A dysfunction of CD4+ T lymphocytes in peripheral immune system of Parkinson’s disease model mice
Yan HUANG, Zhan LIU, Xiao-qin WANG, Yi-hua QIU, Yu-ping PENG
Department of Physiology, School of Medicine, Nantong University, Nantong 226001, China
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by dopamine (DA) depletion in the nigrostriatal system. Pathogenesis of PD in terms of chronic neuroinflammatory process, which may accelerate eventual neurodegeneration, has been well discussed. Evidences of neuroinflammation in the brain, including activation of microglia in the substantia nigra and elevated levels of inflammatory cytokines in the striatum, suggest that immune functions of patients with PD are altered [1, 2].Studies in animal models demonstrate a role of T lymphocytes in development of PD [3, 4]. Depletion of T cells abrogates protection against dopaminergic(DAergic) neuron loss in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD [5]. CD4+T cells are found responsible for this neuroprotection [6]. Specific changes in peripheral blood of PD patients include decrease in CD4+CD45RA+‘naive’ T cells and increases in CD4+CD45RO+‘memory’ T cells and TCRγδ+cells[7, 8].
Naive CD4+T cells may be divided into foursubpopulations, termed as helper T (Th)1, Th2,Th17 and Treg cells by the differences in their cytokine expression profiles [9]. T-bet, a Th1-specific transcription factor, is thought to initiateそ1 development, whereas GATA binding protein 3(GATA3), a Th2-specific transcription factor, plays a pivotal role in development of Th2. Th1 cells are mainly involved in cellular immune responses by secretion of cytokines, such as interleukin (IL)-2,interferon (IFN)-γ and tumor necrosis factor (TNF).そ2 cells mainly mediate humoral immune responses and have anti-inflammatory effects via secretion of IL-4 and IL-5 [10]. そ17 cells are the newest member of effector Th cell family and are characterized by their ability to produce proinflammatory cytokines such as IL-17, IL-22, IL-17F and IL-21 [11, 12].Regulatory T (Treg) cells are known to maintain self-tolerance, prevent autoimmunity, and regulate immune homeostasis by attenuating excessive inflammation caused by pathogens or injuries [13-19]. Treg cells are identified by expression of CD4 and CD25 cell-surface markers and transcription factor forkhead box P3 (Foxp3) in mice [15, 20].In an MPTP mouse model, Th17 cells mediate nigrostriatal degeneration, whereas Treg cells protect DAergic neurons against degeneration [21, 22].
Although the involvement of neuroinflammation in PD pathogenesis has been largely reported, it is less clear what occurs in peripheral immune system of PD. Herein, we employed MPTP-induced mouse model of PD to reveal the changes in function and diあerentiation of the subpopulations of CD4+T cells in lymphoid tissues. そis study may give a new clue for the implication of peripheral immune system in PD pathogenesis.
Materials and Methods
Animals and MPTP administration
Male C57BL/6 mice (8 weeks old, 22-25 g) were obtained from Center of Experimental Animals,Nantong University, China. Mice received 4 intraperitoneal injections at 2-h intervals of either vehicle (saline, 5 ml/kg) or MPTP (20 mg/kg of free base in saline; Sigma-Aldrich, USA). On day 7 after MPTP injection, mice were subjected to motor and behavioral tests, and then sacrificed.Brains, lymphoid tissues and sera were processed for subsequent analyses. All animal procedures in this study were in accordance with guidelines of National Institute of Health and were approved by Animal Care and Use Committee of Nantong University. MPTP handling and safety measures were in accordance with published guidelines [23].
Motor coordination assessment
Rota-rod test is widely used to measure coordinated motor skills [24]. Rota-rod apparatus (Ugo Basile,Italy) consists of a rotating rod (3-cm diameter) and individual compartments for each mouse. Mice were trained for two consecutive days prior to MPTP injection in an acceleration mode (4-40 rpm) during 5 min. On day 7 afer MPTP dosing, the mice were recorded for retention time spent on the rotating rod as latency falling off the rota-rod. The rota-rod test was performed twice every 30 min, and the results were averaged into a single value for each mouse.
Behavioral test
Open-field test was used to assess animals’ motor activity level in an unfamiliar environment. Openfield device is composed of a square open field (50 cm × 50 cm × 50 cm) with a camera above the field.The floor of the arena was divided into 9 identical squares (3 × 3). Mice were carefully placed in center of the open field, and the numbers of squares crossed by the mice during a 2-min period were quantified as behavioral activity.
Immunohistochemistry of tyrosine hydroxylase (TH)in the substantia nigra pars compacta (SNpc)
Mice were intracardially perfused with saline after anesthetized, followed by 4% paraformaldehyde. そe brains were dissected out immediately, postfixed for 4 h at 4°C in the same fixative and dehydrated, and serial coronal sections (25 μm) were cut in a freezing microtome (Leica, Germany) throughout the SNpc and stored in cryoprotectant solution. The sections were treated with phosphate-buffered saline (PBS)containing 3% goat serum and 1% Triton X-100 for 30 min at room temperature to block nonspecific binding sites. Aferward, the sections were incubated overnight at 4°C with mouse anti-TH antibody(1:500, Millipore, USA), followed by incubation of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (1:200, Jackson, USA) at room temperature for 2 h. Images were acquired with a laser scanning confocal microscope (Leica TCS SP2,Germany). Totally, 40 brain sections in the SNpc were cut coronally and therefore these sections covered the entire extent of the SNpc. Every 4th slices in the series of sections were used in the TH immunohistochemistry. そe number of TH-positive cells from the 10 slices was counted in each sample.The data obtained from six animals in each group were statistically analyzed.
