. 4.3.1. Neutrophil Percentage of neutroplil was found to be higher in cows suffering from reproductive diseases during the periparturient period starting from 15 days prepartum to 30 days post partum. The significantly higher percentage of neutrophil for ROP, CM and CE cows at 15 d might be due to the poor migration of neutrophil to the uterus[21]. Neutrophils are the earliest and most important phagocytic cell type recruited from the peripheral circulation to the uterine lumen in response to pathogen challenge[22]. The increased cortisol concentrations in cows that developed ROP has immunosuppressive and inhibitory effects on leukocyte migratory activity[23,24]. The higher percentage of neutrophil observed in cows with PRD might be attributed to the increased cortisol level around the day of calving in the present study[15]. The normal physiological expulsion of the placenta requires the presence of neutrophils at the fetomaternal junction[21]. The significantly lower neutrophil on 15 days pp in normal cows might be due to the migration of neutrophils to the uterus[25] and in case of cows with PRD this mechanism might have failed and due to this the uterus in the disease groups could not clear the bacterial infection by the innate immunity and hence the disease development occurs. The neutrophil percentage increased from 15 days prepartum to the calving day in all groups of cows followed by a general decrease during the post partum days. This might be due to the rise in plasma cortisol around the day of calving[19]. The increase in neutrophil at calving compared to prepartum and post partum days has been reported earlier in cows [12,13,26]. Further, the significantly higher neutrophil percentage in the cows with ROP, CM and CE on 15 d observed in the present study confirms the earlier report of Kim et al.[13] in endometritic cows. This might be due to the poor random migration and activation of neutrophils and monocytes leading to the impairment in diapedesis of the cells to the site of infection in cows suffering from reproductive disease[27]. It is evident from the present study that the neutrophil counts increased in the cows with PRD during periparturient period due to decreased ability of neutrophils to migrate in the uterus. The cows unable to clear the infection and suffer from PRD might also be due to the failure of other mechanism like depressed PMN function and decreased production of proinflammatory cytokine IL-1 as already observed by Islam[15] and Islam et al.[17]. An intensive invasion by pathogens may occur if the migration and functional (phagocytic, cell-killing, oxidative burst) capacity of neutrophils are reduced[3,12,27]. Thus, the increased neutrophil percentage observed at day15 pp in the current study is an indication of PRD in cows. 4.3.2. Lymphocyte The Lymphocyte percentage did not show any clear cut picture either on day -15 or at calving. However, the normal cows showed higher value during the periparturient period, being the significant difference during post partum days. In general, the values decreased from prepartum to calving day in all groups of cows followed by an increase during the post partum period. Decrease in T cells at calving may be linked to systemic immunosuppression, and leads to the appearance of post partum inflammatory diseases like mastitis and puerperal metritis in cows[28]. The decrease is also noted in healthy cows around the calving day[29,30], which supports the present finding. Immunological mechanisms induced by T cells play important role in host defense[31]. The immunosuppression experienced by healthy periparturient cow has been well characterized. The week immediately following calving is marked by a decrease in lymphocyte proliferation[32,33] and the ability of neutrophils to migrate and phagocytise[34,35]. The lower lymphocyte percentage during the pp period in cows with PRD in our study was also reported in cows with DIU[36]. 4.3.3. Neutrophil: Lymphocyte ratio The neutrophil: lymphocyte (N: L) ratio of cows with PRD was higher than the normal during the periparturient period. The increase in N: L ratio on the day of caving due to the increase in neutrophil percentage, which might have occurred due to rise in cortisol around calving day[15,19]. The significant decrease of N: L ratio at post calving days in normal cows as a result of significant decrease in neutrophil percentage, which might be due to the migration of neutrophil to the uterus[25] as a part for initiation of effective innate immunity for microbial clearance against the bacterial contamination obtained during calving and early post calving days. The higher N: L ratio in cows with PRD in the present study might be attributed to the failure in the migration of leukocytes to the cervix and uterus[25]. Leukocytes that have been mobilised into the bovine endometrium from the peripheral blood during parturition are also capable