Stimulation of endothelial non-neuronal muscarinicreceptor attenuates the progression of atherosclerosis via inhibiting endothelial cells activation
Jing-hong ZHOU, Zhi-yuan PAN, Yan-fang ZHANG,, Wen-yu CUI, Chao-liang LONG,, Hai
WANG 1,2
1. Cardiovascular Drug Research Center, Institute of Pharmacology and Toxicology, Beijing 100850; 2. Cardiovascular Drug Research Center, Institute of Health and Environmental Medicine, Beijing 100850; 3. Cardiovascular Drug
Research Center, Thadweik Academy of Medicine, Beijing 100039, China
Introduction
Atherosclerosis, the most common and serious vascular disease, is a special form of chronic inflammatory and immune process resulted from interactions among multiple atherogenic risk factors, cells (including endothelial cells, monocytes/macrophages, T lymphocytes and smooth muscle cells) and the extracellular matrix of the arterial wall[1,2,3]. Notably, endothelial cells play a critical role in vascular intimal inflammatory response during atherosclerosis[4,5]. When activated or injuried by traditional cardiovascular risk factors,endothelium overexpressed multiple cell surface specific adhesion molecules [e.g. vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), etc.] and chemotactic cytokines (e.g. monocyte chemotactic protein-1, MCP-1). These pro-inflammatory molecules and cytokines are mostly controlled by nuclear factor-kappa B (NF-κB) transcription activity, and are able to facilitate the peripheral blood leukocytes firmly adhere to endothelium and subsequently migrate into the intima where they develop into macrophages, which in turn furthersecrete inflammatory cytokines that sustain the stimulus for more leukocytes adherence and recruit circulating T lymphocytes[6,7]. Additionally, the reduced bioavailability of nitric oxide (NO), one of the most important features during endothelial dysfunction, is also responsible for the development of atherosclerosis and other cardiovascular diseases. Endothelial cells activation or endothelial dysfunction ultimately promotes persistent inflammation in vascular wall and contributes to the establishment and progression of atherosclerotic plaque. This progress opens potentially useful avenues of investigation into therapies targeting the endothelium for inhibiting endothelial cells activation and protecting against endothelial dysfunction[5,8, 9], especially in increasing the NO release,suppressing leukocyte-endothelium interaction, and preventing overexpression of adhesion molecules and chemokines[7].
Non-neuronal acetylcholine system (NNAs)plays a crucial role in some diseases[10,11]. In endothelial cells, there are also two major types of cholinergic receptors, the non-neuronal muscarinic receptors (NNMR) and the nicotinic receptors[12].NNMR plays a fundamental role in regulating blood flow, basal vasomotor tone, and immune and inflammatory responses[13,14]. In physiological condition, activation of NNMR in endothelial cells by endogenous or exogenous acetylcholine can increase the production of vasoactive mediators, such as NO.Despite extensive research into these processes, the role of endothelial NNMR in atherosclerosis remains poorly understood. Recently, it was reported that pharmacological stimulation of cholinergic system by acetylcholinesterase inhibitors, where the increased availability of acetylcholine stimulated both the muscarinic and the nicotinic receptors, strikingly attenuated atherogenesis in ApoE-/-mice fed on a high-fat diet, and its underlying mechanisms were still unclear [15].
Interestingly, our previous studies demonstrated that arecoline, a natural product in the areca nut which is recreationally used in many Asian countries,protected against endothelial dysfunction induced by various atherogenic risk factors including oxidized low density lipoprotein (ox-LDL), high concentration of D-glucose, or homocysteine in cultured rat aortic endothelial cells (RAECs). Arecoline could also inhibit the mRNA expression of MCP-1, ICAM-1, and VCAM-1 by activating endothelial NNMR[16,17,18].
Based on these intriguing findings, we hypothesize that activation of endothelial NNMR by arecoline can inhibit ox-LDL-induced inflammatory response in endothelial cells and suppress monocyte-endothelium interaction. To examine this assumption, we investigated the effects of arecoline in regulating NO production and MCP-1 secretion in RAECs.Moreover, we used atropine, a broad-spectrum muscarinic receptor antagonist, to confirm whether the eあects of arecoline on endothelial cells were due to stimulation of NNMR. Furthermore, to validate the protection of arecoline against atherosclerosis in vivo, we also measured serum NO production, serum total cholesterol status, aortic atherosclerotic plaque areas, pro-inflammatory molecules expression and NF-κB transcription activity in the aortae of ApoE-/-mice fed on a high fat diet.
Here, for the first time, we report that stimulation of NNMR can attenuate the development and progression of atherosclerosis via inhibiting endothelial cells activation.
