Investigation of in vivo neuropharmacological effect of Alpinia nigra leaf extract
Farjana Sharmen, Adnan Mannan, Md. Mominur Rahman, Md. Ashraf Uddin Chowdhury, Muhammad Erfan Uddin, A. M. Abu Ahmed*
1Department of Genetic Engineering and Biotechnology, University of Chittagong, Chittagong-4331, Bangladesh
2Department of Pharmacy, International Islamic University Chittagong, Chittagong, Bangladesh
Investigation of in vivo neuropharmacological effect of Alpinia nigra leaf extract
Farjana Sharmen1, Adnan Mannan1, Md. Mominur Rahman2, Md. Ashraf Uddin Chowdhury2, Muhammad Erfan Uddin2, A. M. Abu Ahmed1*
1Department of Genetic Engineering and Biotechnology, University of Chittagong, Chittagong-4331, Bangladesh
2Department of Pharmacy, International Islamic University Chittagong, Chittagong, Bangladesh
PEER REVIEW
Peer reviewer
Imtiaj Hasan, Assistant Professor, Department of Biochemistry and Molecular Biology, Rajshahi University, Rajshahi-6205, Bangladesh.
Tel:+880-721-711109
Fax: +880-721-711114
E-mail: hasanimtiaj@yahoo.co.uk
Comments
It is an interesting paper in which the authors tried to focus on the neuropharmacological effect of A. nigra leaf extract on mice. The anxiolytic activity was determined by wellestablished protocols that signify an anti-depressant effect found in this leaf extract. It is stimulating enough for further research works to develop a new therapeutic agent for anxiety and depression.
Details on Page 141
Objective:To analyze in vivo neuro-pharmacological effects of Alpinia nigra as anxiety is a particular form of behavioral inhibition that occurs in response to novel environmental events.
Sedative, Elevated plus maze, Anxiolytic, Antidepressant activity, Alpinia nigra
1. Introduction
Anxiety and depression are the most common psychiatric disorders. Over 20% of the adult population suffer from these illnesses at some time during their lives[1-3]. It has become an important area of research interest in psychopharmacology during this decade[4].
Benzodiazepines are among the most prescribed and effective antianxiety drugs used worldwide[5]. But these are being slowly replaced by antidepressants, which are not only efficacious in depression, but also in the acute and long-term treatment of several anxiety disorders[6]. Consumption of these drugs is believed to double every five years[7]. Most of these drugs, however, have an unfavorable risk and benefit ratio, and their prominent side effects still represent a barrier to long-term treatment with these drugs[8]. In addition, the risk of interaction with other substances is high, particularly with alcohol[9]. Hence, thereis an urgent need to search for newer, better-tolerated, and more efficacious therapeutic agents, for better management of anxiety and depression.
Alpinia nigra(A. nigra) (Gaertn.) Burtt (Zingiberaceae) commonly known as galangal, black-fruited, kala is an aromatic, perennial and rhizomatous herb. It is closely related to the galangal curcuma and ginger[10]. It is widely distributed in Yunnan and Hainan Province of China, Thailand and other Southeast Asian countries[11].
A. nigrahas two flavone glycosides, astragalin (1) and kaempferol-3-O-glucuronide (2)[12]. A number of studies revealed that 1 and 2 possesses several biological activities,e.g., antibacterial[13], antioxidant[14-16], antiprotozoal[17], hepato-protective[18,19] and glycation inhibitory effects[20]. The crude shoot extract is reported to cause destruction of surface tegument leading to paralysis and death of intestinal parasite[21]. The aqueous extract of shoot and rhizome ofA. nigra(common name “Tora”) has been used in Assam for curing health problems like bone weakness, irregular menstruation, jaundice and gastric ulcers[22].
However, so far, its effect on central nervous system (CNS) activity has not been studied. Therefore, we undertook the study to evaluate the anxiolytic potential ofA. nigra, by using different animal models and studying the effect of the plant on their exploratory behavior.
