Efficacy of seed extracts of Annona squamosa and Annona muricata (Annonaceae) for the control of Aedes albopictus and Culex quinquefasciatus (Culicidae)
Lala Harivelo Raveloson Ravaomanarivo, Herisolo Andrianiaina Razafindraleva, Fara Nantenaina Raharimalala, Beby Rasoahantaveloniaina, Pierre Hervé Ravelonandro, Patrick Mavingui,4
1Department of Entomology, Faculty of Sciences, University of Antananarivo, Po Box 906, Antananarivo (101), Madagascar
2International Associated Laboratory, Research and Valorization of Malagasy Biodiversity Antananarivo, Madagascar
3Research Unit on Process and Environmental Engineering, Faculty of Sciences, University of Antananarivo, Po Box 906, Antananarivo (101), Madagascar
4UMR CNRS 5557, USC INRA 1364, Vet Agro Sup, Microbial Ecology, FR41 BioEnvironment and Health, University of Lyon 1, Villeurbanne F-69622, France
Efficacy of seed extracts of Annona squamosa and Annona muricata (Annonaceae) for the control of Aedes albopictus and Culex quinquefasciatus (Culicidae)
Lala Harivelo Raveloson Ravaomanarivo1*, Herisolo Andrianiaina Razafindraleva1, Fara Nantenaina Raharimalala2, Beby Rasoahantaveloniaina1, Pierre Hervé Ravelonandro3, Patrick Mavingui2,4
1Department of Entomology, Faculty of Sciences, University of Antananarivo, Po Box 906, Antananarivo (101), Madagascar
2International Associated Laboratory, Research and Valorization of Malagasy Biodiversity Antananarivo, Madagascar
3Research Unit on Process and Environmental Engineering, Faculty of Sciences, University of Antananarivo, Po Box 906, Antananarivo (101), Madagascar
4UMR CNRS 5557, USC INRA 1364, Vet Agro Sup, Microbial Ecology, FR41 BioEnvironment and Health, University of Lyon 1, Villeurbanne F-69622, France
PEER REVIEW ABSTRACT
Peer reviewer
Dr. Delatte Hélène, UMR Peuplements Végétaux et Bio-agresseurs en Milieu Tropical, CIRAD-3P7, Chemin de l’IRAT, Ligne Paradis, 97410 Saint Pierre, La Réunion, France. Tel: 026249 9235, Fax: 026249 9200, E-mail: delatte@cirad.fr
Co-reviewer: Prof. Victor Jeannoda, Antananarivo, Madagascar.
Comments
This is a good research work in which authors have demonstrated the insecticide activity of two Annonaceae (A. squamosa and A. muricata) seed extracts against two mosquito species of health importance. The activity was assessed based on biochemical parameters and in vivo tests. A. squamosa and A. muricata were found to be promising candidate plants for future insecticide use on mosquitoes.
Details on Page 805
Objective:To evaluate the potential efficacy of seed extracts of Annona squamosa and Annona muricata used as natural insecticides to control adult and larvae of the vectors Aedes albopictus and Culex quinquefasciatus under laboratory conditions.
Methods:Aqueous and oil extracts of the two plants were prepared from dried seeds. Preliminary identifications of the chemical components of each seed extracts were performed using microreactional and GCP techniques. Larvae and adults of Aedes albopictus and Culex quinquefasciatus were collected from the breeding sites in coastal and highlands regions of Madagascar. WHO standardized tests of susceptibility for larvae and imaginal stage of mosquitoes were realized to determine mortality and LC50of mosquitoes.
Results:Chemical identifications showed that these extracts contain alkaloids and flavonoids compounds that probably confer their biological insecticidal proprieties. CPG analysis showed also the presence of various fatty acids. On adult mosquitoes, significant insecticidal effects were observed with both aqueous and oil extracts of the two plant seeds compared to mortality induced by deltamethrin, an insecticide used as reference. Extracts of Annona muricata induced high mortality rate to both species of mosquito compared to extracts of Annona squamosa at all concentrations tested. The LC50of seed extracts ranged from 1% to 5% for adults and 0.5% to 1% for larvae.
Conclusions:The seed extracts of these two plants may be used as mosquito controlling agents and offer a new approach to a less costly, practical and environmentally friendly control of vector borne diseases.
Annonaceae, Seed extracts, Biological insecticides, Chikungunya, Rift Valley fever, Vector control
1. Introduction
Since 2006, Madagascar was affected by successive outbreaks of chikungunya and Rift Valley fever[1-3]. The chikungunya virus (CHIKV) was detected in the coastal regions of Madagascar such as Toamasina, Antsirananaand Taolagnaro, whereas the Rift Valley fever virus (RVFV) has emerged in several regions of the highlands, including Anjozorobe, Manjakandrina, Antananarivo, Ambatondrazaka, Amparafaravola, and the Haute Matsiatra region (Fianarantsoa I, II, Ambalavao)[2-5].Aedes albopictus(Ae. albopictus) has been identified as the main vector of CHIKV and dengue virus in urban areas, because of its distribution which coincided to the epidemic areas and its abundance during periods of outbreaks[1,6].Ae. albopictuswere found ovipositing and breeding in microhabitats located in the vicinity of human habitations, including natural and/or artificial pounds, crevices, tree trunk excavations and other artificial containers such as discarded cans, used tires and abandoned buckets[1,6,7]. The most important vectors for RVFV areAedesandCulexmosquitoes[8,9].Culexis widely distributed in urban areas of Madagascar[4]. Transmission is facilitated by the feeding behavior ofCulexwhich is both endophilic and exophilic. Larval stages are known to breed in large range of polluted waters with lentic facies[6,7].
As no vaccine and efficient therapeutic treatments are currently available for CHIKV (Alphavirus), dengue virus (Flavivirus) or RVFV (Bunyavirus), various methods have been applied to control populations of vectors but none of them were able to eradicate these vectors in Madagascar. Among the wide range of biological methods, natural enemies such as predators of mosqitoes at different developmental stages have been used. Aquatic larvae ofCulex tigripesplay an important role in regulating populations of vectors by consuming the larvae of other species of mosquitoes in natural pounds[6]. However, it has been demonstrated that the most efficient method that may control the numbers of larvae, remains the environmental sanitation by removing any potential breeding sites of the vectors[1], although its application at a large scale is restricted.
Conventional method using chemical insecticides, including organochlorides, pyrethroids mainly the deltamethrin and organophosphates such as malathion and fenthion were still applied as last resort for vector control[9]. These synthetic compounds are not only environmentally polluting but also have concomitant hazardous effects to non-target organisms and to human health[6].
Phenomenon of resistance to the widely used chemical insecticides were found in many vectors of disease, particularly in the case ofCulex quinquefasciatus(Cx. quinquefasciatus)[10-13] causing sudden epidemic spreads of Rift Valley fever and the resurgence of the dengue and chikungunya. Resistance to chemical insecticides in other vectors such asAe. albopictusandAedes aegyptiwas also confirmed[9-15].