Methods
Chemical compounds and reagents
Arecoline hydrobromide, atropine sulphate, and NG-nitro-L-arginine methylester (L-NAME) were purchased from Sigma-Aldrich (St. Louis, Mo,USA). Ox-LDL was provided by Institutes of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China). Fetal bovine serum (FBS)and endothelial cell basal medium (M199 and RPMI 1640) were purchased from Gibco-BRL (USA).Serum total cholesterol (TC) was measured using a commercial kit (BioSino Bio-technology and Science Inc. Beijing, China). The NO assay kit was product of the Institute of Nanjing Jiancheng Biology Engineering (Nanjing, P.R. China). The MCP-1 protein assay kit (enzyme-linked immunosorbent assay, ELISA) was purchased from R&D System, Inc.,USA. All antibodies were commercially obtained:anti-MCP-1 antibody (Abcam Inc., Cambridge,Mass., USA), anti-IκB-α antibody (Santa Cruz Biotechnology, Inc., USA), anti-P65 antibody(Beijing Biosynthesis Biotechnology Co. LTD,Beijing, China), antibody against phospho-P65 (Cell Signaling Technology Inc., Denver, Colo., USA),and β-actin monoclonal antibody (Sigma Chemical Inc., St. Louis, Mo., USA). The fluorescent dye 2,7-bis(2-carboxyethyl)-5(6)- carboxyfluorescein acetoxymethylester (BCECF-AM) was obtained from Acros, Geel, Belgium. All other chemicals and materials were obtained from local commercial sources.
Animals
Eight-week-old ApoE-/-mice on a C57BL/6J background and wild-type C57BL/6J mice weighing 25±2 g were obtained from the Department of Laboratory Animal Science, Peking University Health Science Center, Beijing, China, and housed in groups of eight, under a 12-hour light/dark cycle at a temperature of (24±1)°C and relative humidity of(50±10)%, with free access to water and food during the experimental periods. All animal procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and approved by the local animal care and use committee.
Experimental atherosclerosis model and treatments
Experimental atherosclerosis model was established in 36 male ApoE-/-mice by a hypercholesterolemic diet (containing 1% cholesterol and 15% pork lard)for 7 weeks, and 12 male wild-type mice fed on a standard chow without any drug intervention were used as control. During these experimental periods,ApoE-/- mice were randomized to receive a low dose of arecoline (7 mg/kg/d, n=12), a high dose of arecoline (21 mg/kg/d, n=12), or no treatment(n=12) while still fed on the hypercholesterolemic diet. All the medications were given daily by gavage.Arecoline was freshly dissolved in saline (0.9% NaCl)every day. Afer 7 weeks of treatment, all mice were weighed, and blood samples for determination of cholesterol and NO were collected and centrifuged at 3 000×g for 15 minutes at 4°C to isolate serum.Serum samples were frozen at -70°C until the assay was performed. Animals then were sacrificed and aortic tissues were rapidly harvested for further analysis: six samples of them were fixed in 10%buffered formaldehyde solution for histopathology studies, and the others were snap frozen in liquid nitrogen for molecular biology studies.
Serum total cholesterol and NO measurement
Serum total cholesterol was measured using a commercially available kit according to the manufacturer’s protocols. Because of its instability in solutions, most of NO is quickly converted to nitrite(NO2?) and further to nitrate (NO3?). Therefore,serum NO level was determined as NO2?/NO3?concentrations using the NO assay kit following the manufacturer’s instructions.
Aortic morphology
The fixed aortic tissues from each group were embedded in paraffin, cut into 3-μm sections, and stained with hematoxylin and eosin [19]. Six slices of each group were randomly chosen, and three to four fields of each slice were used for image analysis. Integral optical density (IOD) of the atherosclerotic plaque area, the total cross-sectional area of blood vessel, intimal plaque area, intima and media thicknesses were detected respectively with the CMIAS-II type true-color pathological image analysis system (version 4.0, developed by Beijing Aeronautic and Astronautic University, China.)under a 10×40 field of a light microscope. Under the same parameter conditions, the IOD value of all the slices was analyzed. The fraction area of lesion (%)was expressed as the area ratio of plaque area to the total cross-sectional area of blood vessel. そe intimal plaque (%) was expressed as the area ratio of intimal plaque area to the total cross-sectional area of blood vessel.
Gene expression of MCP-1 and adhesion molecules in the aortae of ApoE-/-mice
Total RNA was extracted from pieces of aortic segments using TRIzol reagents according to the manufacturer’s protocols (Invitrogen, Carlsbad, CA,USA), and was converted to cDNA using TaqMan reverse transcription and real-time polymerase chain reaction (PCR). PCR was performed by standard methods, as described in our previous study[20]. そe quantitative expressions of MCP-1, VCAM-1, and ICAM-1 were performed using a real-time PCR Kit(RealMasterMix-SYBR Green, TIANGEN Biotech Co. Ltd., Beijing, China) following the manufacturer’s instructions. The mouse oligonucleotide primer pairs were synthesized according to previous reports[19,21]. Parallel amplification of mouse β-actin gene was selected for reference. The target gene, accession number, nucleotide sequences,amplified product sizes and annealing temperatures for each primer were listed in Table 1. The relative mRNA expression values were calculated by comparative Ct method.