2. Materials and methods
2.1. Preparation of leaf extract
Leaves ofA. nigrawere collected at their full mature form from Bangladesh Centre for Scientific and Industrial Research (BCSIR). The plant was identified and authenticated by standard taxonomical method at BCSIR. The collected leaves were thoroughly washed with distilled water and dried in the sun and mechanical dryer at 60-70 °C. The dried sample was coarsely powdered and extracted with 800 mL methanol for 3 d to allow total extraction process. After that the plant extract was filtered with sterilized cotton filter and the filtrate was collected in a beaker. The plant extract then kept in a water bath at 60 °C to evaporate the solvent from the solution. The container allowed to airtight for 72 h and filtrate thus obtained was concentrated by using a rotary evaporator. The extract was stored in refrigerator at 4 °C until used for treatment[23].
The study was performed to find out if the extract had any effect on central nervous system. Elevated plus maze test was conducted for determination of anxiolytic activity whereas thiopental sodium induced sleeping time test was for sedative activity. Effect on exploratory behavior of mice was evaluated by hole cross test and open field test.
2.2. Drugs and chemicals
The drugs and chemicals used for the experiments were diazepam (Square Pharmaceutical Ltd., Bangladesh), thiopental sodium (Gonoshastho Pharmaceuticals Ltd., Bangladesh), methanol (Sigma Chemicals Co., USA).
2.3. Experimental animals
Swiss albino mice of either sex, weighing between 20-25 g, were collected from Animal Research Branch of BCSIR. Animals were maintained under standard environmental conditions [(24.0±1.0) °C, relative humidity: (55-70)% and 12 h light/12 h dark cycle] and free access to feed and waterad libitum. Prior to experimentation, the animals were acclimatized to laboratory condition for one week. The research was approved by the Institutional Ethics Committee.
2.4. Anxiolytic activity
2.4.1. Elevated plus maze test
In elevated plus maze test, the apparatus was made of wood with two open and two closed arms across each other respectively forming a plus-sign figure. The elevated plus maze (EPM; 30 cm×6 cm×6 cm, each arm) was situated 50 cm above the floor. After administration of the drug, each animal was placed at the center of the maze facing one of the closed arms. The number of open and closed arm entries, plus time spent in open and closed arms was recorded for 5 min at 0, 30, 60, 90, 120 min after administration of the extract (200 and 400 mg/kg), diazepam (1 mg/kg) and vehicle (1% Tween 80 in water). The whole test was carried out in a sound attenuated room[24]. Entry into an arm was defined as the point when the animal placed all four paws onto the arm.
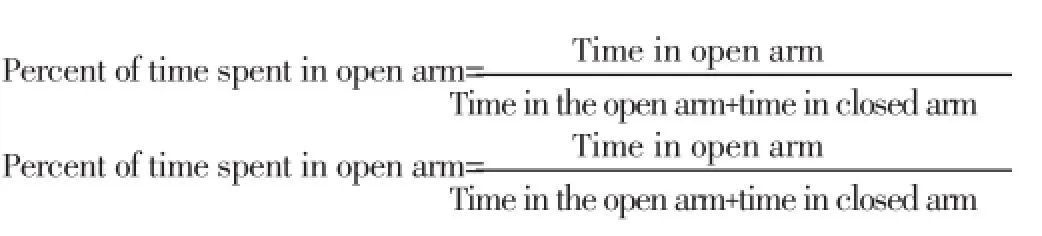
This test has been widely validated for measuring anxiolytic- and anxiogenic-like activities in rodents[25,26].
2.5. Sedative activity
2.5.1. Thiopental sodium induced sleeping time test
For the experiment, the animals were randomly assigned to four groups, each with 5 mice. The test groups were giventhe leaf extract ofA. nigraat doses of 200 and 400 mg/kg body weight, while the positive control was treated with diazepam (1 mg/kg) and control group with vehicle (1% Tween 80 in water). Thirty minutes later, thiopental sodium (40mg/kg) was administered to each mouse to induce sleep. The animals were observed by placing them on separate chambers for the latent period (time between thiopental administrations to loss of righting reflex) and duration of sleepi.e.time between the loss and recovery of righting reflex. The onset of sleep and total sleeping time were recorded for control, positive control and test groups[27].
