Formulation and evaluation of novel stomach specific floating microspheres bearing famotidine for treatment of gastric ulcer and their radiographic study
Rishikesh Gupta, Sunil Kumar Prajapati, Snigdha Pattnaik, Peeyush Bhardwaj
1Institute of Pharmacy, Bundelkhand University, Jhansi, Uttar Pradesh, India
2School of Pharmaceutical Sciences, Siksha O Anusandhan University, Bhubaneswar, Orissa, India
Formulation and evaluation of novel stomach specific floating microspheres bearing famotidine for treatment of gastric ulcer and their radiographic study
Rishikesh Gupta1*, Sunil Kumar Prajapati1, Snigdha Pattnaik2, Peeyush Bhardwaj1
1Institute of Pharmacy, Bundelkhand University, Jhansi, Uttar Pradesh, India
2School of Pharmaceutical Sciences, Siksha O Anusandhan University, Bhubaneswar, Orissa, India
ARTICLE INFO
Article history:
Received 20 Feb 2014
Received in revised form 26 Mar 2014
Accepted 15 May 2014
Available online 28 Jun 2014
Floating microspheres
Objective:To develop and characterize multiple-unit-type oral floating microsphere of famotidine to prolong gastric residence time and to target stomach ulcer.
1. Introduction
Oral rout of administration is the most convenient and widely used method of drug administration, and the development of stomach specific oral controlled-release drug delivery systems is a challenging job due to the variation of pH in different segments of the gastrointestinal tract, the fluctuation in gastric emptying time and the difficulty of localizing an oral delivery system in a selected region of the gastrointestinal tract. Rapid gastrointestinal transit can prevent the absorption of complete drug in the absorption zone and reduce the efficacy of the administered dose since the majority of drugs are absorbed in stomach or the upper part of small intestine[1,2].
To overcome the above discussed issues, many types of oral controlled drug delivery systems having prolonged gastric residence times have been reported such as: floating drug dosage systems (FDDS)[3-7], swelling or expanding system[8], mucoadhesive systems[9,10], modified-shape systems[11], high-density systems and other delayed gastric emptying devices[12].
FDDS have lower density than gastric fluids and thus remain buoyant in the stomach fluid without affecting the gastric emptying for a prolonged period of time. While the system is floating in the gastric fluid, the drug is released slowly from the system at a desired rate. Materials used for FDDS include carbon dioxide gas-forming agents (carbonate or bicarbonate compounds)[8,13], highly swellable hydrocolloids and light mineral oils[14,15]. Multiple unit systems and floating systems prepared by solvent evaporation methods have also been developed[12,16-20].
However, it has been shown that products based on a multiple unit system comprising many small units haveadvantages over single-unit preparations such as matrix tablets[21]. The gastric emptying of multiple unit dosage forms occur gradually, in a more consistent manner, with less individual variation[2,22]. Multiple unit dosage forms also have the potential to distribute widely over a large area in the stomach and small intestine, thus yielding a more predictable drug release by suppressing the effect of many variables in the gastrointestinal environment. As multiple unit dosage forms consist of many small units, less risk of dosage dumping is expected[23].
Famotidine, a competitive histamine H2-receptor antagonist is used to treat gastrointestinal disorders such as gastric or duodenal ulcer, gastroesophageal reflux disease, and pathological hypersecretory conditions. Famotidine inhibits many of the isoenzymes of the hepatic CYP450 enzyme system. Other actions of famotidine include an increase in gastric bacterial flora such as nitrate-reducing organisms[24].
Famotidine is widely used as the treatment of peptic ulcer disease and gastroesophageal reflux disease. Famotidine binds competitively to H2-receptors located on the basolateral membrane of the parietal cell, blocking histamine affects. This competitive inhibition results in reduced basal and nocturnal gastric acid secretion and a reduction in gastric volume, acidity, and amount of gastric acid released in response to stimuli including food, caffeine, insulin, betazole and pentagastrin[25].
Floating microspheres are one of the multiparticulate delivery system and are prepared to obtain prolonged or controlled drug delivery to improve bioavailability and to target drug to specific sites. Microspheres can also offer advantages like limiting fluctuation within therapeutic range, reducing site effects, decreasing dosing frequency and improving patient compliance[26].
In the present study, the multiple-unit-type oral floating microspheres bearing famotidine were developed by modified solvent evaporation technique. Eudragit S-100 was used as polymer.
The high surface tension of stirring phase causes the solidification and aggregation of polymer on the surface when the polymeric solution is poured from upside. To minimize the contact of polymer solution with interface, a new method of introducing the polymer solution into stirring phase was adopted as established by Leeet al[27].
2. Materials and methods
Famotidine was obtained as a gift sample from Cadila Pharmaceutical Ltd., Mumbai (Maharastra). Eudragit S100 (Rohm Pharma GmbH, Germany) was used as polymers. All other chemicals and reagents were of analytical grade and were used without further purification.
2.1. Preparation of floating microspheres
Floating microspheres were prepared by the method reported by Kawashimaet al. with slight modifications[19]. Ten formulations of floating microspheres were developed. Different ratios of famotidine and Eudragit S-100 were mixed in a mixture of dichloromethane (DCM) and ethanol (Table 1). The resulting suspension was added slowly into stirring to 0.25% w/v 400 mL solution of polyvinyl alcohol (PVA) at room temperature from the bottom side as shown in Figure 1[27]. The stirring was continued for 2 h by mechanical stirrer equipped with four blade propellers in order to evaporate the solvent. After evaporation of solvent, microspheres were collected by filtration and washed repeatedly with water. The collected microspheres were dried at room temperature and stored in a desiccator.