MCP-1 protein detection and NF-κB activity
measurement by Western blot analysis
Cytoplasmic and/or nuclear protein extracts of thoracic aorta tissues were prepared using a commercial kit, according to the manufacturer’s instructions (Viagene Biotech Co. Ltd., Ningbo,China, Catalog number: SINP001). The protein concentration in extracts was measured by a bicinchoninic acid (BCA) Protein Assay Kit (Sangon Biotech Co. Ltd., Shanghai, China). Target proteins in the cytoplasmic and/or nuclear fractions of the aortic tissues were determined by Western blot analysis using the following antibodies: anti-MCP-1 antibody, anti-IκB-α antibody, anti-P65 antibody, antibody against phospho-P65, and β-actin monoclonal antibody. そe MCP-1 protein detection was performed by standard method, while NF-κB activity was indirectly reflected by determination of the expression of IκB-α, P65, and phospho-P65, as described in previous reports[22,23]. The density of the bands was analyzed by CMIAS as mentioned above and normalized by β-actin expression.

Tab. 1 Oligonucleoほde primers used for RT-PCR.
Cell culture
Rat aortic endothelial cells (RAECs) were prepared as described previously[20]. In brief, RAECs were isolated from normal Sprague—Dawley rat aorta.The aorta was dissected out, cut into pieces, and placed inner surface down in T25 flasks with 3 ml of M199 medium containing 20% fetal calf serum without growth supplement. On the third day, the aorta pieces were rinsed and the collected cells were cultured in M199 medium containing 10% fetal calf serum. RAECs were grown to confluence at 37°C in a humidified atmosphere of 95% air and 5% CO2.Positive immunofluorescence with antibodies against von Willebrand factor, Factor VIII-related antigen,identified that the cells were endothelial.
Preparation of ox-LDL
Native LDL was isolated from normal human plasma by density gradient centrifugation. Native LDL(200 μg protein/mL) was oxidized by exposure to CuSO4(10 μmol/L free Cu2+) in phosphate-buあered saline (PBS) at 37°C for 24 hours, then was dialyzed against PBS containing 10 mg/L EDTA at 4°C for 24 hours. Control incubations were done in the presence of 200 μmol/L EDTA without CuSO4. The degree of LDL oxidation was determined by analysis of malonaldehyde bis (dimethyl acetal) (MDA)equivalents by using the thiobarbituric acid reactive substance assay. Protein content was determined by a BCA protein assay kit (Pierce) with the use of bovine serum albumin as the standard. そe MDA content of ox-LDL was 3.2 mol/L versus 0.6 mol/L in the native LDL preparation.
Quantization of NO production and MCP-1 secretion in RAECs
To assess the eあects of arecoline on NO production and MCP-1 secretion, the cultured cells from passage three were seeded onto 12-well plates, and cultured in M199 medium for experiments. When the cells grew to 75% confluence, the culture medium was changed to a phenol red-free M199 medium supplemented with 1% FBS and 1% penicillin—streptomycin and maintained for 6 hours. そen cells were pretreated with arecoline (1.0~100 μmol/L final concentration) for 12 hours. Finally, the cells were exposed to 100 μg/ml ox-LDL for another 12 hours and medium was harvested for NO or MCP-1 protein assay. In order to investigate the eあects of atropine, a broad-spectrum muscarinic receptor antagonist, on endothelial NNMR activation, the cells were treated with atropine (1 μmol/L) 1 hour before 10 μmol/L arecoline administration. In addition, to determine whether endothelium-eNOS-NO signal pathway is involved in the eあects of arecoline or not, L-NAME,an inhibitor of endothelial NO synthase, was also added 1 hour before 10 μmol/L arecoline treatment and was continued during the subsequent 24 hours in combination with arecoline and ox-LDL. そe NO production in supernatant was determined using a commercially available kit as described above.
The MCP-1 concentration in culture medium was measured by enzyme-linked immunosorbent assay,and a curve was calibrated from MCP-1 standard according to the manufacturer’s instructions. Each assay was performed in triplicate.
Cell adhesion assay
そe cell adhesion assay was carried out as previously described by Wang L., et al.[24]. In brief, monocytic U937 cells were labeled with the fluorescent dye 2,7-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethylester at a 10 μmol/L final concentration in RPMI 1640 medium containing 10% FBS at 37°C for 1 hour. The labeled cells were harvested by centrifugation and washed with PBS before resuspension in the medium. HUVECs were seeded onto 24-well culture plates and left to grow to 75% confluent monolayers, and pre-incubated with arecoline (0.1~100 μmol/L final concentration)for 6 hours, and then stimulated by 150 μg/ml ox-LDL for 18 hours. Atropine (1 μmol/L) was added 1 hour before arecoline administration. The labeled U937 cells were then added to each well and allowed to interact for 60 minutes at 37°C. Unbound cells were removed by gently washing with PBS. U937 cells adhered to HUVECs were lysed with 50 mmol/L Tris-HCl, pH 8.0, containing 0.1% SDS.The quantitative results were obtained by using an ELISA plate reader at 485 nm excitation and 535 nm emission wavelengths.
Statistical analysis
Data are represented as the mean±SD. One-way ANOVA and the Student’s t-test were used to analyze the data by the Statistical Package for Social Sciences(SPSS) version 9.0 for Windows. P < 0.05 was considered to be statistically significant.