2.6. Exploratory activity
2.6.1. Open field test
The method was adopted as described by Guptaet al[28]. In open field test, the animals were divided into control, positive control and test groups containing 5 mice each. The test groups received extract ofA. nigraat the doses of 200 and 400 mg/kg body weight orally whereas control group received vehicle (1% Tween 80 in water). The floor of half square meter open field was divided into a series of squares each alternatively colored black and white. The apparatus had a 40 cm height wall. The number of squares traveled by the animals was counted for 5 min at 0, 30, 60, 90, 120 min after oral administration of both doses of the extract.
2.6.2. Hole cross test
The apparatus was a cage of 30 cm×20 cm×14 cm with a steel partition fixed in the middle, dividing the cage into two chambers. A hole of 3.5 cm diameter was made at a height of 7.5 cm in the center of the cage. Animals were randomly divided into control, positive control and test groups containing 5 mice each. The test groups were treated with extract ofA. nigraat the doses of 200 and 400 mg/kg body weight orally whereas positive control group with diazepam (1 mg/kg) and control group with vehicle (1% Tween 80 in water). Number of passages of the animals through the hole from one chamber to the other was counted for 5 min at 0, 30, 60, 90 and 120 min after oral administration of the extract as well as diazepam and vehicle[29]. The apparatus was thoroughly cleaned after each trial.
2.7. Statistical analysis
The data were expressed as mean±standard error of mean (S.E.M.). Statistical comparisons were performed using One way ANNOVA followed by Dunnett’s multiple comparison test. The values obtained were compared with the vehicle control group and were considered statistically significant whenP<0.05.
3. Results
In the EPM, the behavior of mice model, as observed, confirmed the anxiolytic activity of diazepam as reported previously. The methanol extract ofA. nigraat the dose of 400 mg/kg (P<0.01), significantly increased the percentage of entries of mice into the open arms, and the percentage of time spent in the open arms of the EPM as shown in Table 1. The effects of treatment of mice at the dose of 200 mg/kg on open arm entries and time spent in open arms were dosedependent. The number of closed arm entries and time spent in the closed arms were decreased significantly in the extract treated groups which was comparable with the standard diazepam. The effect of the methanolic extract ofA. nigraon sodium thiopental induced hypnosis in mice is shown in Table 2.
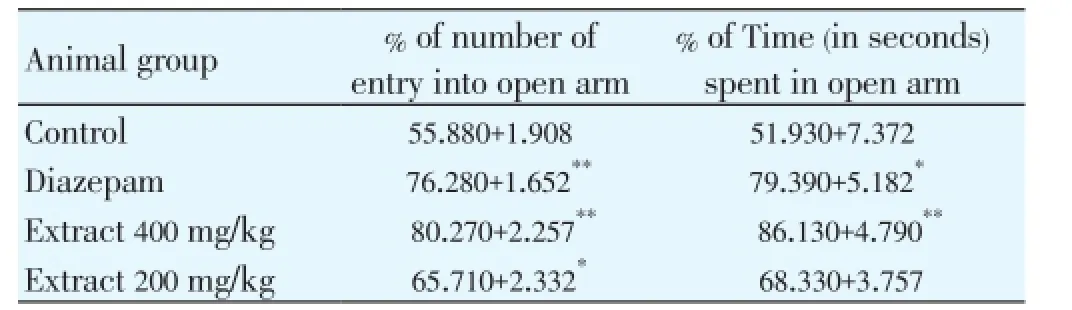
Table 1 Effect of methanolic extract of A. nigra on EPM test during 5 min test session.

Table 2 Effect of methanolic extract of A. nigra on thiopental sodium induced sleeping time.
In the thiopental induced hypnosis test, the extract at doses, 200 and 400 mg/kg showed a significant reduction in the time of onset of sleep in a dose-dependent manner (Table 2). The effect of the extract (200 and 400 mg/kg) on the onset of sleep were comparable to that of standard. Both doses of the extract potentiated the duration of thiopental sodium induced sleeping time in test animals compared to controls(Table 2).
Open field test ofA. nigratreated groups (200 and 400 mg/ kg body weight) showed significant and dose-dependentreduction of movement from its initial value at 0 to 120 min (Figure 1). The number of squares traveled by the mice was decreased significantly from its initial value at 0 to 90 min at the dose level of 400 mg/kg body weight (P<0.01) of the methanol extract from the leaves ofA. nigra(Figure 1).