To face the increasing emergence of mosquito resistance to chemical insecticides, a sound option lays on the use of natural products. In this study, seed extracts ofAnnonasquamosa(A. squamosa) andAnnona muricata(A. muricata) were used to control adult mosquitoes as well as larval stages of the vectorsAe. albopictusandCx. quinquefasciatus.
Annonaceae are empirically known to elicit insecticidal activities[12,16]. Plant species in this family contain an array of toxic compounds such as acetogenins, alkaloids, flavonoids that confer to these plants their insecticidal proprieties[12]. Both seeds ofA. squamosaandA. muricatacontain a great amount of acetogenins[16]. This special group of chemical compounds is known as mitochondrial complex I inhibitor[12,17,18]. Some alkaloids were found inA. muricata. And extracts ofA. muricatanot only affect the mortality rate of pupal and adult stages of mosquitoes but also reduce the reproductive success of surviving adults by decreasing fecundity and egg hatchability[19].
Petroleum ether leaf extracted from leaves ofA. squamosawere reported to possess an insecticidal and growth regulating activities on three mosquito species namelyAnopheles stephensi,Cx. quinquefasciatusandAedes aegypti[19].
The aim of this study was to evaluate the actual efficacy of seed extracts ofA. squamosaandA. squamosaunder laboratory conditions in order to assess their potential uses as natural insecticides to control adult mosquitoes as well as larval stages of the vectorsAe. albopictusandCx. quinquefasciatus. A preliminary screening using various micro-reactional techniques and also GC analyses were also performed to identify the range of chemical groups of ingredients in the compositions of the aqueous and oil extracts of the two seeds.
2. Materiels and methods
2.1. Collection of seeds and preparation of plant extracts
A. squamosaandA. muricataare commonly planted and harvested in the East coast of Madagascar. Their fruits produce a large quantity of seeds that can be used as insecticides. Fruits of the two species were collected in Manakara (22°8’45.68” S; 48°0’15.9” E) at the south east region of Madagascar.
Prior to chemical extraction, seeds were completely dried under a ventilated hood then grinded to powder. A total of 200 g of each grinded seeds of the two plants were then soaked and macerated separately in 1 L of distilled water overnight. Each macerated solution was diluted with distilled water to obtain different lower concentrations (0.5%=1 g/ L, 1%=2 g/L, 5%=10 g/L, 10%=20 g/L and 20%=40 g/L). The aqueous extracts were quoted as EAAS forA. squamosaand EAAM forA. muricata. Similar procedure used to obtain aqueous extracts was adopted to obtain oil extracts bysoaking 200 g of seed powder separately into 1 L of various solvents with different polarity. Three different solvents were used for extractions: ethanol, dichloromethane, and acetone. The oil extracts were quoted EET1 and EET2 for ethanol extracts, DCM1 and DCM2 for dichloromethane extracts ofA. squamosaandA. muricatarespectively; and ACE1 for acetone extract ofA. squamosa.
2.2. Chemical analysis
Preliminary identifications of the chemical ingredients in the composition of the two aqueous extracts from the two seeds were performed using various micro-reactional techniques to detect specific chemical compound families[20] (Table 1). In contrast, oil extracts were injected into Shimazu GCP JC-17A (Version 3 equipped with capillary column: Tracsil TR-wax, 30 m×0.32 m×0.25 m; Injection temperature: 260 °C) for chemical identification of the components of the oil extracts.
2.3. Mosquito collection
Mosquitoes were collected in the coastal regions of Toamasina (18°08’50” S; 49°23’43” E; 10 m), Mahajanga (15°45’39.83’’ S; 46°20’9.38” E; 18 m), and Manakara (22°8’45.68” S; 48°0’15.9” E; 20 m) as well as in the highland regions of Antananarivo (18°52’47” S; 47°34’35” E; 1245 m), Ambositra (20°54’211” S; 47°24’159” E; 1310 m) and Fianarantsoa (21°27’34.05” S; 47°6’33.75” E; 1280 m). Adults ofCx. quinquefasciatuswere collected in human habitations and bedrooms of hospitals early in the morning, whereasAe. albopictusadults were captured with small nets in their natural habitats and shrubs during daytime while flying. Investigations of all potential larval pounds and breeding sites of the two species were also performed and specimens collected as well.
2.4. Mosquito rearing
Cx. quinquefasciatusandAe. albopictuscaught in the wild were transported to the insectarium of Department of Entomology and were reared following WHO procedures and laboratory rearing method[11,21]. The egg strips and rafts were immersed in natural water which is collected in ponds nearby and placed into plastic bowls (dim: 6 cm diameter). Synchronous hatched larvae were transferred into samebowl containing natural water to maintain synchronous development of colonies. Male mosquitoes were fed on 6% sucrose imbibed cotton pad. The females were blood-fed twice a week on laboratory mice. Insectarium temperature was maintained at (25±2) °C, relative humidity at (80±2)% and photoperiod 12 h/12 h.