Measurement of DA content in the striatum
そe striatum was dissected on ice and homogenized in 0.5 mol/L perchloric acid (1:10, w:v). そe mixture was centrifuged at 15,000 rpm for 15 min at 4°C.DA was measured by high performance liquid chromatography (HPLC) coupled with reverse phase C-18 column (Atlantis dC18, 4.6 mm × 150 mm, 5 μm beads, Waters, USA) and electrochemical detector (Waters, USA). そe mobile phase consisting of sodium dihydrogen phosphate buあer was pumped at 1.0 ml/min, and the working voltage was set at 0.42 V. DA content was quantified by using the peak area of a standard curve.
Real-time quantitative polymerase chain reaction(PCR)
The spleen and mesenteric lymph nodes were harvested from the anesthetized mice by celiotomy.Mononuclear cells in the spleen or mesenteric lymph nodes were isolated by percoll density centrifugation.Total RNA was extracted from the mononuclear cells using Trizol (Invitrogen) and cDNA was synthesized from 2 μg of total RNA using cDNA reverse transcription kit (Invitrogen, USA) in 20 μl reaction volume as described by the manufacturer. Real-time PCR was performed with Universal SYBR Green Master Mix (Roche, Germany), 0.4 μmol/L primer and 2 μl cDNA. Amplification was run in a Rotor-Gene 3000 Real-time Cycler at 95°C for 10 min followed by 35 cycles of 94°C for 15 s and 60°C for 60 s. Following amplification, a melting curve analysis was performed by heating the reactions from 72°C to 95°C at 1°C intervals. そe analysis confirmed a single PCR product at the predicted melting temperature.β-actin was used for sample normalization. The cycle at which each sample crossed a fluorescence threshold was determined. Relative gene expression levels were calculated by comparative cycle threshold method. The murine-specific primers of the cytokines and transcription factors were summarized in Table 1.
Western blot analysis
Protein extracts (40 μg/lane) of mononuclear cells isolated from the spleen or mesenteric lymph nodes were separated by 12% SDS-PAGE and transferred for 45 min onto 0.2 μm PVDF membranes (Millipore,USA). The membranes were probed with rabbit antibodies to IL-2 (1:100, Santa Cruz Biotechnology,USA), IL-4 (1:200, Abcam, UK), transforming growth factor-β (TGF-β, 1:500, Abcam, UK), ROR-γt (1:500, Abcam, UK), IL-17 (1:200, Santa Cruz Biotechnology, USA), or IL-22 (1:200, Santa Cruz Biotechnology, USA), or with monoclonal mouse antibodies to T-bet (1:100, Santa Cruz Biotechnology,USA), GATA3 (1:100, Santa Cruz Biotechnology,USA), Foxp3 (1:200, Santa Cruz Biotechnology,USA), IFN-γ (1:200, Santa Cruz Biotechnology,USA), or IL-10 (1:200, Santa Cruz Biotechnology,USA). Appropriate IRDye 800-conjugated secondary antibodies (1:5000, Rockland Immunochemicals,USA) were used to visualize blots using Odyssey laser scanning system (LI-COR Inc, USA). Immunoblots were stripped and reprobed with antibody to β-actin(1:1000, Sigma, USA) as an internal control.
Quantization of cytokine levels by enzyme-linked immunosorbent assay (ELISA)
Blood samples were taken from the orbital sinus of each mouse. After incubated at 37°C for 1 h, the blood samples were centrifuged at 3 000 rpm for 15 min at 4°C and sera were collected. Cytokine concentrations in the sera were detected by ELISA kits according to the manufacturer’s protocols(eBioscience, USA). Briefly, plates were coated with capture antibodies overnight at 4°C and washed with 0.5% Tween-20/PBS. Nonspecific binding was blocked by the addition of 1% bovine serum albumin in PBS and by the incubation at 37°C for 1 h. Serum samples and diluted standards were added to each well, which was incubated at 37°C for 1 h, followed by the incubation with biotinylated secondary antibodies. The plates were washed and incubated with horseradish peroxidase-conjugated streptavidin. The plates were developed using 3,3’,5,5’-tetramethylbenzidine as the substrate and absorbance was determined with a multimode microplate reader (Bio Tek, USA) at 450 nm. A standard curve was plotted from the mean absorbance of each standard sample and the cytokine levels were calculated according to the standard curve.
Flow cytometric assay
Mononuclear cells isolated from the spleen or mesenteric lymph nodes were stained for 30 min at 4°C with different combinations of the following surface markers: anti-CD3-FITC, anti-CD4-allophycocyanin (APC), and anti-CD25-phycoerythrin (PE) (all from BD PharMingen, USA).For intracellular cytokine staining, these cells were cultured at 37°C in round bottom plates (2 × 106 cells/well) and stimulated with 50 ng/ml PMA, 1 μmol/L ionomycin and 2 μmol/L monensin for 5 h. Afer harvesting, the cells were stained for surface marker with APC-labeled anti-CD4 (BD PharMingen, USA),fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences, USA). Subsequently the cells were stained intracellularly with anti-IFN γ, anti-IL 4,or anti-IL 17 monoclonal antibodies conjugated with PE (all from BD PharMingen, USA). All samples were analyzed using a FACSCalibur flow cytometer with CellQuest sofware (BD Bioscience, USA).