Results
Arecoline had no eあect on serum total cholesterol levels
As shown in Table 2, there was no significant difference in each group between initial and final body weight during the experimental periods.At the end of the study, the administration of a hyperlipidemic diet to ApoE-/-mice induced a sharp increase in serum total cholesterol levels (wild-type control: 2.41 ± 0.51 mmol/L vs untreated ApoE-/-mice:34.75 ± 8.69 mmol/L, P<0.01), and both arecoline 7 and 21 mg/kg/d treatments had no effect on the raised serum total cholesterol levels.
Arecoline prevented progression of atherosclerotic plaques
Following 7 weeks of a high fat diet, percentage of atherosclerotic plaque areas, fraction of lesion areas,intimal plaque areas, and intima/media thicknesses ratio were all significantly increased in the aortae of ApoE-/-mice compared with wild-type mice,respectively (Fig. 1A). Arecoline at 7 and 21 mg/kg/d could significantly decrease the plaque areas(arecoline-7 mg/kg/d: 93552 ± 23463 μm2, n=6,P<0.05; arecoline-21 mg/kg/d: 33563 ± 9572 μm2,n=6, P<0.01), compared with untreated ApoE-/-mice(176265 ± 13341 μm2, n=6), respectively (Fig.1B).Effects of arecoline on lesion areas, intimal plaque areas, and intima/media thicknesses ratio were all similar to that on plaque areas (Fig.1C, D and E).These results showed that arecoline had modest attenuating eあects at a dose of 7 mg/kg/d, and could significantly block the formation and progression of atherosclerosis at a dose of 21 mg/kg/d.
Arecoline inhibited the expression of MCP-1 and adhesion molecules in aortic tissues
To further explore the underlying molecular mechanism of anti-atherogenic effects of arecoline,mRNA expressions of adhesion molecules and chemoattractants in the aortae were determined by RT-PCR. Treatment with a hyperlipidemic diet significantly increased ICAM-1, VCAM-1 and MCP-1 mRNA expressions in the aortae of ApoE-/-mice. Arecoline apparently inhibited the mRNA overexpressions of ICAM-1, VCAM-1 and MCP-1(Fig.2A).
As one of the most important chemoattractants,MCP-1 was mainly responsible for recruiting monocytes into the intima. Therefore, the relative protein abundance of MCP-1 in the aortae was further investigated. Western blot analysis showed that MCP-1 protein expression was increased in ApoE-/-mice fed on a high fat diet and was significantly reduced after arecoline administration at 7 and 21 mg/kg/d (Fig.2B).
Arecoline modulated the NF-κB activity
The NF-κB signaling pathway activation has been considered to promote the expression of pro-inflammatory cytokines including adhesion molecules (VCAM-1 and ICAM-1), and chemokines(e.g. MCP-1), and plays a crucial role in the pathogenesis of atherosclerosis[25,26]. Western blot analysis revealed that IκB-α protein expression was significantly decreased, while the P65 subunit of NF-κB and phospho-P65 protein were both increased in the aortae of untreated ApoE-/-mice with respect to wild-type mice respectively (P<0.01, Fig.3).Treatment with arecoline at 7 or 21 mg/kg/d could up-regulate IκB-α protein expression and downregulate P65 and phospho-P65 protein expression respectively (Fig.3B, C, D). These results indicatethat arecoline can normalize the activated NF-κB signal pathway via increasing IκB-α degradation and inhibiting P65 phosphorylation and translocation.
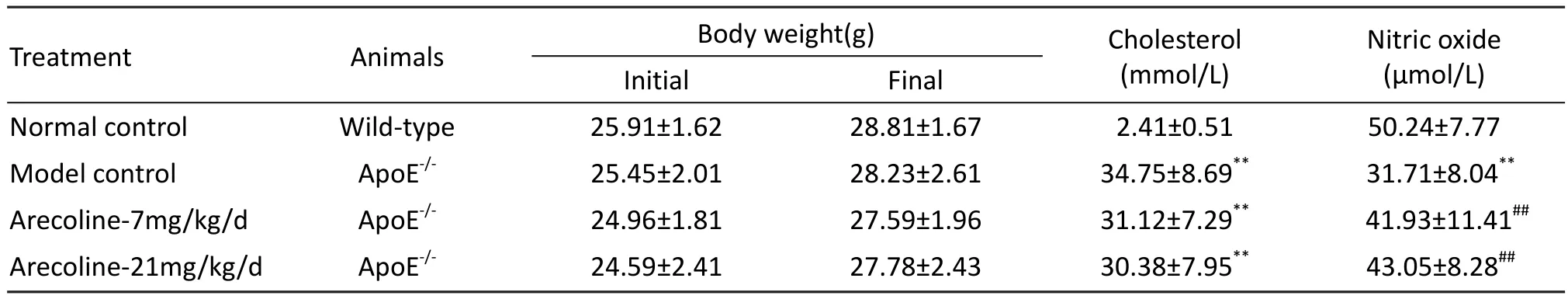
Tab. 2 Effects of arecoline on body weight, serum cholesterol, and serum nitric oxide in ApoE-/- mice fed on a high cholesterol diet.