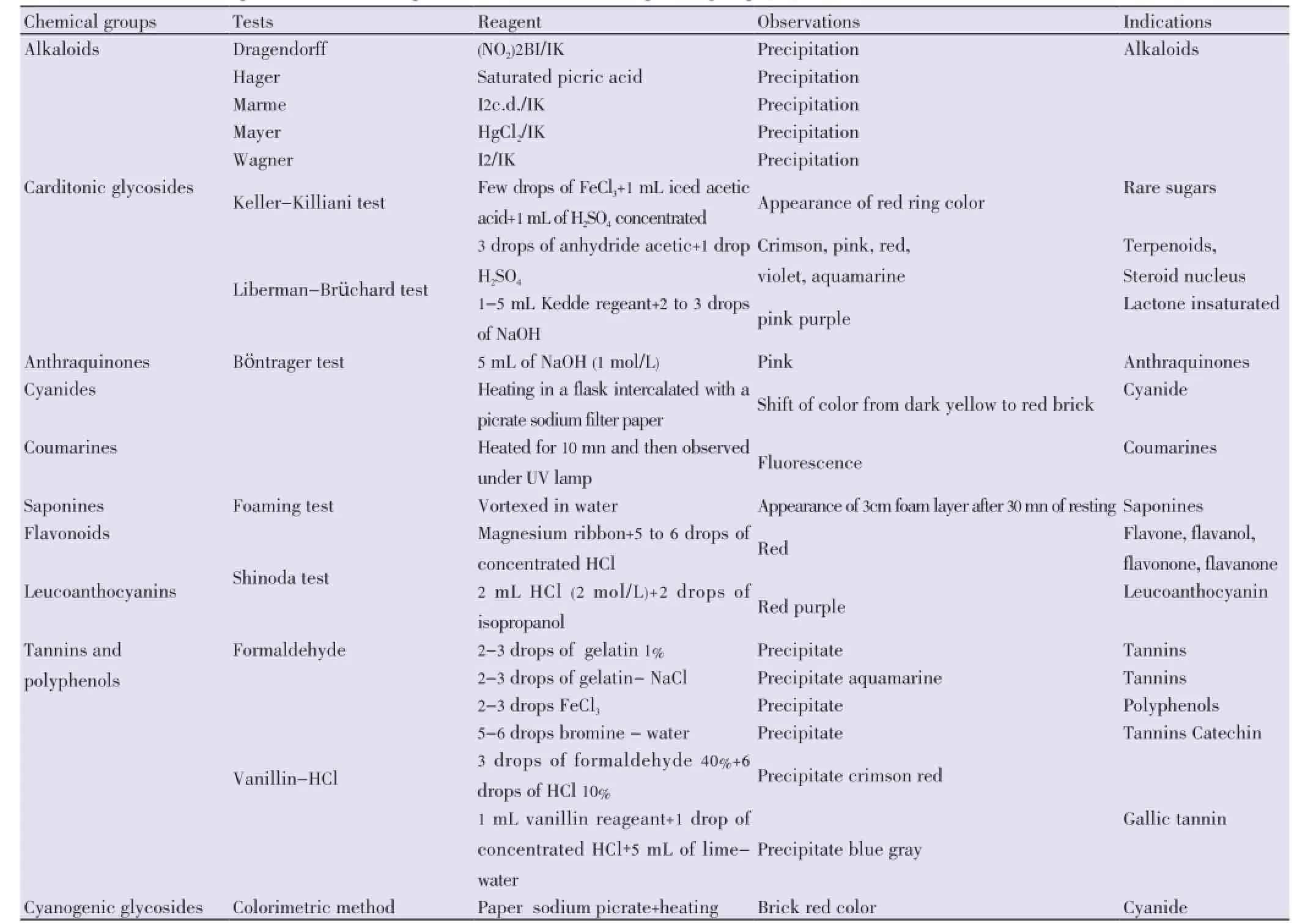
Table1 Micro-reactional techniques used to detect presence of chemical compound groups[20].
2.5. Bioassays for adults
WHO standardized method was used to test the susceptibility ofAe. albopictusandCx. quinquefasciatusadults to different concentrations of each seed extracts[21]. Bioassays were performed using adapted exposure tubes (WHO tubes, dim: L: 12 cm×4.5 cm diameter) having two adjacent compartments, the exposure compartment and the observation compartment, interconnected by a sliding glass separator that allowed mosquitoes to be grouped within one of the two compartments at a time. Filter papers (0.10 mm thick and 6 cm diameter) were impregnated with 5 mL of aqueous extracts with the respective concentrations of 1%, 5%, 10% and 20% or 1 mL of crude oil extracts, and introduced and walled the exposure tube individually. A number of 25 unfed adult mosquitoes were released into a tube corresponding to a replicate. Four replicates were performed. Time of exposure to the extract was standardized to one hour for each replicate then mosquitoes were transferred into the adjacent observation tube and monitored for 24 h. Controls comprised two different treatments; one using filter paper impregnated with distilled water and another with a filter paper impregnated with deltamethrin 2% taken as the positive reference. During observation, test and control tubes were covered with wooden wall box with aeration aperture and moisten tissues to maintain appropriate humidity. After 24 h, falling or immobile mosquitoes were counted as dead. If mortality rate of mosquitoes of the non-impregnated control tube exceeded 20% (4 mosquitoes) within the 24 h of incubation, the whole batch of test was considered as invalid then repeated.
2.6. Bioassays for larvae
Three different concentrations 0.5%, 1% and 2% of each plant aqueous extract were used for bioassays[22]. For each concentration prepared, a batch of two beakers of 100 mL was used. Two controls were run: one blank control with 100 mL of water, and one with 100 mL water supplemented with 1 mL of ethanol that was used as the positive reference. For oil extracts, 1 mL acetone was added to 100 mL of natural breeding pound water prior to adding 1 mL crude oil seed extract of each plant. Twenty-five third instar larvae ofAe. albopictusorCx. quinquefasciatuswere introduced in each beaker and kept immersed for 24 h.
After incubation time-period, mortality of larvae in each beaker was recorded. Each experiment comprised a minimum number of 4 replicates. Immobile and moribund larvae were counted as dead.
2.7. Statistical analysis
The average values of mortality of mosquitoes were calculated and mortality curves were drawn on log-log graph paper to find out LC50for each extract. Data were analysed using SPSS. 10 (Version, 2010). Mortality rates of different treatments were subjected to ANOVA (α=0.05). When ANOVA results were significant, means were separated using Tukey-Kramer test (α=0.05).
3. Results
3.1. Major families of chemical compounds identified in extracts
Similar families of chemical compounds ranging from flavonoids, leucoanthocyanes, triterpenes, unsaturated sterols, polyphenols and polysaccharides (Table 2) were identified in the aqueous extracts. Alkaloids were present in aqueous extract ofA. muricatabut absent inA. squamosaand that was the converse with tannin.