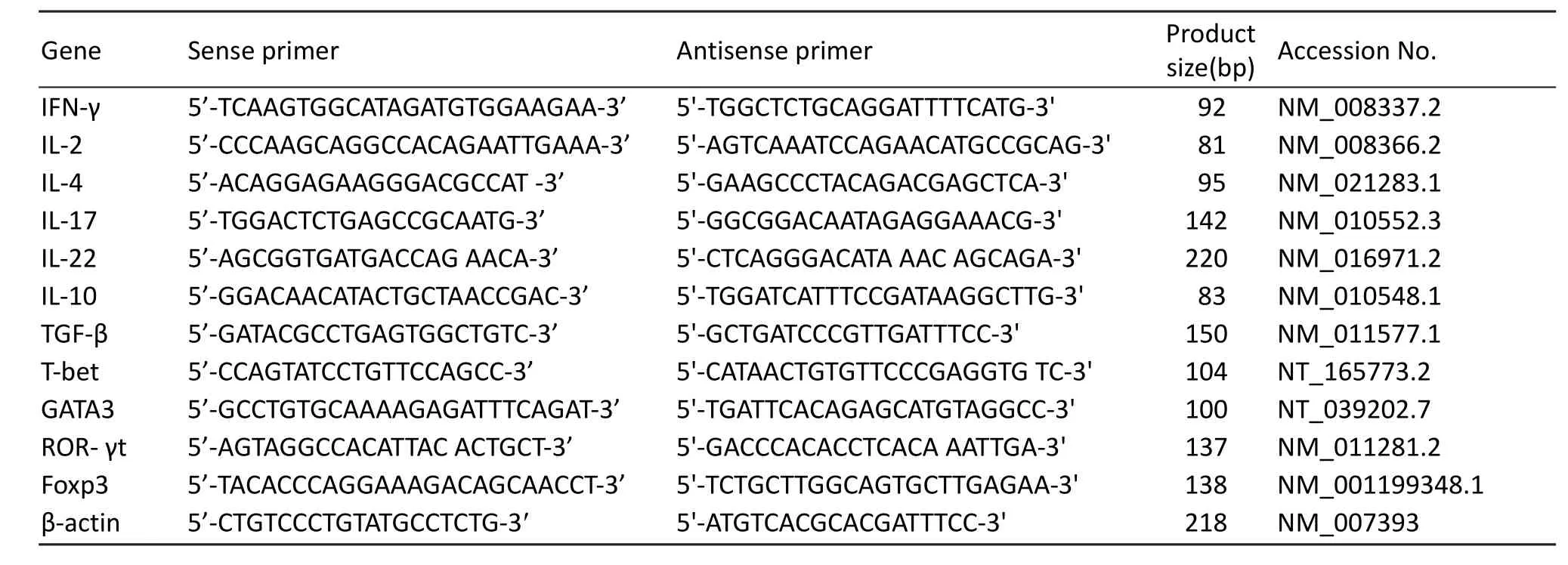
Tab. 1 Sequences of PCR primers.
Statistical analysis
Data were expressed as means ± SD. Statistical analyses were performed with the Statistics Package for Social Science (SPSS, 12.0). The data were subjected to the one-way analysis of variance(ANOVA), followed by Student-Newman-Keul’s test to compare the data of all groups relative to each other. Differences were considered statistically significant at P<0.05.
Results
Nigrostriatal impairments and behavioral deficits in MPTP-treated mice
To confirm MPTP-induced PD-like changes, we determined immunoreactive cells of TH(the ratelimiting enzyme of DA synthesis) in the SNpc on day 7 afer intraperitoneal injection of MPTP. Compared with intact or saline-treated controls, MPTP-treated mice were significantly decreased in the numbers of TH-immunoreactive neurons in the SNpc (Fig.1A). In addition, DA content in the striatum was remarkably lower in the MPTP-treated group than that in the saline-treated group (Fig. 1B). In openfield test, the numbers of moving through grids for the MPTP-treated mice were reduced relative to those for the intact or saline-treated animals (Fig.1C). In rota-rod test, average latency time falling oあthe rotated rod in MPTP-treated group was shorter than that in intact or saline-treated group (Fig. 1D).
Changes in levels of CD4+T cell-related cytokines in lymphoid tissues and serum of PD mice
In the spleens of PD mice, the expression levels of pro-inflammatory cytokines including IFN-γ, IL-17 and IL-22, were upregulated at both mRNA and protein levels comparing with those in intact or saline-treated animals (Fig. 2A, B). In the mesenteric lymph nodes of PD mice, besides the three above- mentioned pro-inflammatory cytokines, the proinflammatory IL-2 was also upregulated (Fig. 2A,B). However, all the examined anti-inflammatory cytokines including IL-4, IL-10 and TGF-β, were not altered in either of the lymphoid tissues in PD progress(Fig. 2A, B). In sera of PD mice,concentrations of the pro-inflammatory IFN-γ and IL-17 were elevated, but the anti-inflammatory TGF-β1 was not changed (Fig. 2C).