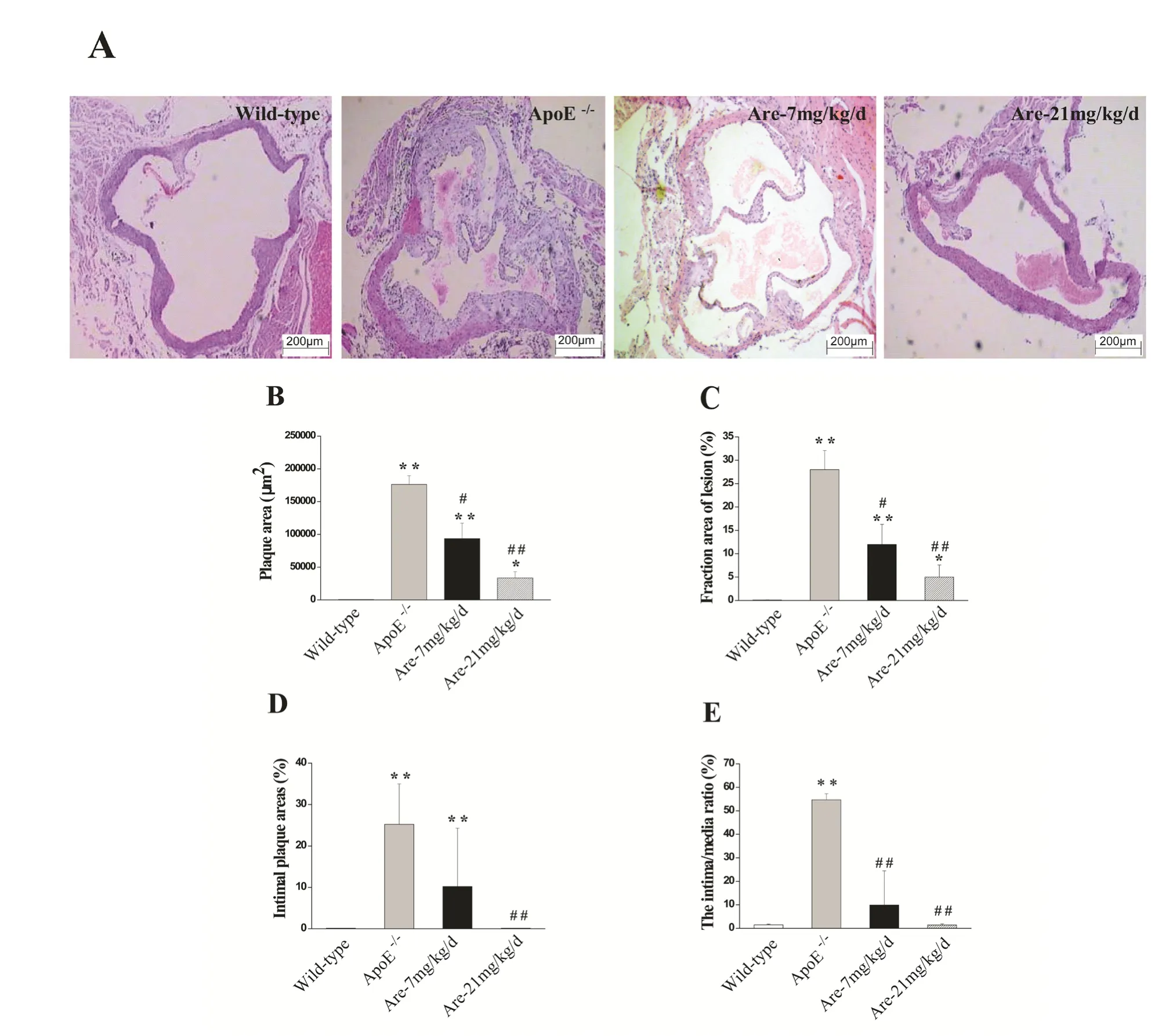
Fig. 1 Effects of arecoline on atherosclerotic plaque lesions in the aortae of ApoE-/- mice fed on a high cholesterol diet. A:Representative atherosclerotic plaques stained with hematoxylin and eosin (Original magnification: ×40) were obtained from the aortic roots of wide-type and ApoE-/- mice treated without or with arecoline at 7 mg/kg/d or 21 mg/kg/d for 7 weeks. B, C,D, and E: Plaque areas, fraction area of lesion, intimal plaque areas, and intima/media ratio from each group were performed by computer associated morphometry. No lesion can be observed in wild-type mice control group. Data were represented as mean±SD, n=6. *P<0.05, **P<0.01 vs wild-type mice with normal diet; # P<0.05, ## P<0.01 vs ApoE-/- mice with a highcholesterol-food diet.