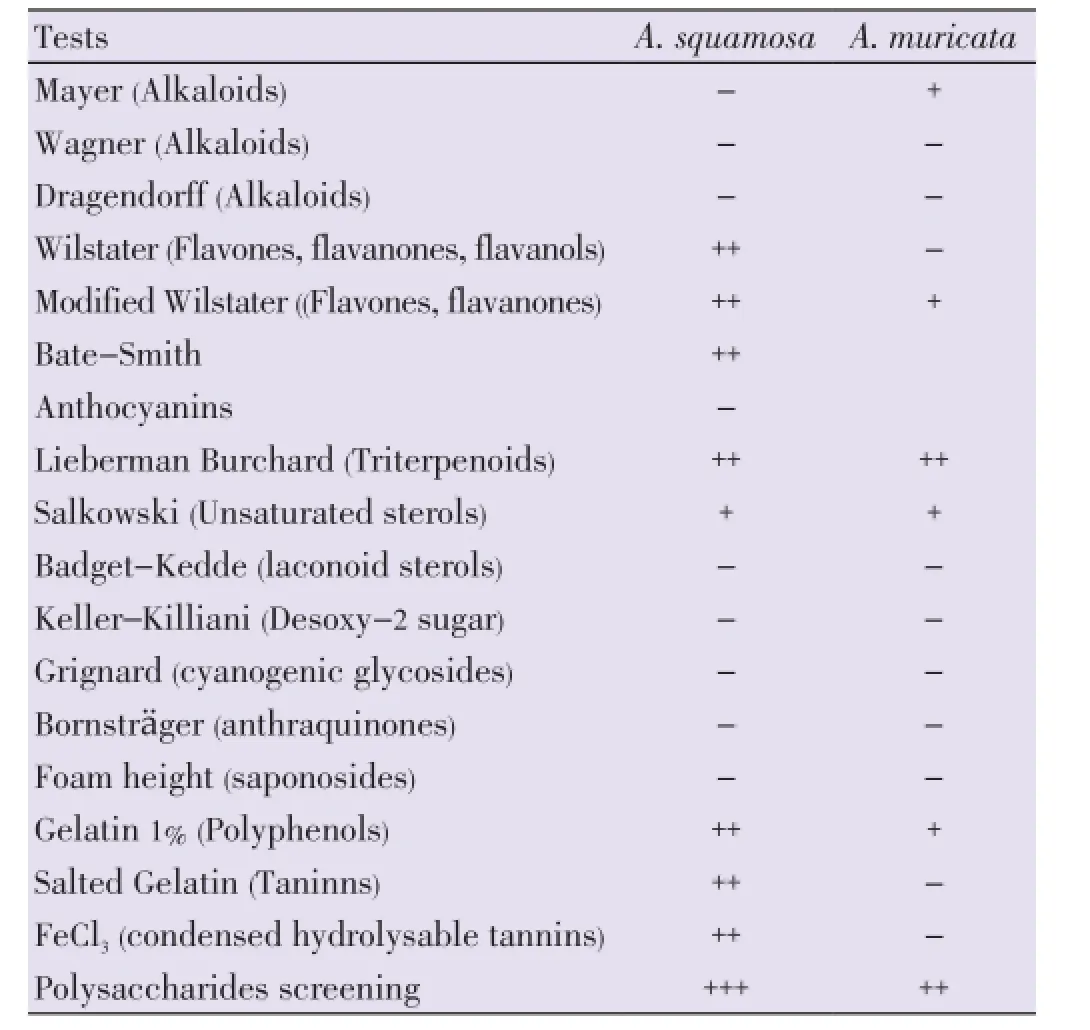
Table 2 Main chemical groups found in aqueous seed extracts of A. squamosa and A. muricata.
Various fatty acids ranging from palmitic acid, oleic acid, linoleic acid were identified in the oil extract ofA. squamosa(Table 3). In addition to the range of major fattyacids found inA. squamosa, the oil extract ofA. muricatacontained more specific fatty acids including myristic acid, palmitoleic acid, stearic acid, linolenic acid, arachidic acid and others acids with C18, C19 and C20 (Table 3).
3.2. Efficacy of A. squamosa and A. muricata extracts to adult mosquitoes Cx. quinquefasciatus and Ae. albopictus
3.2.1. Aqueous extracts
By using aqueous extracts ofA. squamosa, numbers of adult mosquitoesCx. quinquefasciatuskilled after 24 h were proportional to the concentrations of the extracts: 10 ±2, 13±2, 18±2 and 23±2 (means±SE) for the concentrations 1%, 5%, 10% and 20%, respectively. When aqueous extracts ofA. muricatawere used, mortalities to adultsCx. quinquefasciatuswere 18±2, 20±2, 23±2 and 23±2 (means±SE) for the concentrations of 1%, 5%, 10% and 20%, respectively (Table 4). Mortalities of adult mosquitoes using EAAS and EAAM were significantly superior to mortalities observed in the deltamethrin reference tests (14±2) (means±SE) (F(7,111)=22.18,n=118,P=0.0001, ANOVA, Tukey-Kramer test). By plotting average values of mortalities on log-log graph, the LC50was found to be at the concentration of 1% for EAAM and 5% for EAAS.
In adults ofAe. albopictus, mortalities after 24 h exposure to 1% concentration of EAAS and of EAAM were respectively 18±2 and 24±1 (means±SE) (Table 4). Mortalities of adult mosquitoes treated by EAAM were similar to mortalities observed in the deltamethrin reference tests (23±2) (means ±SE), whereas those ofA. squamosawere lower (18±2) [F(3.36)=44.72,n=39,P=0.0001, ANOVA, Tukey-Kramer test]. The LC50was found to be lower than the concentration of 1% for both EAAS and EAAM.
3.2.2. Oil extracts
Mortalities ofCx. quinquefasciatusadults after 24 h were 23±2, 22±3, 19±3, 22±3 and 21±4 (means±SE), respectively for DCM1, DCM2, EET1, EET2 and EAC1 (Table 5). Mortalities ofmosquitoes using oil extracts ofA. squamosa(DCM1, EET1 and EAC1) andA. muricata(DCM2 and EET2) were significantly superior to mortalities observed in deltamethrin reference tests (14±2) (means±SE) [F(5,44)=5.89,n=49,P=0.0003, ANOVA, Tukey-Kramer test].
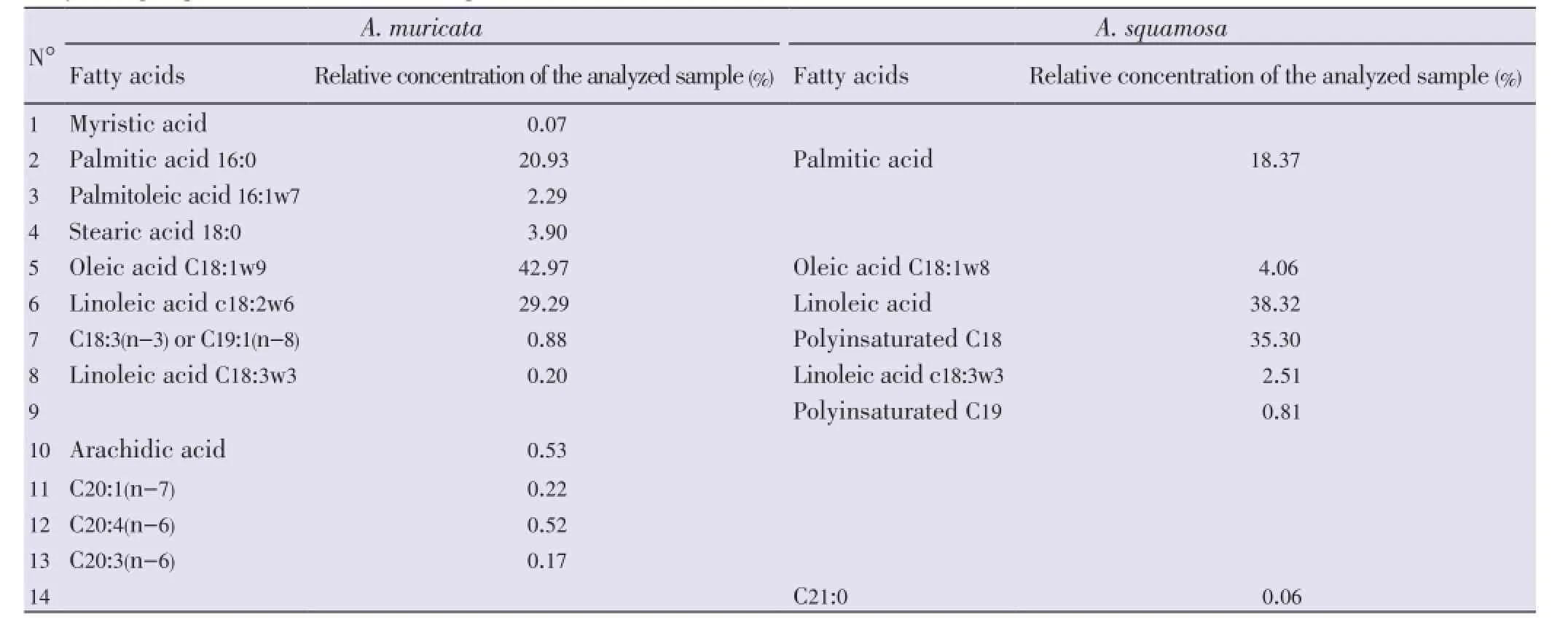
Table 3 Fatty acid groups for A. muricata and A. squamosa.