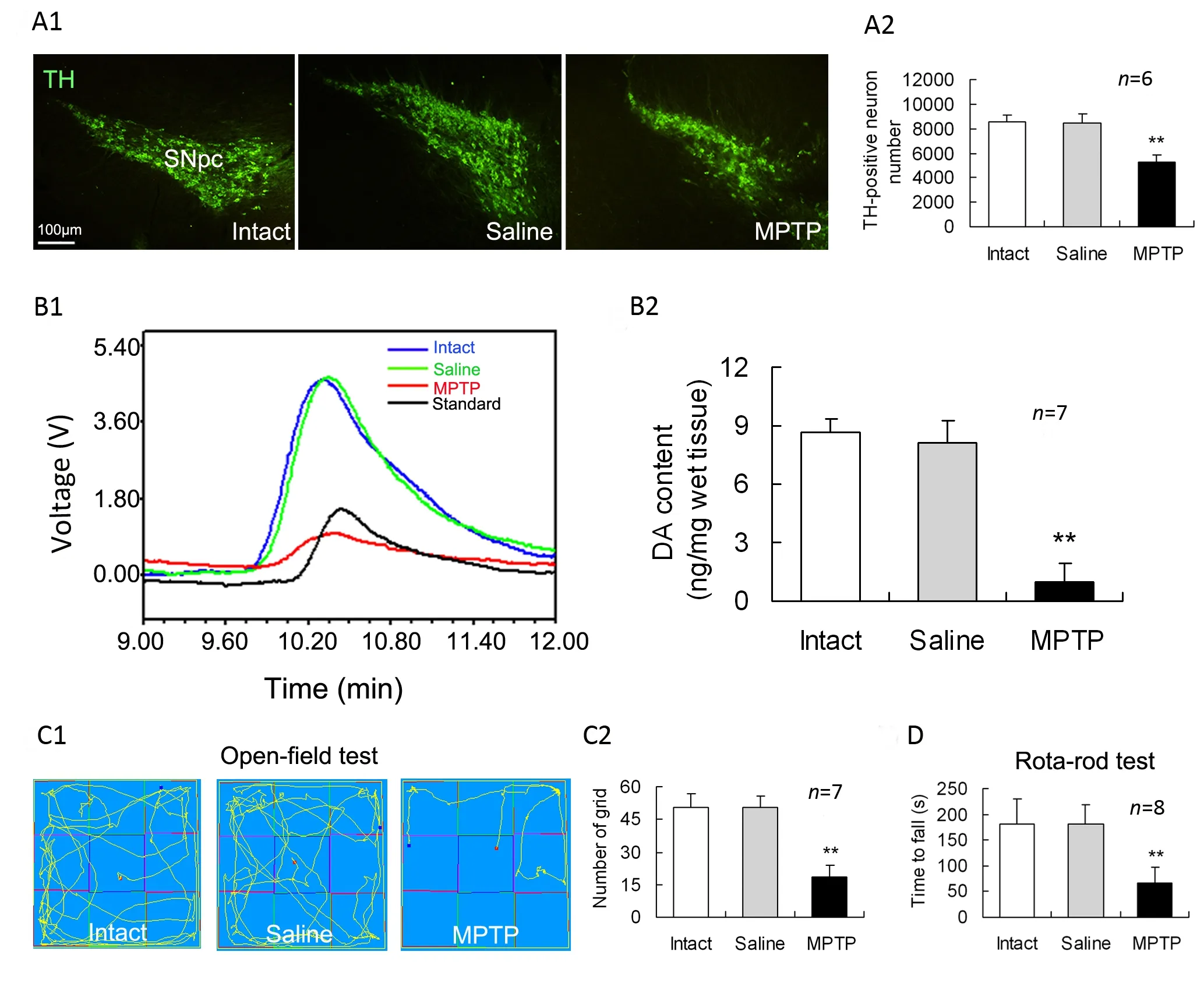
Fig. 1 Nigrostriatal impairments and behavioral deficits in MPTP-treated mice. MPTP was intraperitoneally injected into mice,and seven days later these measurements were performed. (A1) Representative photomicrographs of tyrosine hydroxylase(TH) staining in the substantia nigra pars compacta (SNpc). (A2). The numbers of TH immunoreactive neurons in the SNpc from the first (rostral) and every 4th coronal sections in a series of 40 brain frozen sections , which cover the entire extent of the SNpc, were counted as a sum. (B1) Typical HPLC spikes of DA in the striatum of mice. (B2) Statistical histogram showing dopamine (DA) content in the striatum. (C1) Movement orbits in open-field test. (C2) Numbers of squares crossed by mice.(D) Average retention time on the rota-rod. **P<0.01 vs intact or saline controls.