of synthesising pro-inflammatory cytokines[3,37] which may be responsible for the clearance of bacterial infection. The immune system remains suppressed in order to prevent premature rejection of fetus by the maternal system during pregnancy; however, significant changes occur during term pregnancy and peripartum period, when massive invasion of leukocytes occurs in the cervix and uterus[38]. At parturition, massive blood supply to the gravid uterus, coupled with the migration of white cells from the circulation to the uterine lumen, enables vigorous and active phagocytosis of bacteria to occur[13]. This might be the reason for the decreased neutrophil percentage and N: L ratio in normal cows compared to the PRD group around calving which is physiological due to the migration of leukocytes into the uterus[13,33] to boost up innate immunity required for clearance of bacterial infection from the uterus. The failure in this migration process of leukocytes might cause disturbance in innate immunity leading to the development of the reproductive diseases during postpartum in this study. It is evident from the present study that the neutrophil counts increased in the cows with PRD during periparturient period due to decreased ability of neutrophils to migrate in the uterus. Thus, the increased neutrophil percentage and neutrophil: lymphocyte ratio observed at day15 postpartum in the current study is an indication of PRD in cows. TLC did not show any indication regarding the pathogenesis of the inflammatory uterine diseases in cow. However, the significant rise in TLC on the day of calving than prepartum and post partum days only in normal cows indicates healthy immune status of the cow and helping cows to remain free form PRD. It is also clear that cows suffering from ROP most likely to suffer from other post partum reproductive diseases. The authors declare that they do not have any conflict of interest. [1] Goff JP, Horst RL. Physiological changes at parturition and their relationship to metabolic disorders. J Dairy Sci 1997; 80: 1260-1268. [2] Singh J, Murray RD, Mshelia G, Woldehiwet Z. The immune status of the bovine uterus during the peripartum period. The Vet J 2008; 175: 301-309. [3] Sheldon IM. The post-partum uterus. Vet Clin North America Food Anim Practice 2004; 20: 569-591. [4] Griffin JFT, Hartigan PJ, Nunn WR. Nonspecific uterine infection and bovine fertility. Theriogenology 1974; 1: 107-114. [5] Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 2002; 123: 837-845. [6] Sheldon IM, Lewis GS, LeBlanc SJ, Gilbert RO. Defining postpartum uterine disease in cattle. Theriogenology 2006; 65: 1516-1530. [7] LeBlanc SJ, Osawa T, Dubuc J. Reproductive tract defense and disease in postpartum dairy cows. Theriogenology 2011; 76: 1610- 1618 [8] Hussain AM, Daniel RCW. Bovine normal and abnormal reproductive and endocrine functions in the postpartum period: a review. Reprod Domest Anim 1991; 26: 101-111. [9] Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ. Defining postpartum uterine disease and the mechanisms of infection andimmunity in the female reproductive tract in cattle. Biol Reprod 2009; 81: 1025-1032. [10] Zerbe H, Schneider N, Leibold W, Wensing T, Kruip TAM, Schuberth HJ. Altered functional and immunophenotypical properties of neutrophilic granulocytes in postpartum cows associated with fatty liver. Theriogenology 2000; 54: 771-786. [11] Moreira da Silva F, Burvenich C, Massart Leen AM, Brosse L. Assessment of blood neutrophil oxidative burst activity in dairy cows during the period of parturition. Anim Sci 1998; 67: 421-426. [12] Mateus L, Lopes da Costa L, Bernardo F, Silva JR. Influence of puerperal uterine infection on uterine involution and postpartum ovarian activity in dairy cows. Reprod Dom Anim 2002; 37: 31-35. [13] Kim IH, Na KJ, Yang MP. Immune responses during the peripartum period in dairy cows with postpartum endometritis. J Reprod Dev 2005; 51: 757-64. [14] Hammon DS, Evjen IM, Dhiman TR, Goff JP, Walters JL. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet Immunol Immunopathol 2006; 113: 21-29. [15] Islam R. Studies on immuno-endocrine profile of peripartum cows in relation to postpartum reproductive health. Ph.D. Thesis; submitted to Indian Veterinary Research Institute, Izatnagar, Bareilly, India, 2012. [16] Islam R, Kumar H, Nandi S, Rai RB. Determination of antiinflammatory cytokine in periparturient cows for prediction of postpartum reproductive diseases. Theriogenology 2013; 79(6): 974-979. [17] Islam R, Kumar H, Nandi S, Mehrotra S. Circulatory level of interleukin-1 in periparturient cows with or without postpartum reproductive diseases. Asian Pacific J Reprod 2013; 2(4): 316-320. [18] Gautam G, Nakao T, Yusuf M, Koike K. Prevalence of endometritis during the postpartum period and its impact on subsequent reproductive performance in two Japanese dairy herds. Anim Reprod Sci 2009; 116: 175-187. [19] Preisler