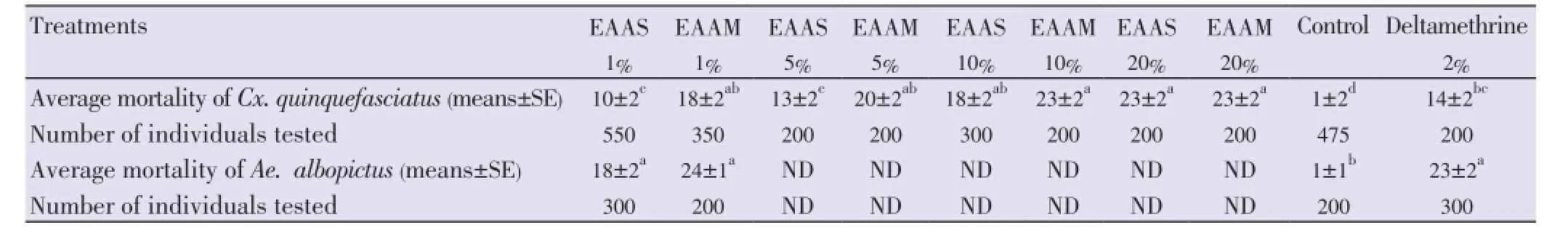
Table 4 Mortality of adult mosquitoes induced by aqueous seed extracts of A. squamosa and A. muricata.
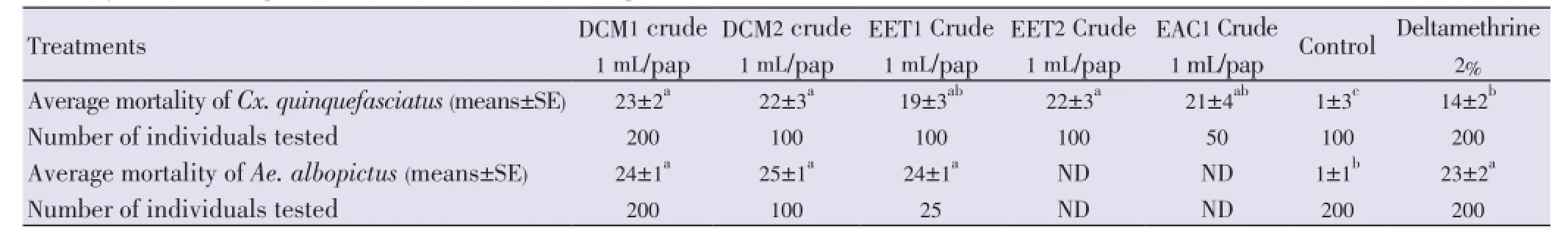
Table 5 Mortality of adult mosquitoes to oil seed extracts of A. squamosa and A. muricata.
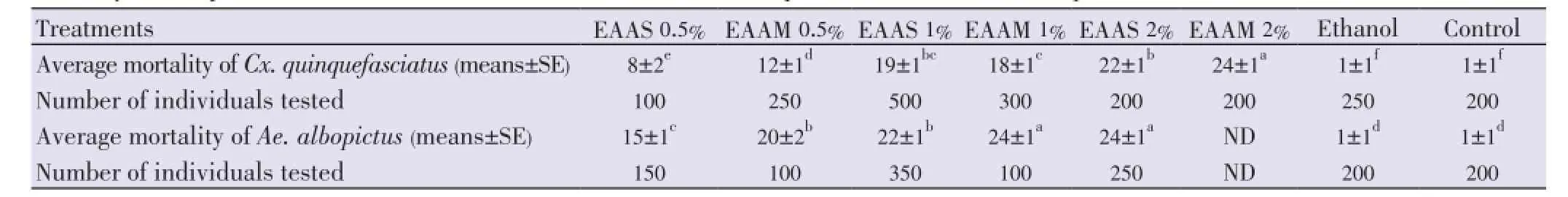
Table 6 Mortality of mosquito third instar larvae to different concentration of aqueous seed extracts of A. squamosa and A. muricata.
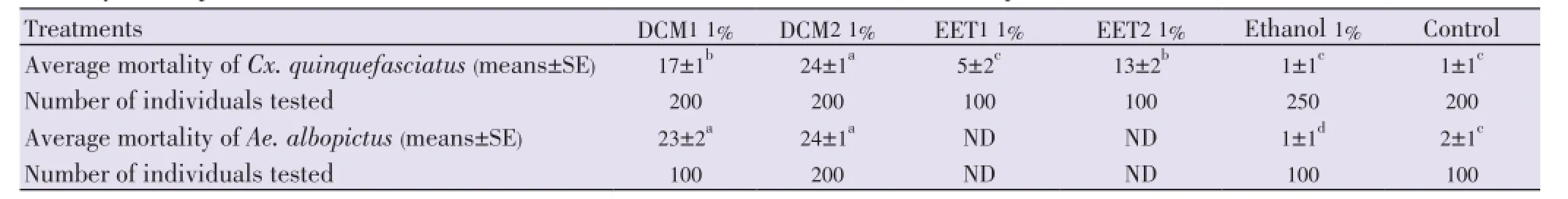
Table 7 Mortality of mosquito third instar larvae to different concentration of oil seed extracts of A. squamosa and A. muricata.
ForAe. albopictusadults, mortalities after 24 h were 24±1, 25±1, 24±1 (means±SE) respectively to DCM1, DCM2 and EET1 (Table 5). There were no difference in mortalities between extracts ofA. squamosa(DCM1, EET1) andA. muricata(DCM2) to that induced by deltamethrin (23±2) (F(4,21)=56.33,n=25;P=0.0001, ANOVA, Tukey-Kramer test).