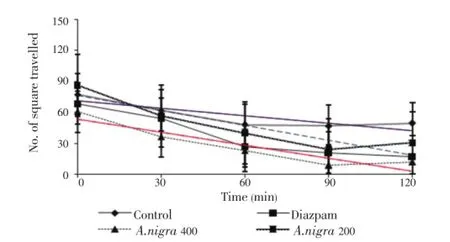
Figure 1. Effect of methanolic extract of A. nigra on exploratory behaviour open field test in mice.Values are mean±S.E.M., (n=5);*P< 0.05,**P< 0.01, Dunnet test as compared to control (Vehicle=0.4 mL/mouse).
The number of hole crossed from one chamber to another by mice of the control group was similar from 30 to 120 min (Figure 2). Hole cross test ofA. nigratreated groups showed decrease of movement from its initial value at 0 to 90 min. But, at doses of 400 mg/kg (P<0.01), maximum suppression of locomotor activity was displayed which was comparable to the reference drug diazepam (Figure 2).
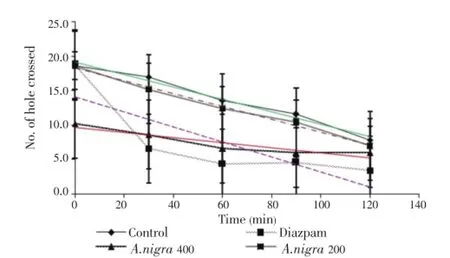
Figure 2. Effect of methanolic extract of A. nigra on exploratory behaviour (hole cross test).Values are mean±S.E.M., (n=5);*P<0.05,**P<0.01. Dunnet test as compared to control (Vehicle=0.4 mL/mouse).
4. Discussion
This study examined some neuropharmacological effects ofA. nigraand established that it has anxiolyticand antidepressant-like activities. The EPM is one of the most widely validated tests and is highly sensitive to the influence of both anxiolytic and anxiogenic drugs acting at the gamma aminobutyric acid type A (GABAA)-benzodiazepine complex[30]. In EPM, normal mice will normally prefer to spend much of their allotted time in the closed arms. This preference appears to reflect an aversion towards open arms that is generated by the fears of the open spaces. Drug like diazepam that increases open arm exploration are considered as anxiolytic and the reverse holds true for anxiogenics[31]. In this study, we observed that the administration of different doses (200 and 400 mg/ kg body weight) of methanolic extract ofA. nigrainduced an anxiolytic-like effect in mice, as it increased open arm entries and the time spent in the open arms of the EPM when compared to the control animals.
Earlier reports showed a significant decrease in the locomotor score for diazepam when compared to the control animals. Locomotor activity is considered as an index of alertness and a decrease in that indicates a sedative effect[32]. Both the doses (200 and 400 mg/kg body weight) of theA. nigrashowed a decrease in the locomotor score and produced a significant increase in the hypnotic effect induced by the thiopental sodium, in a dosedependent manner, thus suggesting a profound sedative activity. Thiopental is basically a hypnotic agent, given at appropriate dose, induced hypnosis by potentiating GABA mediated post synaptic inhibition through allosteric modification of GABAA receptors. Substances which possess CNS depressant activity either decrease the time for onset of sleep or prolong the duration of sleep or both[33,34]. In addition, the study on locomotor activity, as measured by hole cross and open field tests, showed that both doses of methanol extract from the leaves ofA. nigradecreased the frequency and the amplitude of movements.
The method employed for this assay is considered as a very sensitive way to detect agents with CNS depressant activity[35]. The sedative effect recorded here may be related to an interaction with benzodiazepines and related compounds that bind to receptors in the CNS and have already been identified in certain plant extracts. Phytochemical analysis of the plant extract reveals thatA. nigracontains alkaloids, glycosides, cardiac-glycosides, flavonoids, steroids, tannins, anthraquinone glycosides and saponins. Many flavonoids and neuro-active steroids were found to be ligands for the GABAA receptors in the CNS; which led to the hypothesis that they act as benzodiazepine-like molecules[36-38]. This is supported by the present study on the behavioral effects in animal models of anxiety and sedation.