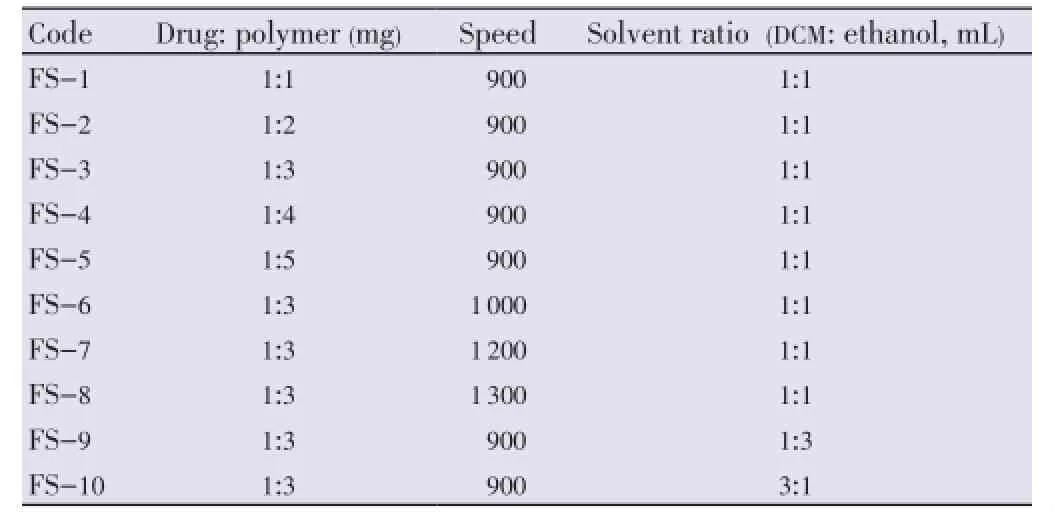
Table 1 Batch specifications for floating microspheres.
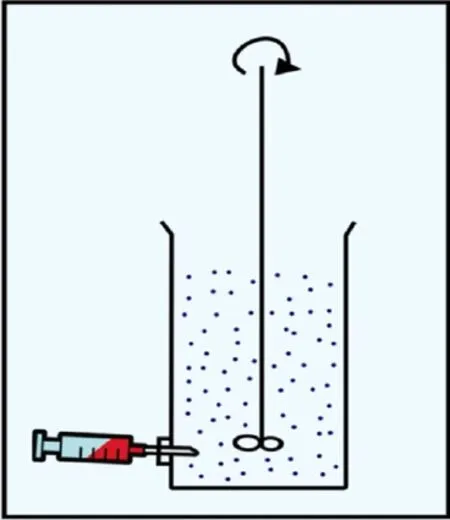
Figure 1. Method of injection of volatile phase into PVA solution.
2.2. Characterization of floating microspheres
2.2.1. Morphology
The morphology of microspheres was studied by scanning electron microscopy (SEM). The samples for SEM were prepared by sprinkling the microspheres on a both side adhesive tape stuck to a stub. Gold palladium coating on the prepared stub was done by using sputter coater (POLARON model SC-76430). The thickness of coating was 200 ?. The coated stubs were randomly scanned under electron microscope (LEO-430, UK). The photomicrograph of prepared microspheres are presented in Figure 2.

Figure 2. Scanning electron micrograph of floating microsphere.A: Photo micrograph showing population of batch FS-3; B: Photo micrograph showing a floating cavity and porous surface in microsphere of batch FS-5.
2.2.2. Particle size analysis
The size of microspheres was determined by using an optical microscope (Magnus MLX-DX, Olympus, India) fitted with an ocular micrometer and a stage micrometer. The mean particle size was calculated by measuring 200-300 particles.
2.2.3. Micromeritic properties
The prepared microspheres were characterized for their micromeritic properties such as true density, tapped density and % compressibility index. The tapping method was used to calculate tapped densities and % compressibility index using following equations[28]:
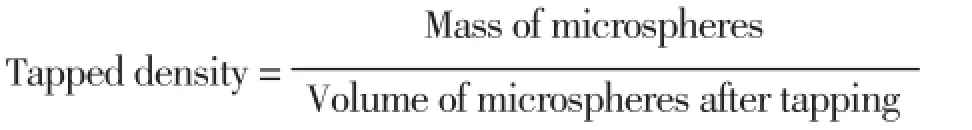
Compressibility index (%) =(1-V/VO)×100
Where V and Voare the volumes of the samples after and before the standard tapping respectively.
True density of floating microspheres was determined by immersing the microparticles in 0.02% Tween 80 solution for three days in a metal mesh basket. The microparticles that were sunk after the process were used for density measurement as carried out by the displacement method[29].
2.2.4. Angle of repose
Flow characteristic such as angle of repose (θ) of the microspheres, which measures the resistance to particle flow, was determined by fixed funnel method using following equation[28]:
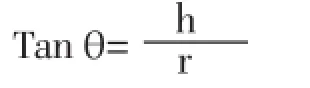
Where, h is the height of pile, r is the radius of the base of pile on the graph paper.