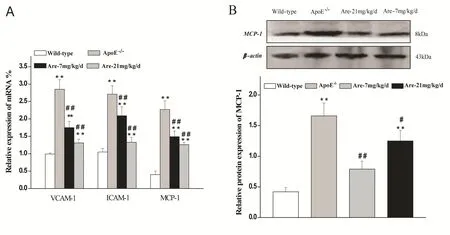
Fig. 2 Effects of arecoline on the expressions of adhesion molecules and chemotactic cytokines in the aortae of ApoE-/- mice fed on a high cholesterol diet. A: The mRNA expressions of VCAM-1, ICAM-1 and MCP-1 determined relative to the mRNA expression of β-actin were observed in wild-type C57BL/6J mice fed on normal chow and ApoE-/- mice fed on a high cholesterol diet treated without or with arecoline at 7 mg/kg/d and 21 mg/kg/d for 7 weeks. B: Effects of arecoline on the protein expression of MCP-1 determined by Western blot analysis. Protein band density was normalized by β-actin. Data were expressed as mean±SD,n=6. * P<0.05, **P<0.01 vs wild-type mice; # P<0.05, ## P<0.01 vs ApoE-/- mice treated without arecoline.
Arecoline increased NO production in vivo and in vitro Incubation of RAECs with ox-LDL (100 μg/ml) for 12 hours significantly decreased the production of NO compared with control group (control group:10.5 ± 2.31 μmol/L vs ox-LDL group: 4.21 ± 1.17 μmol/L, P<0.01). Pretreatment of RAECs with 10 and 100 μmol/L arecoline for 12 hours significantly increased the content of NO in supernatant to 7.92 ±0.97 and 9.29 ± 2.03 μmol/L respectively, compared with ox-LDL group (both P<0.01), whereas lower concentrations (0.1~1.0 μmol/L) had no eあect. When the endothelial NNMR was blocked by 1.0 μmol/L atropine, the enhancement of arecoline (10 μmol/L)on NO release was absolutely abolished. In addition,after pre-incubation with 100 μmol/L L-NAME, an endothelial nitric oxide synthase (eNOS) inhibitor,eあect of arecoline on NO release was also completely abolished (Fig.4A).
To further confirm the eあects of arecoline on NO,the results in our experiments in vivo showed that the administration of a hyperlipidemic diet to ApoE-/-mice induced a dramatic decline in serum NO levels(wild-type control: 50.24 ± 7.77 μmol/L vs untreated ApoE-/-mice: 31.71 ± 8.04 μmol/L, P<0.01), while arecoline could significantly enhance the serum NO production at 7 and 21 mg/kg/d (arecoline-7mg/kg/d: 41.93 ± 11.41 μmol/L; arecoline-21 mg/kg/d: 43.05± 8.28 μmol/L, P<0.01) compared with untreated ApoE-/-mice respectively (Tab.2). As the decreased serum NO content indicates that endothelial dysfunction has occurred in ApoE-/-mice afer a long term hyperlipidemic diet feeding, our results suggest that arecoline can improve endothelial dysfunction in atherosclerosis.
Arecoline decreased MCP-1 secretion in RAECs