3.3. Efficacy of A. squamosa and A. muricata extracts to third instar nymph mosquitoes Cx. quinquefasciatus and Ae. albopictus
3.3.1. Aqueous extracts
For the three concentrations 0.5%, 1% and 2%, the mortalities of third instar larvae ofCx. quinquefasciatusafter 24 h were respectively 8±2, 19±1 and 22±1 (means±SE) for the aqueous extracts ofA. squamosa(EAAS), and 12±1,18±1 and 24±1 (means±SE) for the aqueous extracts ofA. muricata(EAAM), respectively (Table 6). Mortalities of larvae using EAAS and EAAM were significantly superior to mortalities observed in the ethanol and control reference tests (both 1± 1) (means±SE) [(F(7,88)=320.05,n= 95,P=0.0001, ANOVA, Tukey-Kramer test]. By plotting average values on log-log graph, the LC50was found to be 0.5% for EAAM and 1% for EAAS.
For the two concentrations 0.5% and 1% after 24 h of exposure, third instar larvae ofAe. albopictusexhibited mortalities of 15±1cand 22±1b(means±SE) for the aqueous extracts ofA. squamosa(EAAS), and 20±2 and 24±1 (means ±SE) for the aqueous extracts ofA. muricata(EAAM), respectively (Table 6). Mortalities of larvae using EAAS and EAAM were significantly higher than mortalities observed in the ethanol and control reference tests (both 1±1) (means±SE) [F(6,47)=284.50,n=54,P=0.0001, ANOVA, Tukey-Kramer test]. The LC50was found to be lower than 0.5% for both EAAS and EAAM.
3.3.2. Oil extracts
Mortalities of third instar larvae ofCx. quinquefasciatusafter 24 h were respectively 17±1, and 5±2c(means±SE) for the oil extracts DCM1 and EET1, and 24±1 and 13±2 (means± SE) for the oil extracts DCM2 and EET2 respectively (Table 7). Mortalities of mosquitoes under oil extracts ofA. muricata(DCM2 and EET2) were significantly higher than mortalities observed in the ethanol and the control reference tests (both 1±1) (means±SE) whereas oil extracts ofA. squamosa(DCM1 and EET1) induced a mild mortality to third instar larvae ofCx. quinquefasciatus(F(7,48)=58.97,n=55,P=0.0001, ANOVA test, Tukey-Kramer test). Mortalities of third instar larvae ofAe. albopictusby using oil extracts ofA. squamosa(DCM1) andA. muricata(DCM2) were significantly higher than mortalities observed in the ethanol and the control reference tests (1 ±1 and 2±1 respectively) (means±SE) [F(5,24)=650.73,n=30,P=0.0001, ANOVA, Tukey-Kramer test].
Overall, the results showed thatAe. albopictuspopulations were more sensitive to the seed extracts than populations ofCx. quinquefasciatus.
4. Discussion
Chemical screening and GC analyses results showed a range of potent and bioactive compound groups such as flavonoids, and fatty acids in bothA. muricataandA. squamosaaqueous and oil seed extracts. Chemical screening was in agreement with the results obtained by different authors who have already identified a broad range of alkaloids, flavonoids and acetogenins compounds produced in seeds ofA. squamosa[16,17,19] andA.muricata[16,23] suggesting their probable role in insecticidal activities[23]. Alkaloid compounds including nicotine found in tobacco, have been demonstrated to inhibit the active site of acetylcholine in many organisms[10,19,24]. These alkaloids were only found inA. muricataaqueous extracts. Acetogenins were discovered in 1982 by Joladet al., they are specific of Annonaceae[17,23]. These compounds are derivatives of 32 to 37 carbons long chain fatty acids[12]. The major acetogenins in seeds are respectively annonacin inA. muricata[23] and squamocin inA. squamosa[17]. Although typical acetogenins were not detected with the two methods used, an in-depth identification of the factual nature of the active ingredients belonging to all the chemical families using GC-MS will enable further determination of their precise roles in the insecticidal activities of the extracts.
Numerous types of toxicity result from physical proprieties of fatty acids: toxicity by inhalation due to volatile organic compounds, toxicity by contact due to aggregation and formation of thin film at the surface of water which does not allow respiration of aquatic insects, and by penetration due to the amphibolic propriety of certain molecules; Oleic acid C18 and undecylenic acid C11 possess direct insecticidal activities or may enhance the toxicity of other toxic compounds[25].
Currently, plant-based chemical control may broaden up gradually the existing arsenal of methods in vector control. Many plants are used as biocides in different parts of the world, namingPiper nigrum(black pepper),Nicotiana tabacum(tobacco),Melia azedarach,Derris,LonchocarpusandTephrosiaandAzadirachta indica(neem) [11,12,24,26]. The latter plant is the most widely used biocide as its insecticidal properties have been known for over 300 years and was applied as a foliar spray and vapor to control insects, particularly in greenhouses and other protected crop fields.
In comparison to synthetic deltamethrin, a compound usually taken as standard reference by WHO in susceptibility tests for mosquitoes, a higher insecticidal effect on both adult and larvae ofAe. albopictusandCx. quinquefasciatuswas observed when using the aqueous and oil seed extracts ofA. squamosaandA. muricata. This higher efficacy opens the possibility to exploit these extracts as biological insecticides to control the two tested mosquito species, and extendedly likely to other mosquito vector taxa. Results of bioassays likely were in concordance with those obtained on larva ofCx. quinquefasciatus. Thus, they confirm the insecticidal activities ofA. muricata[16] andA. squamosa[12,16,19]. Strikingly, the present study also brought up additional evidence and support concerning the prevalence of resistance to deltamethrin, the insecticide reference advocated by WHO[10,12].
It is also found that the aqueous extracts ofA. muricataelicited higher adulticidal activities inAe. albopictusthan those ofA. squamosaat the same concentration. Similar trend was observed when using both aqueous and oil extracts on the larvae ofCx. quinquefasciatus. This difference of efficacy between the two species is probably due to the alkaloids present inA. muricatawhich are not found inA. squamosa[16]. Aqueous extracts were all efficient at a very low dose (0.5%) and with a proportional increase of the lethal activity following the gradual increase of extract concentrations. At the highest dose (2%), all mosquitoes tested were killed during the tests. Although extracts obtained from all organic solvent elicited adulticidal activities, dichloromethane extracts of both seed plants were significantly potent (DCM1, DCM2) compared to ethanolic extracts, in particular for larval stages. In contrast, only ethanolic extracts (EET2) ofA. muricataelicited adulticidal activities againstCx. quinquefasciatus, with regard to oil extracts.