2.2.5. Yield of microspheres
The prepared microspheres were collected and weighed. The actual weight of obtained microspheres divided by the total amount of all drug and polymer solid material that was used for the preparation of the microspheres[30]:
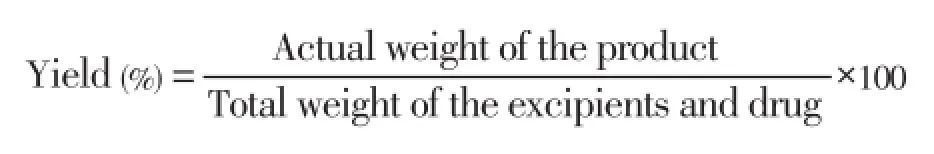
2.2.6. Incorporation efficiency
To determine the incorporation efficiency 50 mg microspheres were taken and dissolved in 25 mL of methanol. The solution was filtered to separate shell fragments. The estimation of drug was carried out at the λmax of 264 nm by using a UV double-beam spectrophotometer (Shimadzu UV-1 800 series). The incorporation efficiency was calculated using following equation[30]:

2.2.7. In-vitro floating ability
Floating microspheres (50 mg) were placed in 100 mL beaker. A total of 10 mL of 0.1 mol/L HCl containing 0.02% Tween 20 was added to that. The beaker was shaken horizontally in a water bath at (37.0±0.1) °C. Floated particles were collected after 20 h and dried in a desiccator till constant weight. This process was applied to all the batches. Floating microspheres are shown in Figure 3. The percentage of floating microspheres was calculated using following equation[30]:

Figure 3. Floating microspheres.
2.3. In-vitro drug release
In-vitrodrug release studies were performed in 0.1 mol/L HCl (pH 1.2) for floating microspheres. The drug release rate of the floating microspheres was determined in 900 mL 0.1 mol/L HCl at 100 r/min by using USP XXIII dissolution apparatus (paddle type). A weighed amount of floating microspheres equivalent to 50 mg drug was placed in a non-reacting muslin cloth having a smaller mesh size than the microspheres. The mesh was tied with a nylon thread to avoid the escape of any microspheres and a glass bead was placed in the mesh to induce sinking of microspheres in the dissolution medium[31]. The temperature of dissolution medium was maintained at
(37.0±0.5) °C.
At specified time intervals, 5 mL aliquots were withdrawn, filtered, diluted with the same medium and assayed at 264 nm for famotidine using a UV double-beam spectrophotometer (Shimadzu UV-1 700 series). Samples withdrawn were replaced with equal volume of the same dissolution medium. All the experiments as specified above were conducted in triplicate.
2.4. Kinetics of drug release
The zero-order rate (equation 1) describes systems where drug release is independent of its concentration and this is applicable to the dosage forms like transdermal system, coated forms, osmotic system as well as matrix tablets with low soluble drugs. The first-order equation (equation 2) describes systems in which the release is dependent on its concentration (generally seen for water-soluble drugs in porous matrix). The Higuchi model describes the release of the drug from an insoluble matrix to be linearly related to the square root of time and is based on Fickian diffusion (equation 3). The Hixson-Crowell cube root law (equation 4) describes the release of drug from systems where it depends on the change in surface area and diameter of the particles or tablets with time and mainly applies in the case of systems that dissolute or erode over time. In order to authenticate the release model, dissolution data can further be analyzed by Peppas and Korsmeyer equation (equation 5).
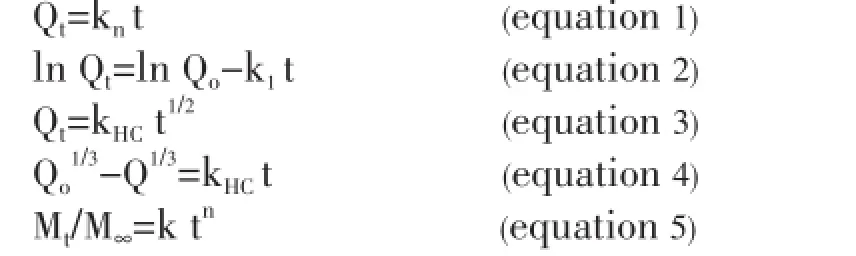
Where Qtis the amount of drug released at time t; Q0is the initial amount of the drug in the formulation; k0, k1, kH, and kHCare release rate constants for zero-order, firstorder, Higuchi model and Hixson-Crowell rate equations. In equation 5, Mtis the amount of drug released at time t, and M∞is the amount of drug released at time ∞; k is the kinetic constant, and n is the diffusion coefficient[32].
The release data of various formulations were fitted in various models to ascertain the mechanism of drug release.
2.5. Preparation of dosage form for radiographic study
The optimized formulations which showed goodinvitrobuoyancy and sustained release behavior were finally selected for radiography study. The drug in all selected formulations was replaced with the same amount of barium sulphate while all other ingredients were kept constant. These formulations were analyzed for their physical properties. The analysis confirmed that the developed dosage forms were similar to those containing drug.
2.5.1. Radiographic studies
The experimental protocol to carry outin-vivoradiographic studies were reviewed and approved by the Institutional Animals Ethical Committee of Institute of Pharmacy, Bundelkhand University, Jhansi, India (Registration No. BU/ Pharm/IAEC/10/031). Thein-vivoradiographic studies were conducted in young and healthy male albino rabbits weighing 2.0 kg to 2.2 kg. The animals were kept under standard laboratory conditions [temperature (25±2) °C]. Rabbits were kept for one week in animal house to acclimatize them and were fed a fixed standard diet. The 4 healthy male albino rabbits were used to monitor thein-vivotransit behavior of the prepared floating microspheres. None of the animal had symptoms or past history of gastro-intestinal disease. In order to standardize the conditions of gastrointestinal motility, the animals were fasted for 12 h prior to the commencement of each experiment. In each experiment, the first radiographic image of the animal subjects was taken to ensure the absence of radio-opaque material in the gastrointestinal tract. One of each dosage form (capsule containing microspheres) prepared for radiography was orally administered to rabbits with sufficient amount of water. During the study the rabbits were not allowed to eat, but water available withad libitum[33,34].