Alterations in expression levels of specifictranscription factors of CD4+T-cell subsets in lymphoid tissues of PD mice
The expression levels of Th1-, Th2-, Th17- and Treg-transcription factors, T-bet, GATA3, ROR-γt and Foxp3 in mononuclear cells derived from the spleen or mesenteric lymph nodes were measured.Compared with intact or saline controls, PD mice had a downregulated T-bet expression and an upregulated Foxp3 expression at both gene and protein levels in either of the spleen and mesenteric lymph nodes (Fig. 3). However, neither GATA3 nor ROR-γt was altered at gene or protein expression levels in the two lymphoid tissues of PD mice in comparison with intact or saline-treated animals(Fig. 3).
Decrease in IFN-γ-producing cells and increase in CD4+CD25+cells in lymphoid tissues of PD mice
In order to show changes in diあerentiation of CD4+T cells, the mononuclear cells derived from the spleen or mesenteric lymph nodes were labeled by membrane surface molecules or intracellular cytokines specific for Th1, Th2, Th17 or Treg cells.Percentage of CD3+CD4+cells, which represent total CD4+T lymphocytes, in the mononuclear cells was decreased in both the lymphoid tissues of PD mice relative to that of intact or saline mice (Fig.4). Further analysis indicated that CD4+IFN-γ+cells, which represent Th1 cells, were reduced, but CD4+CD25+cells, which reflect Treg cells, were increased in either of the lymphoid tissues of PD mice (Fig. 4). However, numbers of CD4+IL-4+cells(Th2 cells) or CD4+IL-17+cells (Th17 cells) in the two lymphoid tissues were not aあected by the MPTP treatment (Fig. 4).
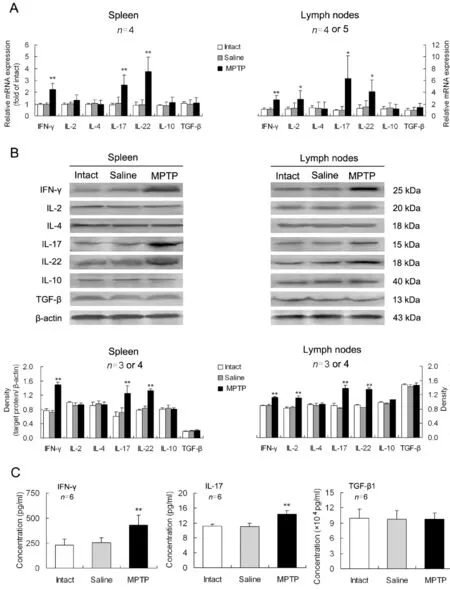
Fig. 2 Changes in levels of CD4+ T cell-related cytokines in lymphoid tissues and serum of Parkinson’s disease (PD) mice.On day 7 after MPTP injection, mononuclear cells were isolated from the spleen or mesenteric lymph nodes, and serum from inner canthus vein blood was collected. Expression levels of the cytokines at mRNA (A) and protein (B) levels and concentrations of the cytokines in serum (C) were measured. *P<0.05, **P<0.01 vs intact or saline-treated mice.
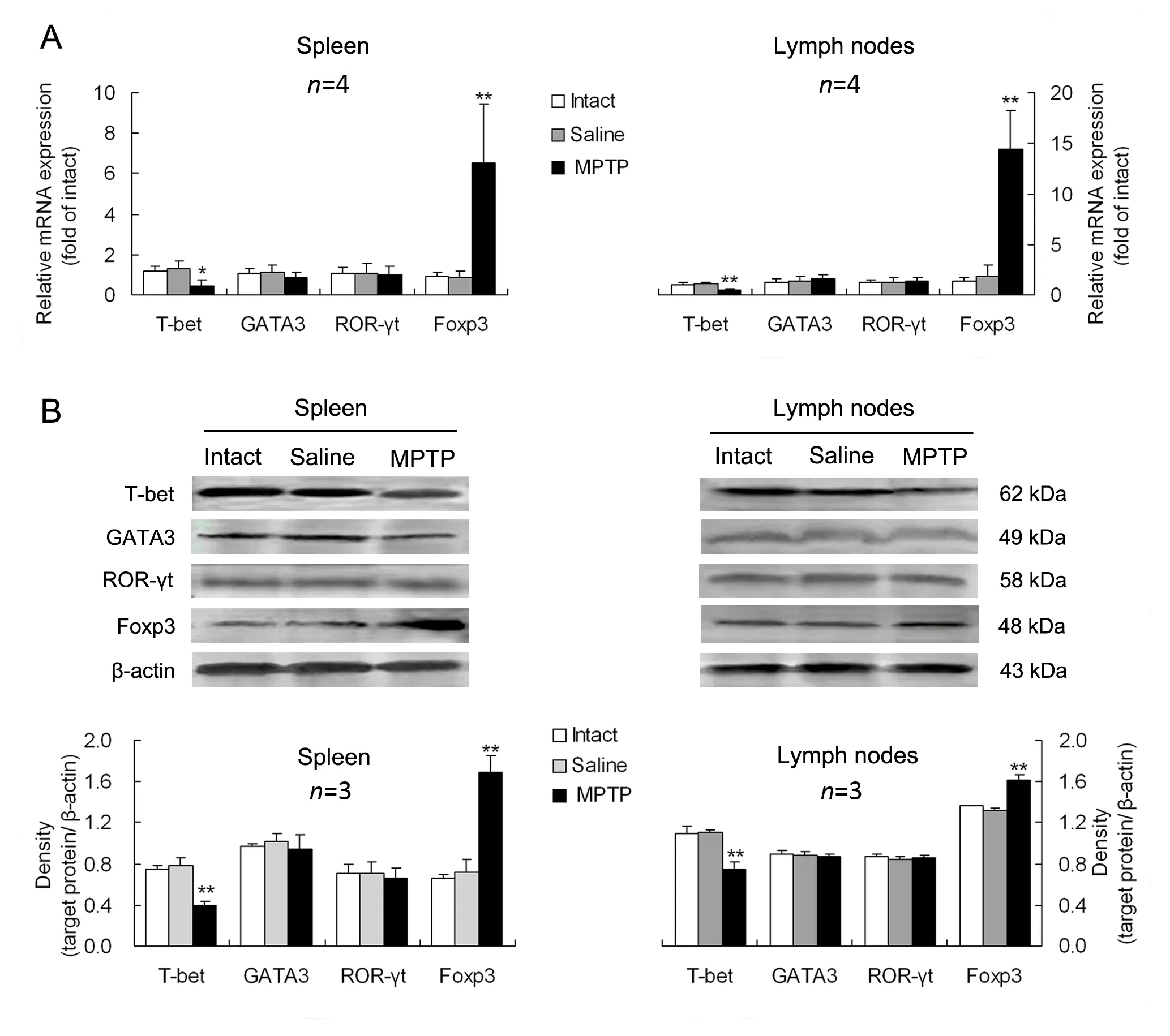
Fig. 3 Alterations in expression levels of specific-transcription factors of CD4+ T-cell subsets in lymphoid tissues of PD mice.The expression levels of the specific-transcription factors including T-box expressed in T cells (T-bet), GATA binding protein 3(GATA3), retinoid-related orphan nuclear receptor γt (ROR-γt) and transcription factor forkhead box p3 (Foxp3), for helper T(Th)1, Th2, Th17 and regulatory T (Treg) cells, were respectively determined in mononuclear cells separated from the spleen or mesenteric lymph nodes at gene (A) and protein (B) levels. *P<0.05, **P<0.01 vs intact or saline-treated animals.
Discussion