MT, Weber PS, Tempelman RJ, Erskine RJ, Hunt H, Burton JL. Glucocorticoid receptor down-regulation in neutrophils of periparturient cows. American J Vet Res 2000; 61: 14-19. [20] Mette C, Dooleweerdt BC, Stine J, Miki BA, Roenn PM, Henrik LJ. Evaluation of the systemic acute phase response and endometrial gene expression of serum amyloid A and pro- and antiinflammatory cytokines in mares with experimentally induced endometritis. Vet Immunol Immunopathol 2010; 138: 95-105. [21] Gunnick JW. Retained placenta and leukocytic activity. Vet Quart 1984; 6: 49-51. [22] Subandrio AL, Noakes DE. Neutrophil migration into the uterine lumen of the cow: the influence of endogenous and exogenous sex steroid hormones using two intrauterine chemoattractants. Theriogenology 1997; 47: 825-835. [23] Roth JA, Kaeberle ML. Effects of in vivo dexamethasone administration on in vitro bovine polymorphonuclear leucocyte function. Infect Immunol 1981; 83: 434-41. [24] Engler H, Baily MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol 2004; 148: 106-115. [25] Guidry AJ, Paape MJ, Pearson RE. Effects of parturition and lactation on blood and milk cell concentrations, corticosteroids and neutrophil phagocytosis in the cow. American J Vet Res 1976; 37: 1195-1200. [26] Mallick S, Prakash BS. Effects of supplementation of Tinospora cordifolia to crossbred cows peripartum. Anim Reprod Sci 2011; 123: 5-13. [27] Paisley LG, Mickelson WD, Anderson PB. Mechanism and therapy for retained fetal membranes and uterine infection of cows: A review. Theriogenology 1986; 25: 353-381. [28] Ohtsuka H, Koiwa M, Fukuda S, Satoh Y, Hayashi T, Hoshi F, et al. Changes in peripheral Leukocyte subsets in dairy cows with inflammatory diseases after calving. J Vet Med Sci 2004; 66: 905-909. [29] Kimura K, Goff JP, Kehrli ME Jr, Harp JA. Phenotype analysis of peripheral blood mononuclear cells in periparturient dairy cows. J Dairy Sci 1999; 82: 315-319. [30] Karcher EL, Beitz DC, Stabel JR. Parturition invokes changes in peripheral blood mononuclear cell populations in Holstein dairy cows naturally infected with Mycobacterium avium subsp. Paratuberculosis. Vet Immunol Immunopathol 2008; 124: 50-62. [31] Hagiwara E, Abbasi F, Mor G, Ishigatsubo Y, Klinman DM. Phenotype and frequency of cells secreting IL-2, Il-4, IL-6, Il-10, IFN and TNF-alpha in human peripheral blood. Cytokine 1995; 7: 815-822. [32] Kehrli Jr ME, Nonnecke BJ, Roth JA. Alterations in bovine lymphocyte function during the periparturient period. American J Vet Res 1989; 50: 215-220. [33] Meglia GE, Johannisson A, Agenas S, Holtenius K, Persson Waller K. Effects of feeding intensity during the dry period on leukocyte and lymphocyte sub-populations, neutrophil function, and health in periparturient dairy cows. Vet J 2005; 169: 376-384. [34] Kehrli Jr ME, Nonnecke BJ, Roth JA. Alterations in bovine neutrophil function during the periparturient period. Am J Vet Res 1989; 50: 207-214. [35] Lee E, Kehrli Jr ME. Expression of adhesion molecules on neutrophils of periparturient cows and neonatal calves. American J Vet Res 1998; 59: 37-43. [36] Levkut M, Pisti J, Revajova V, Choma J, Levkutova M, David V. Comparison of immune parameters in cows with normal and prolonged involution time of uterus. Vet Med Czech 2002; 47: 277-282. [37] Saji F, Samejima Y, Kamiura S, Sawai K, Shimoya K, Kimura T. Cytokine production in chorioamnionitis. J Reprod Immunol 2000; 47: 185-196. [38] Van Engelen E, de Groot MW, Breeveld-Dwarkasing VN, Everts ME, van der Weyden GC, Taverne MA, et al. Cervical ripening and parturition in cows are driven by a cascade of pro-inflammatory cytokines. Reprod Dom Anim 2009; 44: 834-841. ment heading 10.1016/S2305-0500(14)60003-8 *Corresponding author: Rafiqul Islam, Associate Professor & Head, Division of Animal Reproduction, Gynaecology and Obstetrics, Faculty of Veterinary Sciences and Animal Husbandry, S. K. University of Agricultural Sciences and Technology (K), Shuhama, Srinagar-190006, Kashmir, India. Tel.: + 91-9419021798, +91 194 2262208 E-mail: rafiqvet@gmail.com Total leukocyte Differential leukocyte count Post-partum reproductive diseases3. Results
4. Discussion
Conflict of interest statement
 Asian Pacific Journal of Reproduction2014年1期
Asian Pacific Journal of Reproduction2014年1期
- Asian Pacific Journal of Reproduction的其它文章
- Tuberculous orchitis mimicking a testicular tumor: A diagnostic dilemma
- Klinefelter syndrome and its association with male infertility
- Current insights into gonadotropic pituitary function in the polycystic ovary syndrome
- Tranexamic acid reduces blood loss during and after cesarean section: A double blinded, randomized, controlled trial
- Maternal outcome in multiple versus singleton pregnancies in Northern Tanzania: A registry-based case control study
- Soluble fms-like tyrosine kinase-1 and vascular endothelial growth factor: Novel markers for unexplained early recurrent pregnancy loss