It may possible that the mechanism of anxiolytic action ofA. nigramethanol extract could be due to the binding of any of the phyto-constituents to the GABAA-BZD complex. In support of this, it has been found that flavones bind withhigh affinity BZD site of the GABAA receptor[39].
The locomotor activity is a measure of the level of excitability of the CNS and sedation resulting from depression of the central nervous system[40]. The result indicated that the extract significantly decreased the locomotor activity as shown by the results of the open field and hole cross tests. The results were also dose-dependent and statistically significant. Therefore, the use ofA. nigrain folkloric medicine may be due to its CNS action validated by our findings. However, further investigation is necessary to determine the exact phyto-constituents and mechanism of action that are responsible for the biological activities of the methanol extract ofA. nigra.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
Authors would like to thank Department of Genetic Engineering and Biotechnology, University of Chittagong for thesis financial support for conducting this research work (Ref No. GEB/ AC-54/ 18-09-2010). Special thanks to Dr. Md. Atiar Rahman, Assistant Professor, Dept. of Biochemistry and Molecular Biology, University of Chittagong for his guidance during the study.
Comments
Background
Anxiety and pain are neurologic disorders. CNS depressants can reduce muscle tension, pain, insomnia and acute stress reactions. Natural products can be good sources of potent and effective CNS depressants that are needed for various medicinal purposes.
Research frontiers
This study was aimed to investigate thein vivoneuropharmacologic effect ofA. nigraleaf extract at different doses using mice behavioral models. Four different experiments were conducted to observe the CNS depressant effect.
Related reports
Previous studies also claimed similar medicinal impacts ofA. nigra. It contains several compounds having roles in metabolic pathways. It has been reported to possess antioxidant potential besides having folklore use against pain.
Innovations and breakthroughs
Neuropharmacological studies are very significant nowadays due to the lack of effective drugs and medications. Identification of neuropharmacologically active compounds in plants can help to develop new drugs. This research-based study shows the possible role of a medicinal plant in this regard.
Applications
This will help to observe the relation of the identified compounds from this plant with other neurotransmitters and metabolic pathways. Researchers can become interested to find out the active component(s) responsible for anxiolytic effect of this plant.
Peer review
It is an interesting paper in which the authors tried to focus on the neuropharmacological effect ofA. nigraleaf extract on mice. The anxiolytic activity was determined by well-established protocols that signify an antidepressant effect found in this leaf extract. It is stimulating enough for further research works to develop a new therapeutic agent for anxiety and depression.
[1] Buller R, Legrand V. Novel treatments for anxiety and depression: hurdles in bringing them to the market. Drug Discov Today 2001; 6: 1220-1230.
[2] Yadav AV, Kawale LA, Nade VS. Effect of Morus alba L. (mulberry) leaves on anxiety in mice. Indian J Pharmacol 2008; 40: 32-36.
[3] Titov N, Andrews G, Kemp A, Robinson E. Characteristics of adults with anxiety or depression treated at an internet clinic: comparison with a national survey and an outpatient clinic. PLOS ONE 2010; 5(5): e10885.
[4] Woode E, Abotsi WK, Mensah AY. Anxiolytic-and antidepressant-like effects of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt. in mice. J Nat Pharm 2011; 2: 62-71.
[5] Rabbani M, Sajjadi SE, Mohammadi A. Evaluation of the anxiolytic effect of Nepeta persica Boiss. in mice. Evid-Based Complement Alternat Med 2008; 5(2): 181-186.
[6] Raihan MO, Habib MR, Brishti A, Rahman MM. Sedative and anxiolytic effects of the methanolic extract of Leea indica (Burm. f.) Merr. leaf. Drug Discov Ther 2011; 5(4): 185-189.
[7] Rahman H, Elumalai A, Eswaraiah MC, Bardalai D. Evaluation of anxiolytic activity of ethanolic extract of Pisonia grandis R. Br leaves in mice. J Chem Pharm Res 2011; 3(5): 646-652.
[8] Newman M, Lhuillier A, Poulsen AD. Checklist of the Zingiberaceae of Malesia. Blumea Suppl 2004; 16: 166.
[9] Wu DL. Flora of China.Vol.16, Fascicle 2. Beijing: Science Press; 1981, p. 67-106.