For all extracts and at all concentrations tested, the populations ofAe. albopictuscaught from five regions of Madagascar were more susceptible than those ofCx. quinquefasciatus. This higher susceptibility might be due to the intrinsic natural ecology and behavior ofAe. albopictuslarvae that usually breed into relatively clean and less polluted water. This behavior reduced at its minimum their contact to organic compounds into highly polluted waters, which in turn might be among the mechanisms that prevented populations of the species to remain susceptible. In contrast, larvae ofCx. quinquefasciatusare known to breed into polluted water containing a broad variety of organic compounds namely alkaloids and/or derivative compounds. The repeated contacts ofCx. quinquefasciatuslarvae to these compounds might have conferred to these larvae and consequently to adults, a range of adaptations to various chemicals hence their relative moderate sensitivity to these plants extracts.
Nevertheless, the use of plant-based pesticides presents a number of challenges and advantages[9,26] as follows: (i) the active ingredients of many plant-based pesticides are not known, but are essential for the development of specifications. (ii) The active ingredients can vary both in composition and concentration in the same plant species, in different clones, in different parts of the plants, at different stages of plant growth and under different climatic and soil conditions. (iii) The activity may not be due to a single ingredient but to a mixture of compounds, which may act synergistically. (iv) The development of resistance to pesticides containing a mixture of active ingredients may occur less readily. (v) Adequate toxicological and ecotoxicological data are not available for many plant-based pesticides. (vi) Analytical standards of the active ingredients may not be easily obtainable.
As the two tested plants have a wide geographical distribution in eastern region of Madagascar where malariaand other virus-borne vectors are endemic[1], integrating and promoting their use as an alternative plant-based method in vector control could be advocated, especially in the case of Madagascar. If used properly at an efficient dose, these extracts ofAnnonawere revealed to be a low cost and powerful environmentally friendly tool by avoiding accumulation and negative impacts on non-target entomofauna. The possibility of newly synthesized bioactive molecules forming all extracts and that from the different organic solvent used thus remains pivotal in the design of efficient and practical plant extract-based methods in vector control.
The aqueous and oil extracts ofA. squamosaandA. muricataboth showed insecticidal properties though at varying levels. Extracts ofA. muricatadisplayed higher insecticidal potential compared to extracts ofA. squamosa. Besides,Ae. albopictuswas more susceptible thanCx. quinquefasciatusto all extracts. Lethal concentrations (LC50) of all extracts of the two plants tested ranged from less than 1% for adult mosquitoes and 0.5% for larvae ofAe. albopictus, and 1% to 5% for adult mosquitoes and 0.5% to 1% for larvae ofCx. quinquefasciatus. Dichloromethane extracts ofA. muricatashowed high lethal effect to larval and adult stages ofAe. albopictusandCx. quinquefasciatuscompared to deltamethrin which is an OMS insecticide reference product. Hence, this extract might already be considered having high insecticidal potential as an alternative agent in the control of mosquitoes though further studies on identification and chemical characterization of the active ingredients of these two plants are still needed for its complete integration as mosquito controlling agents.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
We are grateful to the Ministry of Water and Forestry and to Madagascar National Parks (formerly ANGAP) for fully authorizing collection of wild mosquitoes within parks. We thank Rahanitriniaina Sahondra and Rajaonera Tahina Ernest for assistance and cooperation, Rajaonarivo Solofoniaina for providing all the plant extracts, and Brown Rakotoarisoa and Ratsimbazafy Jonah for helpful suggestions on the manuscript. This work was funded by the grants FRB-CDAOOI-07-012 and CMIRA Coopera 2011 from Region Rh?ne-Alpes 11MIF-MAVINGUI-10851, and was carried out within the frameworks of GDRI “Biodiversité et Développement Durable à Madagascar”.
Comments
Background
Ae. albopictusandCx. quinquefasciatusare mosquito vectors of several human diseases all over the world. Among them chikungunya and Rift Valley fever diseases have been recently reported for severe outbreaks in several countries, such as the islands of the south west part of the Indian Ocean. Most of the methods used today to eradicate those vector populations are not efficient or too costly for the country. Therefore there is need of new molecules to improve those control methods.
Research frontiers
The present research work depicts insecticide activity of aqueous and oil extracts ofA. squamosaandA. muricata(Annonaceae) seeds against 2 mosquito species of health importance:Ae. albopictusandCx. quinquefasciatusby identifications of the chemical components of each seed extracts andin vivotests of susceptibility for larvae and imaginal stage of mosquitoes (determining mortality and CL 50 of mosquitoes).
Related reports
Annonaceae are empirically known to elicit insecticidal activities. Plant species in this family contain an array of toxic compounds such as acetogenins, alkaloids, flavonoids that confer to these plants their insecticidal proprieties. The folklore medicine has evidence of effectiveness of those extracts as insecticides.
Innovations and breakthroughs
Annonaceae are empirically known to elicit insecticidal activities however in the present study, authors have looked for their different chemical compounds and demonstrated the insecticide activity ofA. squamosaandA. muricataseeds against 2 mosquito species of health importance.
Applications
This scientific study is, as mentioned by author, a preliminary work, screening 2 plant species for their insecticide activity. Nevertheless their results even preliminary are supporting and suggesting these seeds extract for future insecticide use.
Peer review
This is a good research work in which authors have demonstrated the insecticide activity of two Annonaceae (A. squamosaandA. muricata) seed extracts against 2 mosquito species of health importance. The activity was assessed based on biochemical parameters andin vivotests.A. squamosaandA. muricatawere found to be promising candidate plants for future insecticide use on mosquitoes.
[1] Ratsitorahina M, Harisoa J, Ratovonjato J, Biacabe S, Reynes JM, Zeller H, et al. Outbreak of dengue and chikungunya fevers, Toamasina, Madagascar, 2006. Emerg Infect Dis 2008; 14: doi: 10.3201/eid1407.071521.
[2] Raveloson NE, Ramorasata JC, Rasolofohanitrininosy R, Rakotoarivony ST, Andrianjatovo JJ, Sztark F. [Fatal haemorrhagic Rift Valley fever: a case at Madagascar]. Med Trop (Mars) 2010; 70: 177-179. French.
[3] Jeanmaire EM, Rabenarivahiny R, Biarmann M, Rabibisoa L, Ravaomanana F, Randriamparany T, et al. Prevalence of Rift Valley fever infection in ruminants in Madagascar after the 2008 outbreak. Vector Borne Zoonotic Dis 2011; 11: 395-402.
[4] Ratovonjato J, Olive MM, Tantely LM, Andrianaivolambo L, Tata E, Razainirina J, et al. Detection, isolation, and genetic characterization of Rift Valley fever virus from Anopheles (Anopheles) coustani, Anopheles (Anopheles) squamosus, and Culex (Culex) antennatus of the Haute Matsiatra region, Madagascar. Vector Borne Zoonotic Dis 2011; 11: 753-759.