For radiographic imaging, the legs of the rabbit were tied over a piece of plywood (20 inch×20 inch), and location of the formulation in the stomach was monitored by keeping the subjects in front of X-ray machine (Allengers, Bharat Electricals, India, model No. E-080743). The distance between the source of X-rays and the object was kept same during the imaging process. Gastric radiography was done at the intervals of 0 h, 1 h, 3 h, 5 h and 6 h. In between the radiographic imaging, the animals were freed and allowed to move and carry out normal activities but were not allowed to take any food.
3. Results
The formulations of famotidine loaded floating microspheres were developed by modified solvent evaporation method. In this method, the drug-polymeric suspension was introduced into stirring 0.25% w/v solution of PVA from bottom side to reduce the aggregation of polymer on the top of PVA. This technique produced more spherical microspheres and good yield of product could be achieved.
The formulation mechanism of polymeric floating microspheres is well documented in the literature (Figure 4). The polymer and drug were dissolved in solvent mixture suchas dichloromethane and ethanol. The polymeric mixture at different ratio were dissolved in a volatile solvent (ethanol, dichloromethane combined) as given in Table 1. This polymeric drug solution was poured in 0.25% w/v solution of PVA. Thus, an O/W emulsion was formed. The ethanol present in oil droplet diffuse, to water thus polymer and drug mixture solidified on dichloromethane droplet. Now the temperature was raised up to 45 °C-50 °C. After evaporation of solvent, microspheres were collected by filtration, washed with water, and dried at room temperature. As a result, hollow cavity
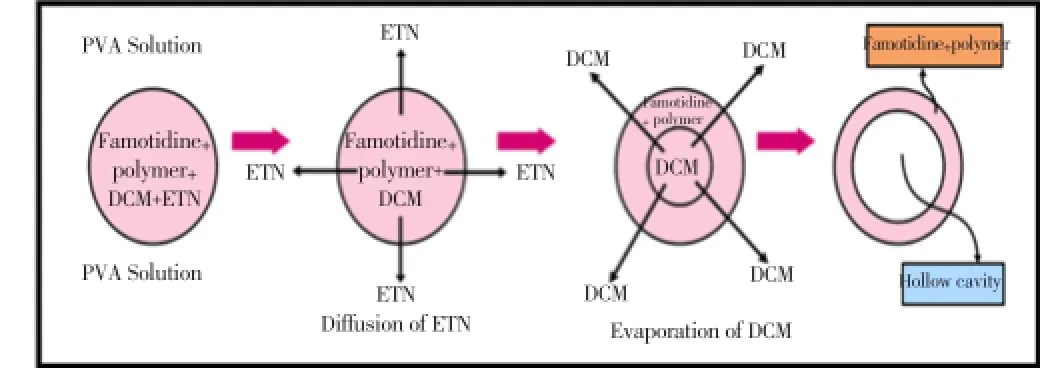
Figure 4. Mechanism of formation of floating microspheres. ETN: Erythrityl tetranitrate; DCM: Methylene chloride.
SEM photo micrograph confirmed that prepared microspheres were spherical with smooth perforated surface (Figure 2). The formation of perforation may be attributed to evaporation of alcohol form embryonic microspheres. The prepared microspheres were found to be spherical in shape with smooth perforated surface as indicated by photomicrographs. Floating cavities could also be observed on some micrographs.
The mean particle size of the floating microspheres was found to be ranging from (52.18±1.82) μm to (91.64±5.16) μm. It was observed that, on increasing the polymer amount, the average particle size increased. This may be due to diminished shearing efficiency at higher concentration of the polymer (higher viscosity). It was also observed that on increasing the volume of DCM, average particle size was found to be increased (Table 2).
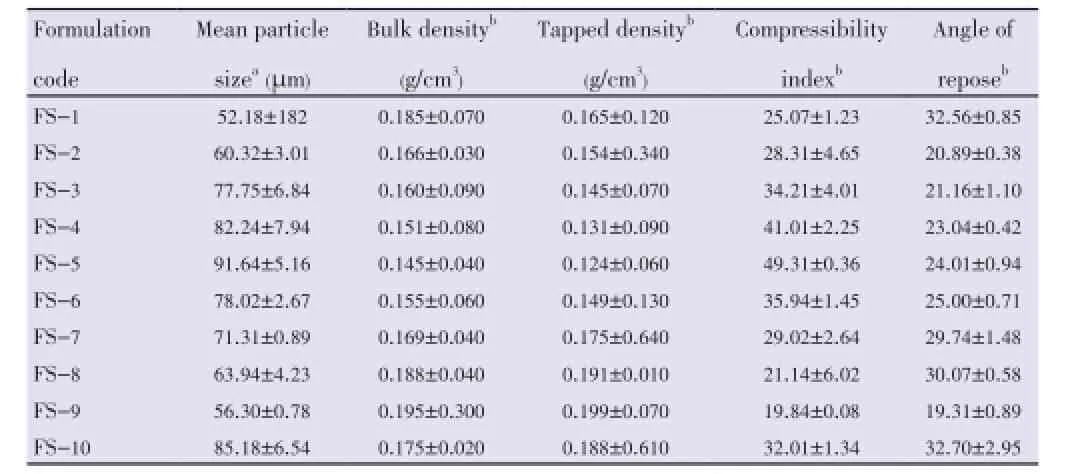
Table 2 Micromeritic properties of floating microspheres.