[10] Nishat FS, Nilima N, Saikia BM. Phytochemical analysis of Lasia spinosa and Alpinia nigra, potential medicinal plants of Assam. Med Plants-Int J Phytomed Relat Ind 2012; 4(3): 170-173.
[11] Panizzi L, Caponi C, Catalano S, Cioni PL, Morelli I. In vitro antimicrobial activity of extracts and isolated constituents of Rubus ulmifolius. J Ethnopharmacol 2002; 79: 165-168.
[12] Qiao CF, Wang ZT, Dong H, Xu LS, Hao XJ. The chemical constituents of blackfruit galangal (Alpinia nigra). Chin Tradit Herb Drugs 2000; 31: 404-405.
[13] Sinha M, Choudhury MD. Antimicrobial activity of some selected plants from Southern Assam. Assam Univ J Sci Tech 2010; 6(1): 58-65.
[14] Das BN, Biswas BK. Anti-inflammatory activity of the rhizome extract of Alpinia nigra. Int Res J Pharma 2012; 2(3): 73-76.
[15] Ghosh S, Padilla-González GF, Rangan L. Alpinia nigra seeds: a potential source of free radical scavenger and antibacterial agent. Ind Crop Prod 2013; 49: 348-356.
[16] Apati P, Houghton PJ, Kery A. HPLC investigation of antioxidant components in Solidago herba. Acta Pharm Hung 2004; 74: 223-231.
[17] Fernando C, Alma DA. Additional antiprotozoal flavonol glycosides of the aerial parts of Helianthemum glomeratum. Phytother Res 2007; 21: 78-80.
[18] Swargiary A, Roy B, Ronghang B. Partial characterisation of alkaline phosphatase in Fasciolopsis buski-an intestinal fluke treated with crude extract of Alpinia nigra (Zingiberaceae). J Pharm Tech Drug Res 2013; DOI:10.7243/2050-120X-2-5.
[19] Singab AN, Youssef DT, Noaman E, Kotb S. Hepatoprotective effect of flavonol glycosides rich fraction from Egyptian Vicia calcarata Desf. against CCl4-induced liver damage in rats. Arch Pharm Res 2005; 28: 791-798.
[20] Kim HY, Moon BH, Lee HJ, Choi DH. Flavonol glycosides from the leaves of Eucommia ulmoides O. with glycation inhibitory activity. J Ethnopharmacol 2004; 93: 227-230.
[21] Roy B, Tandon V. Flukicidal activity of Alpinia nigra (Zingiberaceae) against the trematode, Fasciolopsis buski in humans. Biomedical Lett 1999; 60: 23-29.
[22] Tushar BS, Sarma GC, Rangan L. Ethno-medical uses of Zingiberaceous plants of Northeast India. J Ethnopharmacol 2010; 132(1): 286-296.
[23] Baruah DB, Dash RN, Chaudhari MR, Kadam SS. Plasminogen activators: a comparison. Vascul Pharmacol 2011; 44: 1-9.
[24] Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 1986; 24: 525-529.
[25] Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987; 92: 180-185.
[26] Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 1985; 14: 149-167.
[27] Ferrini R, Miragoli G, Taccardi B. Neuro-pharmacological studies on SB 5833, a new psychotherapeutic agent of the benzodiazepine class. Arzneim-Forsch (Drug Res) 1974; 24: 2029-2032.
[28] Gupta BD, Dandiya PC, Gupta ML. A psychopharmacological analysis of behavior in rat. Jpn J Pharmacol 1971; 21: 293.
[29] Takagi K, Watanabe M, Saito H. Studies on the spontaneous movement of animals by the hole cross test; effect of 2-dimethylaminoethanol and its acyl esters on the central nervous system. Jpn J Pharmacol 1971; 21: 797-810.
[30] Dhonnchadha BAN, Bourin M, Hascoet M. Anxiolytic-like effects of 5-HT2 ligands on three mouse models of anxiety. Behav Brain Res 2011; 140: 203-214.
[31] Subramanian N, Jothimanivannan C, Kumar RS, Kameshwaran S. Evaluation of anti-anxiety activity of Justicia gendarussa burm. Pharmacologia 2013; 4(5): 404-407.