[5] Rakotoarivelo RA, Andrianasolo R, Razafimahefa SH, Randremandranto NS, Randria MJ. [Severe presentations of Rift Valley fever in Madagascar]. Med Mal Infect 2011; 41: 318-321. French.
[6] Raharimalala FN, Ravaomanarivo LH, Ravelonandro P, Rafarasoa LS, Zouache K, Tran-Van V, et al. Biogeography of the two major arbovirus mosquito vectors, Aedes aegypti and Aedes albopictus (Diptera, Culicidae), in Madagascar. Parasit Vectors 2012; 5: 56.
[7] Zouache K, Raharimalala FN, Raquin V, Tran-Van V, Raveloson LH, Ravelonandro P, et al. Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti, from different geographic regions of Madagascar. FEMS Microbiol Ecol 2011; 75: 377-389.
[8] Heinrich N, Saathoff E, Weller N, Clowes P, Kroid I, Ntinginya E, et al. High seroprevalence of Rift Valley fever and evidence for endemic circulation in Mbeya region, Tanzania, in a crosssectional study. Plos Negl Trop Dis 2012; 6(3): e1557.
[9] Becker N, Petric D, Zgomba M, Boase C, Madon M, Dahl C, et al. Mosquitoes and their control. 2nd ed. London: Springer Science; 2010.
[10] Tantely ML, Tortosa P, Alout H, Berticat C, Berthomieu A, Rutee A, et al. Insecticide resistance in Culex pipiens quinquefasciatus and Aedes albopictus mosquitoes from la Réunion Island. Insect Biochem Mol Biol 2010; 40: 317-324.
[11] Azmi MA, Naqvi SN, Ahmad I, Tabassum R, Anbreen B. Toxicity of neem leaves extract (NLX) compared with malathion (57 E.C.) against late 3rd instar larvae of Culex fatigans (wild strain) by WHO method. Turk J Zool 1998; 22: 213-218.
[12] Grzybowski A, Tiboni M, da Silva MA, Chitolina RF, Passos M, Fontana JD. The combined action of phytolarvicides for the control of dengue fever vector, Aedes aegypti. Rev Bras Farmacogn 2011; doi: 10.1590/S0102-695X2012005000026.
[13] Kamgang B, Marcombe S, Chandre F, Nchoutpouen E, Nwane P, Etang J, et al. Insecticide susceptibility of Aedes aegypti and Aedes albopictus in Central Africa. Parasit Vectors 2011; 4: 79.
[14] Khan HA, Akram W, Shehzad K, Shaalan E. First report of field evolved resistance to agrochemicals in dengue mosquito, Aedes albopictus (Diptera, Culicidae), from Pakistan. Parasit Vectors 2011; 4: 146.
[15] Marcombe S, Darriet F, Agnew P, Etienne M, Yp-Tcha MM, Yébakima A, et al. Field efficacy of new larvicide products for control of multi-resistant Aedes aegypti populations in Martinique (French West Indies). Am J Trop Med Hyg 2011; 84: 118-126.
[16] Das NG, Goswami D, Rabha B. Preliminary evaluation of mosquito larvicidal efficacy of plant extracts. J Vector Borne Dis 2007; 44: 145-148.
[17] Araya H, Sahai M, Singh S, Singh AK, Yoshida M, Hara N, et al. Squamocin-O1 and squamocin-O2, new adjacent bistetrahydrofuran acetogenins from the seeds of Annona squamosa. Phytochemistry 2002; 61: 999-1004.
[18] Tormo JR, González MC, Cortés D, Estornell E. Kinetic characterization of mitochondrial co mplex I inhibitors using Annonaceous acetogenins. Arch Biochem Biophys 1999; 369: 119-126.
[19] Kaushik R, Saini P. Growth inhibiting effects of Annona squamosa leaf extract on vector mosquitoes. J Exp Zool 2009; 12: 395-398.
[20] Fong EH, Tin-Wa M, Farnsworth NR, Dobberstein RH. Phytochemical screening methods (Laboratory manual). Chicago: College of Pharmacy, University of Illinois at the Medical Center; 1977.
[21] World Health Organization. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Geneva: World Health Organization; 2013. [Online] Available from: http:// apps.who.int/iris/bitstream/10665/80139/1/9789241505154_eng.pdf [Accessed on 27 October, 2013]
[22] World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. Geneva: World Health Organization; 2005. [Online] Available from: http://whqlibdoc. who.int/hq/2005/who_cds_whopes_gcdpp_2005.13.pdf [Accessed on 27 October, 2013]
[23] Bermejo A, Figadere B, Zafra-Polo MC, Barrachina I, Estornell E, Cortes D. Acetogenins from Annonaceae: recent progress in isolation, synthesis and mechanisms of action. Nat Prod Rep 2005; 22: 269-303.
[24] Scott IM, Jensen HR, Philogène BJ, Arnason JT. A review of Piper spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochem Rev 2008;7: 65-75.
[25] Regnault-Roger C, Philogène BJ, Vincent C. [Biopesticides d’origine végétale]. 2th ed. France: Tec & Doc Lavoisier; 2005, p. 9-10. French.
[26] Kamaraj C, Rahuman AA, Mahapatra A, Bagavan A, Elango G. Insecticidal and larvicidal activities of medicinal plant extracts against mosquitoes. Parasitol Res 2010; 107: 1337-1349.
10.12980/APJTB.4.2014C1264
*Corresponding author: Lala Harivelo Raveloson Ravaomanarivo, Departement of Entomology, Faculty of Sciences, University of Antananarivo, Po Box 906, Antananarivo (101), Madagascar.
Tel: +261320243006
E-mail: lravaomanarivo@gmail.com
Foundation Project: Supported by the grants FRB-CD-AOOI-07-012 and CMIRA Coopera 2011 from Region Rh?ne-Alpes 11MIF-MAVINGUI-10851.
Article history:
Received 27 Nov 2013
Received in revised form 12 Feb, 2nd revised form 19 Mar, 3rd revised form 6 Apr 2014 Accepted 17 May 2014
Available online 27 Aug 2014
 Asian Pacific Journal of Tropical Biomedicine2014年10期
Asian Pacific Journal of Tropical Biomedicine2014年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Disseminated toxocariasis in an immunocompetent host
- Human ophthalmomyiasis externa caused by the sheep botfly Oestrus ovis: a case report from Karachi, Pakistan
- Calcinosis circumscripta in a captive African cheetah (Acinonyx jubatus)
- Production and purification of a bioactive substance against multi-drug resistant human pathogens from the marine-sponge-derived Salinispora sp.
- In vitro antibacterial activity of leaf extracts of Zehneria scabra and Ricinus communis against Escherichia coli and methicillin resistance Staphylococcus aureus
- In vitro antioxidant and anti-inflammatory activities of Korean blueberry (Vaccinium corymbosum L.) extracts