In this study, the intraperitoneal injection of the neurotoxin MPTP resulted in loss of TH-positive neurons in the SNpc and DA depletion in the striatum, suggesting a specific MPTP-induced damage to DAergic neurons in the nigrostriatal system. DA is a main neurotransmitter involved in control of motor function and therefore, alteration in brain DA levels can produce modification in motor function [25]. The MPTP-induced impairments in motor coordination, which was determined by rota-rod test, and in behavioral activity, which was examined by open-field test, confirmed that the PD-like changes were caused by DAergic neuron loss.
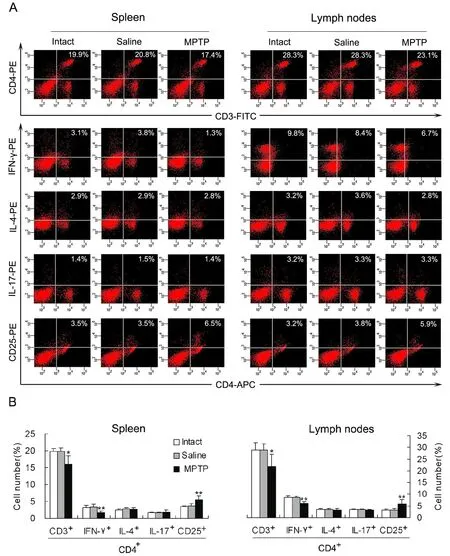
Fig. 4 Decrease in IFN-γ-producing cells and increase in CD4+CD25+ cells in lymphoid tissues of PD mice (n=4). (A) A representative image showing percentages of CD3+CD4+ (CD4+ T lymphocytes), CD4+IFN-γ+ (Th1 cells), CD4+IL-4+ (Th2 cells),CD4+IL-17+ (Th17 cells) and CD4+CD25+ (Treg cells) in mononuclear cells derived from the spleen or mesenteric lymph nodes.(B) Statistical graph of the repeated experiments as displayed in (A). *P<0.05, **P<0.01 vs intact or saline controls.
Importantly, the levels of pro-inflammatory cytokines including IFN-γ, IL-17, IL-22, or IL-2 were elevated in the spleen, mesenteric lymph nodes and serum of PD mice. However, the levels of antiinflammatory cytokines including IL-4, IL-10, or TGF-β were not altered in the lymphoid tissues or blood of PD mice. The elevated pro-inflammatory cytokines levels show an enhanced pro-inflammatory responses in PD pathogenesis. It has been reported that α-synuclein, a chief component of Lewy bodies that is released from aあected DAergic neurons of PD,is nitrated during oxidative stress responses and in its aggregated form, induces inflammatory microglial functions [26]. Notably, immunization of mice with the nitrated α-synuclein elicits peripheral T-cell immune responses to the novel antigenic epitopes that exacerbate neuroinflammation and nigrostriatal degeneration in MPTP model of PD [3]. Accordingly,we presume that the enhanced pro-inflammatory response in the peripheral may be caused by antigens such as the nitrated α-synuclein, which is also found in the cervical lymph nodes [3]. The enhanced pro-inflammatory response in turn aggravates PD progress. On the other hand, the peripheral immune inflammation may be also a factor leading to PD neuroinflammation, since a pro-inflammatory event in the peripheral can induce chronic, self-propelling neuroinflammation in the brain [27-29]. そis suggests a close link between peripheral inflammation and neuroinflammation. The brain can receive information from the immune system and respond to immune-derived signals by the cytokine network[30]. そe entry of pro-inflammatory cytokines to the brain causes the activated microglia to produce more inflammatory cytokines, which lead to neuronal death [31]. そus, peripheral inflammation amplifies neuroinflammation contributing to pathogenesis of the neurodegenerative disease PD, which is previously thought to be purely neuronal mechanism[32, 33].