The measured tapped density was found to be in the range of (0.124±0.06) g/cm3to (0.199±0.070) g/cm3. Compressibility index ranged from (19.84±0.08) to (49.31±0.36) (Table 2).
The angle of repose was found to be in the range of (19.31 ±0.89) to (32.56±0.85). All the batches showed good flow properties except bathes FS-1 and FS-8 which exhibited higher angle of repose (Table 2).
The yield was found to be ranging from (69.74±1.35)% to (95.11±0.35)%. The drug entrapment/incorporation efficiency of microspheres was found to be satisfactory (55.75±2.94)% to (75.35±1.96)% (Table 3).

Table 3 Various formulation parameters of floating microspheres.
All floating microspheres showed good floating ability/ buoyancy (61.64±2.67)% to (95.81±2.46)% up to 20 h. This could be attributed to insolubility of used polymers in the gastric fluid. It was observed that the floating ability increased with increasing average particle size (Table 3).
It was also observed that the formulation prepared with higher volume of DCM (batch FS-10) showed better floating ability as compared to other batches. The reason behind this may be the formation of larger air core, which made them lesser dense than that of gastric fluid.
The release of famotidine from the developed floating microspheres was studied in 0.1 mol/L HCl (pH 1.2) up to 20 h. Since the acrylic polymers are insoluble in acidic medium, the microspheres retained their integrity duringinvitrodissolution studies. The cumulative % drug release for microspheres was found to be between 85-98% (Figures 5- 7).
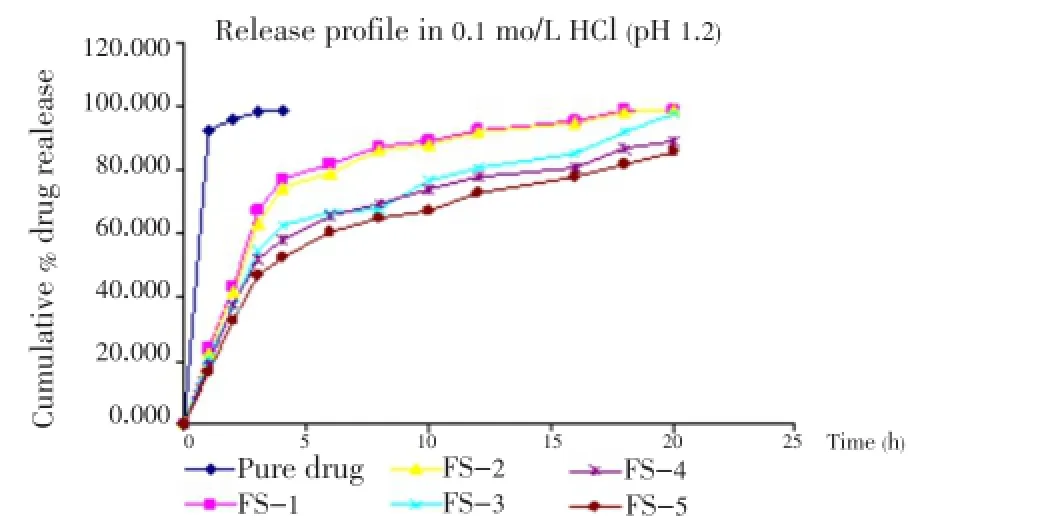
Figure 5. Effect of polymer ratio on release profile of drug in 0.1 mol/L HCl.
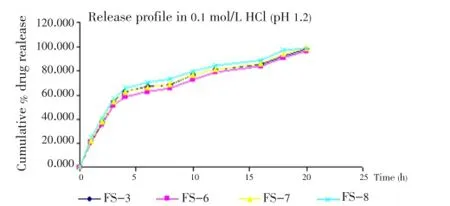
Figure 6. Effect of stirring ratio on release profile of drug in 0.1 mol/L HCl.
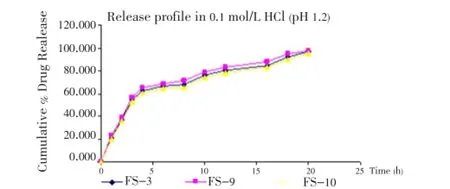
Figure 7. Effect of solvent ratio on release profile of famotidine in 0.1 mol/L HCl.