Incubation of RAECs with ox-LDL strikingly increased the secretion of MCP-1 compared with control group (control group: 80.55 ± 5.91 pg/ml vs ox-LDL group: 205.62 ± 9.57 pg/ml, P<0.01).Arecoline (1.0, 10, 100 μmol/L) could dosedependently decrease the content of MCP-1 in supernatant to 169.36 ± 30.01, 148.77 ± 8.21, and 119.36 ± 17.46 pg/ml, respectively. These effects could be abolished by atropine pretreatment. To further assess whether the decreased MCP-1 levels by arecoline is associated with the endotheliumeNOS-NO signal pathway, we checked the effect of L-NAME, an eNOS inhibitor, on MCP-1 secretion when the cells treated with arecoline. It was showed that afer the eNOS activity was blocked by L-NAME
(100 μmol/L), the inhibition of arecoline on MCP-1 production was also abolished (Fig.4B).
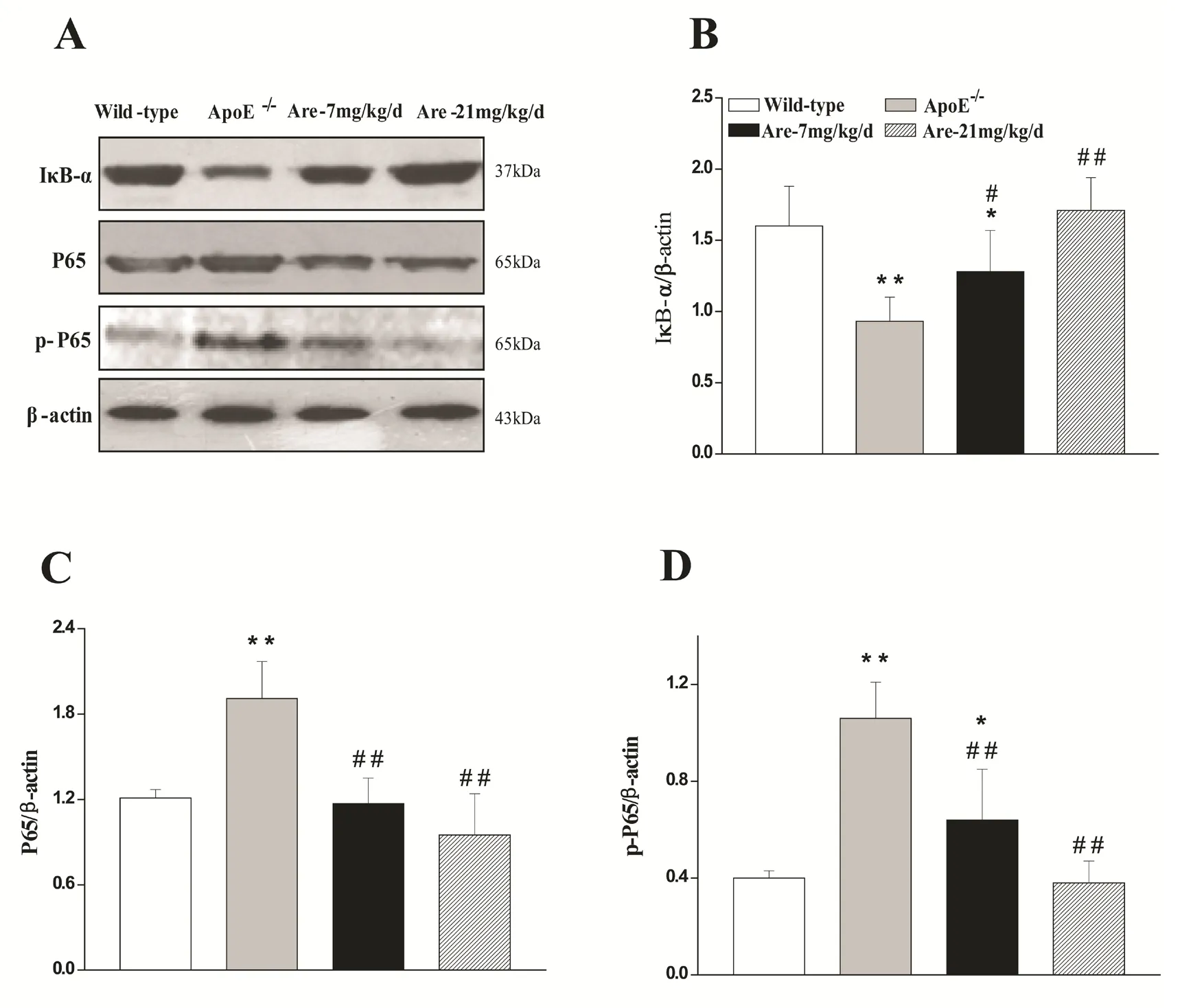
Fig. 3 Effects of arecoline on IκB-α degradation and P65 phosphorylation and translocation in the aortae of ApoE-/- mice.Cytoplasmic extracts and nuclear extracts were prepared from the aortae of wild-type mice and ApoE-/- mice treated without or with arecoline at 7 mg/kg/d and 21 mg/kg/d for 7 weeks (pool of every two arteries in each group). The respective protein was examined by Western blot analysis. The data were visualized with enhanced chemiluminescence (A), and densitometry analysis (B, C, and D). Density of IκB-α band against β-actin and of phospho-P65 and P65 band against β-actin was expressed as mean±SD, n=6. *P<0.05, **P<0.01 vs wild-type mice; #P<0.05, ##P<0.01 vs ApoE-/- mice treated without arecoline.
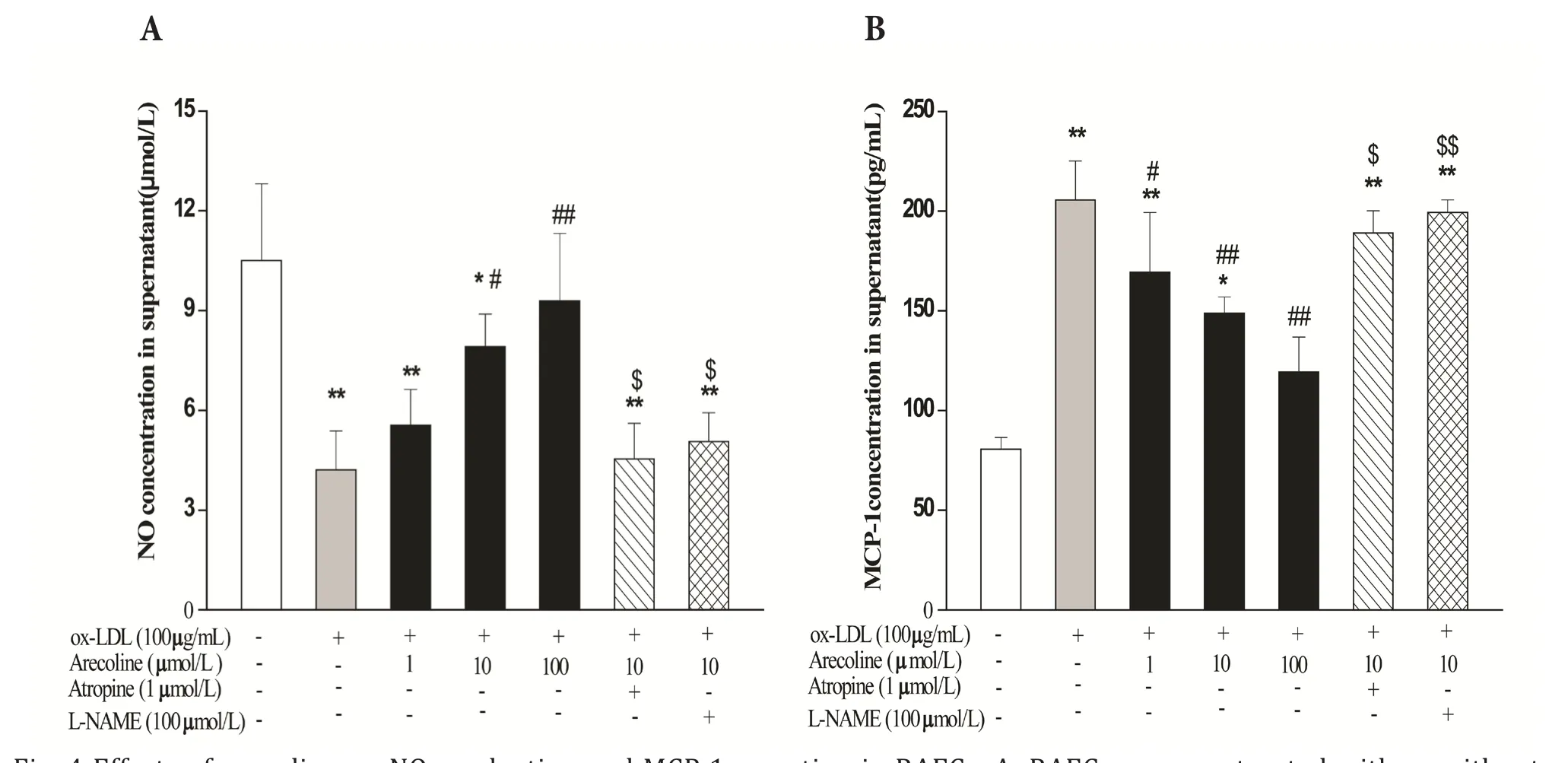
Fig. 4 Effects of arecoline on NO production and MCP-1 secretion in RAECs. A: RAECs were pretreated with or without atropine or L-NAME for 1 hour, and then incubated with arecoline at various concentrations for 12 hours. After exposure to ox-LDL (100 μg/ml) for another 12 hours, the supernatants were collected for measurement of NO concentrations using Griess reagent. B: MCP-1 release into medium was measured by ELISA. Data represent the means±SD, n=4. *P<0.05, **P<0.01,compared with control group; #P<0.05, ##P<0.01 vs only ox-LDL treatment; $P<0.05, $$P<0.01 vs the data obtained in the absence of atropine or L-NAME.
Arecoline suppressed monocyte-endothelium interaction in vitro