In addition to the enhanced levels of proinflammatory cytokines, CD4+T cell diあerentiation was altered in PD progress. The total numbers of CD4+T cells were decreased in lymphoid tissues of PD mice. Furthermore, both the expression of T-bet(Th1-specific transcription factor) and the number of CD4+IFN-γ+cells (Th1 cells) were decreased,whereas both the expression of Foxp3 (Treg-specific transcription factor) and the number of CD4+CD25+cells (Treg cells) were increased in the lymphoid tissues of PD mice. そese consistent results suggested a differentiation bias of CD4+T cells towards Treg cells away from Th1 cells in PD progress. As a support, Hisanaga et al. [34] have reported that PD patients have significantly lower CD4+/CD8+T-cell ratio due to decreased percentage of CD4+CD8-T cells and increased percentage of CD4-CD8+T cells in peripheral blood. Our present data provided further evidences, showing that the decreased CD4+T cells are attributed to the reduction of Th1 cells.そe decrease in peripheral blood CD4+T cells of PD patients was due to an increased possibility of cell apoptosis [35]. Therefore, the reduced CD4+T cells may reflect an increase in apoptosis of the cells [8].In addition, a previously unrecognized inflammation or a postinfectious immune dysfunction was also possible to contribute to the reduction of CD4+T cells [34, 36]. Moreover, the shift of CD4+T cell diあerentiation towards anti-inflammatory Treg cells away from pro-inflammatory そ1 cells in PD progress was beneficial for control of the inflammation and alleviation of the neurodegeneration. As shown by Reynolds et al. [21], adoptive transfer of Treg cells in MPTP-treated mice provided a protection for the nigrostriatal system.
On the other hand, it seems to be inconsistent between the numbers of IFN-γ-producing cells and the concentration of IFN-γ production in PD progress, because the numbers were reduced but the concentration was elevated. Since the IFN-γproducing cells detected in the current study were CD4 positive, the IFN-γ-producing CD4+cells principally represent Th1 cells. Dissimilarly, the IFN-γ concentration in this study reflected IFN-γ production by mononuclear cells rather than only by Th1 cells. Therefore, the present study explained that the enhancement of pro-inflammatory response in peripheral immune system of PD included the activation of other mononuclear cells such as macrophages, natural killer cells and dentritic cells,besides T lymphocytes.
Conclusion
PD has a dysfunction of peripheral immune system.It manifests enhancement of pro-inflammatory response and CD4+T cell diあerentiation bias towards Treg cells away from Th1 cells. These changes may aあect outcome of PD, depending on balance in proinflammatory/anti-inflammatory levels. Thus, a therapeutic strategy targeting the balance will be promising for amelioration of PD.
Acknowledgements
This work was supported by grants 81271323 and 31371182 from the National Natural Science Foundation of China, BK2011386 from the Natural Science Foundation of Jiangsu Province of China,and a project funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
1. Mogi M, Harada M, Kondo T, et al. Interleukin-2 but not basic fibroblast growth factor is elevated in parkinsonian brain[J]. J Neural Transm, 1996, 103(8-9): 1077—1081.
2. Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection[J]? Lancet Neurol,2009, 8(4): 382—397.
3. Benner EJ, Banerjee R, Reynolds AD, et al. Nitrated alphasynuclein immunity accelerates degeneration of nigral dopaminergic neurons[J]. PLoS One, 2008, 3(1): e1376.
4. Cao JJ, Li KS, Shen YQ. Activated immune cells in Parkinson’s disease[J]. J Neuroimmune Pharmacol, 2011,6(3): 323—329.
5. Benner EJ, Mosley RL, Destache CJ, et al. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease[J]. Proc Natl Acad Sci USA,2004, 101(25): 9435—9440.
6. Laurie C, Reynolds A, Cuskun O, et al. CD4+T cells from Copolymer-1 immunized mice protect dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease[J]. J Neuroimmunol, 2007, 183(1-2): 60—68.
7. Fiszer U, Mix E, Fredrikson S, et al. Parkinson’s disease and immunological abnormalities: increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+T cells in peripheral blood[J]. Acta Neurol Scand,1994, 90(3): 160—166.
8. Bas J, Calopa M, Mestre M, et al. Lymphocyte populations in Parkinson’s disease and in rat models of parkinsonism[J].J Neuroimmunol, 2001, 113(1): 146—152.
9. Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone: I. Definition according to profiles of lymphokine activities and secreted proteins[J]. J Immunol, 1986, 136(7): 2348—2357.