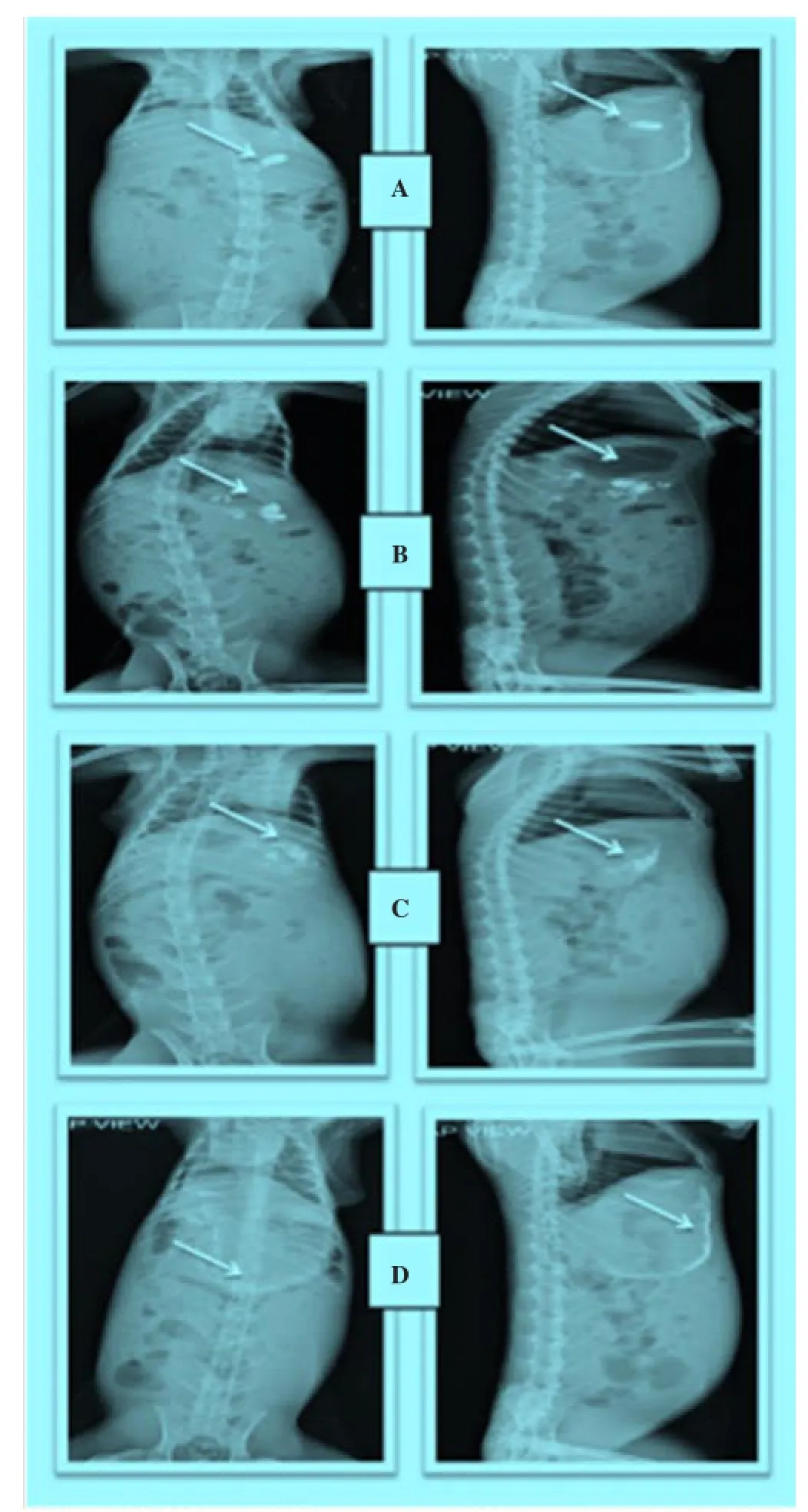
Figure 8. Radiographic images showing the presence of BaSO4loaded floating microspheres in the stomach at different time intervals. Images were taken after a: 0 h, b: 1 h, c: 3 h, d: 5 h.
The release profile data of all the developed formulations were fitted in kinetic model using PCP Disso software. The best model was selected on the basis of goodness of fit the values of residual sum of squares (RSS).
The drug release of batches FS-1, FS-2 and FS-9 showed first order drug release and remaining all the batches showed Higuchi (Matrix) model (Table 7).
The hard gelatin capsule containing BaSO4loaded floating microspheres were clearly visible in stomach after oral administration of dosage form. In radiographic image taken after 1 hour, all microspheres were found to be scattered in stomach. Dense images of microspheres were seen at initial hours, but as time passed on the images of microspheres became lighter. It may be because of distribution and scattering of microspheres within gastrointestinal region. The radiographic images indicted that these floating microspheres retained successfully in the stomach up-to 6 hours (Figure 8).
4. Discussion
Novel floating microspheres were successfully prepared by solvent evaporation method with modified technique of administration of polymeric solution from bottom side, for prolonged as well as stomach specific action of famotidine. Due to their low densities, this multi particulate drug delivery system showed good floating ability.
It was noted, on increasing the polymer ratio the cumulative % drug release decreased gradually. It may be because of increased partitioning of drug in polymer (Eudragit S-100). It was also observed that the release of famotidine decreased significantly (P<0.05) on increasing the amount of polymers.
Further, a significant increase was found in drug release on increasing the stirring rate. It may be because of increased surface area of microspheres due to reduced size.
On increasing the volume of ethanol in solvent phase the release was found to be increased. On the other hand, when the volume of DCM was increased, the release of drug was found to be decreased. The reason behind this may be the size of microspheres which increases on increasing the volume of DCM.
Fromin-vitrodrug release studies, it can be concluded that by changing the ratio of polymer and solvent, drug release can be controlled. These floating microspheres containing famotidine could be dispensed by filling them in the empty capsule shell.
In-vivoradiographic images were also helpful to locate the position of floating micro spheres in gastrointestinal tract. And this study also confirmed that the prepared maicrospheres were able to retain in stomach for prolongedrelease of famotidine.