[32] Thakur VD, Mengi SA. Neuropharmacological profile of Eclipta alba (Linn.) Hassk. J Ethnopharmacol 2005; 102(1): 23-31.
[33] Raquibul Hasan SM, Hossain MM, Akter R, Jamila M, Mazumder EHM, Rahman S. Sedative and anxiolytic effects of different fractions of the Commelina benghalensis Linn. Drug Discov Ther 2009; 3: 221-227.
[34] Sen AK, Bose S, Dutta SK. Comparative evaluation of CNS depressant activity of the flavonoid fractions from the fresh leaves and flowers of Ixora coccinea Linn. J Pharma Sci Tech 2011; 1(1): 54-56.
[35] Hossain MM, Biva IJ, Jahangir R, Vhuiyan MMI. Central nervous system depressant and analgesic activity of Aphanamixis polystachya (Wall.) parker leaf extract in mice. Afr J Pharm Pharmacol 2009; 3(5): 282-286.
[36] Fernández SP, Wasowski C, Loscalzo LM, Granger RE, Johnston GA, Paladini AC, et al. Central nervous system depressant action of flavonoid glycosides. Eur J Pharmacol 2006; 539: 168-176.
[37] J?ger AK, Saaby L. Flavonoids and the CNS. Molecules 2011; 16: 1471-1485.
[38] Johnston GA. GABA(A) receptor channel pharmacology. Curr Pharm Des 2005; 11: 1867-1885.
[39] Hanrahan JR, Chebib M, Johnston GAR. Flavonoid modulation of GABAA receptors. Br J Pharmacol 2011; 163(2): 234-245.
[40] Thirupathy KP, Tulshkar A, Vijaya C. Neuropharmacological activity of Lippia nodiflora Linn. Pharmacognosy Res 2011; 3(3): 194-200.
10.1016/S2221-1691(14)60222-7
*Corresponding author: A. M. Abu Ahmed, Department of Genetic Engineering and Biotechnology, University of Chittagong, Chittagong-4331, Bangladesh.
E-mail: abugebcu@yahoo.com
Foundation Project: Supported by Department of Genetic Engineering and Biotechnology, University of Chittagong. (Grant No.Ref No. GEB/ AC-54/ 18-09-2010).
Article history:
Received 18 Oct 2013
Received in revised form 27 Oct , 2nd revised form 11 Nov, 3rd revised form 7 Dec 2013
Accepted 26 Jan 2014
Available online 28 Feb 2014
Methods:In present study, the extract of Alpinia nigra was evaluated for its central nervous system depressant effect using mice behavioral models, such as hole cross, open field and thiopental sodium induced sleeping time tests for its sedative properties and an elevated plusmaze test for its anxiolytic potential, respectively.
Results:In anxiolytic study, the extract displayed increased percentage of entry into open arm at the dose of 400 and 200 mg/kg. The extract produced a significant (P<0.01) increase in sleeping duration and reduction of onset of sleep compared to sodium thiopental at both doses (200 and 400 mg/kg). The extract (200 and 400 mg/kg) also showed a dose-dependent suppression of motor activity and exploratory activity of the mice in both open field and hole cross test.
Conclusion:This study demonstrates that the treated extract has significant central nervous system depressant effect. Further studies on active constituent of the extract can provide approaches for therapeutic intervention.
 Asian Pacific Journal of Tropical Biomedicine2014年2期
Asian Pacific Journal of Tropical Biomedicine2014年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Prevalence and antibiogram of bacterial isolates from urinary tract infections at Dessie Health Research Laboratory, Ethiopia
- Inhalation of Shin-I essential oil enhances lactate clearance in treadmill exercise
- Anti-inflammatory activity and qualitative analysis of different extracts of Maytenus obscura (A. Rich.) Cuf. by high performance thin layer chromatography method
- Ameliorative effect of alkaloid extract of Cyclea peltata (Poir.) Hook. f. & Thoms. roots (ACP) on APAP/CCl4induced liver toxicity in Wistar rats and in vitro free radical scavenging property
- A pharmacobotanical study of two medicinal species of Fabaceae
- Pancreatic islet regeneration and some liver biochemical parameters of leaf extracts of Vitex doniana in normal and streptozotocin-induced diabetic albino rats