10. D’Ambrosio D, Iellem A, Colantonio L, et al. Localization ofそ cell subsets in inflammation: diあerential thresholds for extravasation of そ1 and そ2 cells[J]. Immunol Today, 2000,21(4): 183—186.
11. Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses[J]. Nat Immunol, 2008, 9(6):650—657.
12. Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-β are required for differentiation of human T(H)17 cells[J]. Nature, 2008, 454(7202): 350—352.
13. Cederbom L, Hall H, Ivars F. CD4+CD25+regulatory T cells down-regulate co-stimulatory molecules on antigenpresenting cells[J]. Eur J Immunol, 2000, 30(6): 1538—1543.14. Kipnis J, Mizrahi T, Hauben E, et al. Neuroprotective autoimmunity: Naturally occurring CD4+CD25+regulatory T cells suppress the ability to withstand injury to the central nervous system[J]. Proc Natl Acad Sci USA, 2002, 99(24):15620—15625.
15. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3[J]. Science,2003, 299(5609): 1057—1061.
16. Sakaguchi S. Naturally arising CD4+regulatory T cells for immunologic self-tolerance and negative control of immune responses[J]. Ann Rev Immunol, 2004, 22: 531—562.
17. Coombes JL, Robinson NJ, Maloy KJ, et al. Regulatory T cells and intestinal homeostasis[J]. Immunol Rev, 2005, 204:184—194.
18. Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice[J]. Nat Immunol, 2007, 8(2): 191—197.
19. Bourreau E, Ronet C, Darcissac E, et al. Intralesional regulatory T-cell suppressive function during human acute and chronic cutaneous leishmaniasis due to Leishmania guyanensis[J]. Infect Immun, 2009, 77(4): 1465—1474.
20. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+regulatory T cells[J]. Nat Immunol, 2003, 4(4): 330—336.
21. Reynolds AD, Banerjee R, Liu J, et al. Neuroprotective activities of CD4+CD25+regulatory T cells in an animal model of Parkinson’s disease[J]. J Leukoc Biol, 2007, 82(5):1083—1094.
22. Reynolds AD, Stone DK, Hutter JA, et al. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease[J]. J Immunol, 2010, 184(5): 2261—2271.
23. Przedborski S1, Jackson-Lewis V, Naini AB, et al.The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety[J]. J Neurochem, 2001, 76(5): 1265—1274.
24. Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease[J]. Behav Brain Res, 2001, 125(1-2): 109—125.
25. Goldberg NR, Haack AK, Lim NS, et al. Dopaminergic and behavioral correlates of a progressive lesioning of the nigrostriatal pathway with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine[J]. Neuroscience, 2011, 180: 256—271.
26. Reynolds AD, Glanzer JG, Kadiu I, et al. Nitrated alphasynuclein-activated microglial profiling for Parkinson’s disease[J]. J Neurochem, 2008, 104(6): 1504—1525.
27. Ferrari CC, Tarelli R. Parkinson’s disease and systemic inflammation[J]. Parkinsons Dis, 2011, 2011: 436813.
28. Cunningham C, Campion S, Lunnon K, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease[J]. Biol Psychiatry, 2009, 65(4): 304—312.
29. Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation aあect chronic neurodegeneration[J]. Nat Rev Immunol, 2007, 7(2): 161—167.
30. Goncharova LB, Tarakano AO. Molecular networks of brain and immunity[J]. Brain Res Rev, 2007, 55(1): 155—166.
31. Qin L, Wu X, Block ML, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration[J].Glia, 2007, 55(5): 453—462.
32. McGeer PL, McGeer EG. The alpha-synuclein burden hypothesis of Parkinson disease and its relationship to Alzheimer disease[J]. Exp Neurol, 2008, 212(2): 235—238.
33. Dobbs RJ, Charlett A, Purkiss AG, et al. Association of circulating TNF-alpha and IL-6 with ageing and parkinsonism[J]. Acta Neurol Scand, 1999, 100(1): 34—41.
34. Hisanaga K, Asagi M, Itoyama Y, et al. Increase in peripheral CD4 bright+CD8 dull+T cells in Parkinson disease[J]. Arch Neurol, 2001, 58(10): 1580—1583.
35. Calopa M, Bas J, Callen A, et al. Apoptosis of peripheral blood lymphocytes in Parkinson patients[J]. Neurobiol Dis,2010, 38(1): 1—7.
36. Fiszer U. Does Parkinson’s disease have an immunological basis? The evidence and its therapeutic implications[J].BioDrugs, 2001, 15(6): 351—355.
- 中國應(yīng)用生理學(xué)雜志的其它文章
- Pathophysiological changes in mitochondria of mammalian exposed to hypoxia at high altitude
- Microvesicles derived from hypoxia/reoxygenation-treated human umbilical vein endothelial cells impair relaxation of rat thoracic aortic rings
- Stimulation of endothelial non-neuronal muscarinicreceptor attenuates the progression of atherosclerosis via inhibiting endothelial cells activation
- Effect of acclimation training on physiological changes in a randomized controlled trial in hot-humid environment
- Scene-trait coping style of military rescuers in Wenchuan earthquake
- Plasma endothelin-1 and nitric oxide correlate withligustrazine alleviation of pulmonary artery hypertension in patients of chronic cor pulmonale from high altitude plateau during acute exacerbation