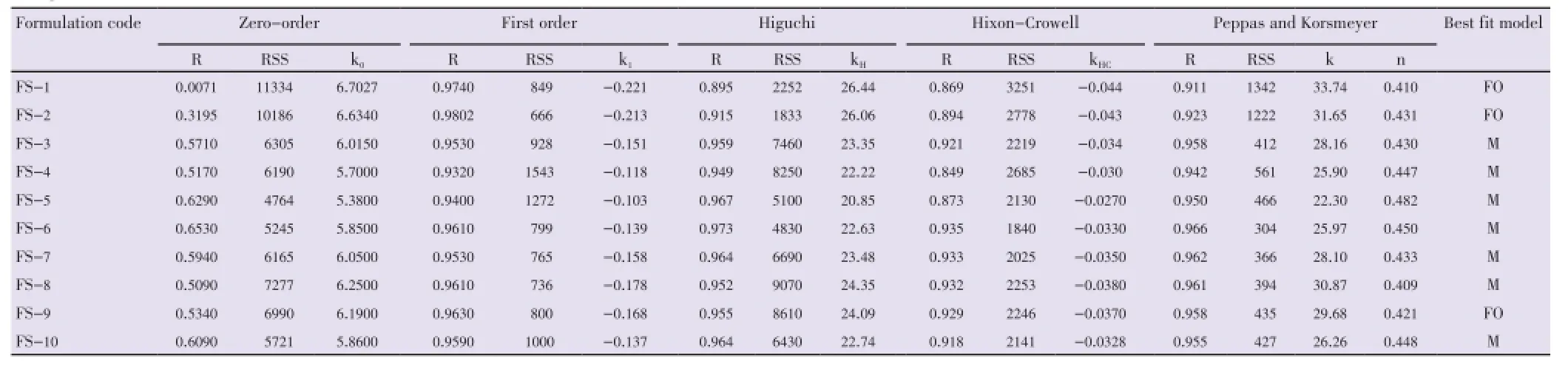
Table 7 Drug release kinetics for different formulations.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
We are thankful to Cadila Pharmaceutical Ltd., Gujarat, for providing gift sample of famotidine and Degussa India Pvt. Ltd. for providing Eudragit S-100 polymer as a gift sample. We also wish to thank to the Director, Birbal Sahni Institute of Palaeobotany, Lucknow, India, for providing us SEM facility.
[1] Kaushik K, Chaurasia D, Chaurasia H, Mishra SK, Bhardwaj P. Development and characterization of floating alginate beads for gastroretentive drug delivery system. Acta Pharma Sci 2011; 53(4): 551-562.
[2] Tadros MI. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: development, optimization and in vitro-in vivo evaluation in healthy human volunteers. Eur J Pharm Biopharm 2010; 74(2): 332-339.
[3] Baumgartner S, Kristl J, Vrecer F, Vodopivec P, Zorko B. Optimisation of floating matrix tablets and evaluation of their gastric residence time. Int J Pharm 2000; 195(1-2): 125-135.
[4] Bulgarelli E, Forni F, Bernabei MT. Effect of matrix composition and process conditions on casein-gelatin beads floating properties. Int J Pharm 2000; 198(2): 157-165.
[5] Davoudi ET, Noordin MI, Kadivar A, Kamalidehghan B, Farjam AS, Javar HA. Preparation and characterization of a gastric floating dosage form of capecitabine. Biomed Res Int 2013; doi: 10.1155/2013/495319.
[6] Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release 2000; 63(3): 235-259.
[7] Garg R, Gupta G. Progress in controlled gastroretentive delivery systems. Trop J Pharm Res 2008; 7(3): 1055-1066.
[8] Chen J, Park K. Synthesis of fast-swelling, superporous sucrose hydrogels. Carbohyd Polym 2000; 41(3): 259-268.
[9] Shaikh R, Singh TRR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci 2011; 3(1): 89-100.
[10] Boddupalli BM, Mohammed ZNK, Nath RA, Banji D. Mucoadhesive drug delivery system: an overview. J Adv Pharm Technol Res 2010; 1(4): 381-387.
[11] Kedzierewicz F, Thouvenot P, Lemut J, Etienne A, Hoffman M, Maincent P. Evaluation of peroral silicone dosage forms in hymans by gamma-scintigraphy. J Control Release 1999; 58(2): 195-205.
[12] Rouge N, Allémann E, Gex-Fabry M, Balant L, Cole ET, Buri P, et al. Comparative pharmacokinetic study of a floating multiple-unit capsule, a high density multiple-unit capsule and an immediaterelease tablet containing 25 mg atenolol. Pharm Acta Helv 1998; 73(2): 81-87.
[13] Prajapati PH, Nakum VV, Patel CN. Formulation and evaluation of floating matrix tablet of stavudine. Int J Pharm Investig 2012; 2(2): 83-89.
[14] Chaturvedi K, Umadevi S, Vaghani S. Floating matrix dosage form for propranolol hydrochloride based on gas formation technique: development and in vitro evaluation. Sci Pharm 2010; 78(4): 927-939.
[15] Murata Y, Sasaki N, Miyamoto E, Kawashima S. Use of floating alginate gel beads for stomach-specific drug delivery. Eur J Pharm Biopharm 2000; 50(2): 221-226.
[16] Jagtap YM, Bhujbal RK, Ranade AN, Ranpise NS. Effect of various polymers concentrations on physicochemical properties of floating microspheres. Indian J Pharm Sci 2012; 74(6): 512-520.
[17] Jain AK, Jain CP, Tanwar YS, Naruka PS. Formulation, characterization and in vitro evaluation of floating microspheres of famotidine as a gastro retentive dosage form. Asian J Pharm 2009; 3(3): 222-226.
[18] El-Kamel AH, Sokar MS, Al Gamal SS, Naggar VF. Preparation and evaluation of ketoprofen floating oral delivery system. Int J Pharm 2001; 220(1-2): 13-21.
[19] El-Kamel AH, Sokar MS, Al Gamal SS, Naggar VF. Development of floating microspheres to improve oral bioavailability of cefpodoxime proxetil. Int J Pharma 2001; 220: 13-21.
[20] Wakde PH, Kasliwal RH, Mane SB. Design and fabrication of gastroretentive bilayer floating tablet of propronolol HCl using natural polymer. Int J Pharm Sci Res 2013; 4(12): 4715-4728.
[21] Pandya N, Pandya M, Bhaskar VH. Preparation and in vitro characterization of porous carrier-based glipizide floating microspheres for gastric delivery. J Young Pharm 2011; 3(2): 97-104.
[22] Jagdale SC, Agavekar AJ, Pandya SV, Kuchekar BS, Chabukswar AR. Formulation and evaluation of gastroretentive drug delivery system of propranolol hydrochloride. AAPS PharmSciTech 2009; 10(3): 1071-1079.
[23] Hussain MN, Masum MAA, Akhter S, Sharmin F, Reza MS. Formulation and evaluation of gastro retentive floating tablets of simvastatin using hydrophilic rate retardant. Bangladesh Pharm J 2012; 15(2): 119-126.
[24] Al-Omar MA, Al-Mohizea AM. Famotidine, analytical profile. In: Brittain HG, editor. Profiles of drug substances, excipients and related methodology. Burlington: Academic Press; 2009, p. 115-151.
[25] Muzib YI, Ch Rekha. Development and evaluation of mineral oil entrapped emulsion gel beads of famotidine. Int J Pharmacol Technol 2011; 3(2): 83-90.
[26] Haznedar S, Dortunc B. Preparation and in vitro evaluation of eudragit microspheres containing acetazolamide. Int J Pharm 2004; 269(1): 131-140.
[27] Lee JH, Park TG, Choi HK. Development of oral drug delivery system using floating microspheres. J Microencapsul 1999; 16(6): 715-729.
[28] Bhardwaj P, Singh R, Swarup A. Development and in-vitro evaluation of floating microspheres of 5-fluorouracil. Int J Pharm Sci Res 2012; 3(3): 934-941.
[29] Soppimath KS, Kulkarni AR, Aminabhavi TM. Development of hollow microspheres as floating controlled-release systems for cardiovascular drugs: preparation and release characteristics. Drug Dev Ind Pharm 2001; 27(6): 507-515.
[30] Patel A, Ray S, Thakur RS. In-vitro evaluation and optimization of controlled release floating drug delivery system of metformin hydrochloride. DARU 2006; 14(2): 57-64.
[31] Chaurasia D, Kaushik K, Bhardwaj P, Chaurasia H, Jain SK, Shobhna S. Development and in-vitro characterization of floating microsphere bearing tramodol HCl. Acta Pol Pharm-Drug Res 2011; 68(5): 795-801.
[32] Bhardwaj P, Chaurasia D, Chaurasia H, Gupta R, Prajapati SK. Development and in-vitro evaluation of site-specific floating miltiparticulate drug delivery system for stomach. Int J Pharmagenesis 2011; 2(1): 5-13.
[33] Jagdale SC, Bari NA, Kuchekar BS, Chabukswar AR. Optimization studies on compression coated floating-pulsatile drug delivery of bisoprolol. Biomed Res Int 2013; doi: 10.1155/2013/801769.
[34] Shah SS, Pandya SJ, Waghulade MK. Development and investigation of gastro retentive dosage form of weakly basic drug. Asian J Pharm 2010; 4(1): 11-16.
10.12980/APJTB.4.201414B73
*Corresponding author: Rishikesh Gupta, Institute of Pharmacy, Bundelkhand University, Jhansi, Uttar Pradesh, India.
Tel: 09450040567
E-mail: rishikeshgupt@gmail.com
Foundation Project: Supported by Institute of Pharmacy, Bundelkhand University, Jhansi (U.P.), India (Grant No. BU/SF/PHARM/IAEC/10/031).
Famotidine
Floating drug delivery
Stomach targeting
Methods:The floating microspheres were prepared by modified solvent evaporation method. Eudragit S-100 was used as polymer. Microspheres were characterized for the micromeritic properties, floating behavior, entrapment efficiency and scanning electron microscopy. The invitro release studies and floating behavior were studied in simulated gastric fluid at pH 1.2. Different drug release kinetics models were also applied for all the batches. Selected formulations were also subjected for X-ray radiographic study.
Results:Floating microspheres were successfully prepared by modified solvent evaporation technique. Microspheres showed passable flow properties. The maximum yield of microspheres was up to (95.11±0.35)%. On the basis of optical microscopy particle size range was found to be ranging from (52.18±182.00) to (91.64±5.16) μm. Scanning electron microscopy showed their spherical size, perforated smooth surface and a cavity inside microspheres. Microspheres were capable to float up to 20 h in simulated gastric fluid. X-ray radiographic studies also proved its better retention in the stomach.
Conclusions:On the basis of the results, such dosage forms may be a good candidate for stomach targeting and may be dispensed in hard gelatin capsules.
 Asian Pacific Journal of Tropical Biomedicine2014年9期
Asian Pacific Journal of Tropical Biomedicine2014年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A case report of cutaneous larva migrans in a Mexican population of high marginalization
- Acute brucellosis as unusual cause of immune thrombocytopenia: a case report and review of the literature
- Chemical composition and larvicidal activity of essential oil of Origanum majorana (Lamiaceae) cultivated in Morocco against Culex pipiens (Diptera: Culicidae)
- Hematological and serum biochemical aspects associated with a camel (Camelus dromedarius) naturally infected by Trypanosoma evansi with severe parasitemia in Semnan, Iran
- Phytochemical screening and antioxidant activity of ethanol extract of Tithonia diversifolia (Hemsl) A. Gray dry flowers
- Rate of carcass and offal condemnation in animals slaughtered at Yazd Slaughterhouse, central Iran