Monocytes adhesion to endothelial cells is an initiating event in the formation of atherosclerotic plaque. そerefore, we explored the eあect of arecoline on U937 cell adhesion to the ox-LDL-stimulated HUVECs (Fig.5A). After HUVECs were exposed to 150 μg/mL ox-LDL for 18 hours, U937 cellendothelium adhesion enhanced substantially(P<0.01), and the enhanced adhesion of U937 cells to the ox-LDL-stimulated HUVECs could be inhibited by arecoline from 0.01 to 100 μmol/L in a dose-dependent manner (P<0.05 or P<0.01).Pretreatment with atropine at 1.0 μmol/L could block the inhibition of 10 μmol/L arecoline on monocytesendothelial cells adhesion (Fig.5B). These results indicate that arecoline can efficiently suppress ox-LDL-exposed endothelial cells activation and cells adhesion, and inhibit dysfunction-induced persistent inflammatory response in endothelial cells.
Discussion
Atherosclerosis, the principal cause of heart attack and stroke, remains a major contributor to morbidity and mortality worldwide. Accumulating studies in humans and animals show that inflammation drives all phases of atherosclerosis, including initiation,progression, and thrombotic complications of lesion[27]. However, currently available antiinflammatory agents do not appear promising as antiatherosclerotic interventions[27]. In spite of extensive studies into inflammatory cytokines, there are still no specific anti-cytokines therapies for the treatment of atherosclerosis in this emerging field[7].Because endothelial cells activation or endothelial dysfunction can initiate sustained vascular inflammation and accelerate atherogenesis via upregulating the expressions of adhesion molecules and chemoattractants[4,5], searching for a promising therapeutic drug targeting endothelial cells to inhibit inflammatory response is ongoing[27,28].
In the present study, we have demonstrated that the systemic administration of arecoline efficiently attenuated atherosclerotic plaque areas, and suppressed overexpression of adhesion molecules and chemokines as well as nuclear factor-kappa B activity in the aortae of ApoE-/-mice fed on a high cholesterol diet. In vitro, arecoline increased the NO production and inhibited the MCP-1 secretion in ox-LDL injured RAECs in a concentration-dependent manner, and suppressed the adhesion of monocytic U937 cells to ox-LDL-exposed HUVECs, which all could be abolished by atropine. These findings suggest that activation of non-neuronal muscarinic receptors in endothelial cells may be a novel strategy for the treatment of atherosclerosis and its complications.
Our histopathology studies demonstrated that arecoline administrations could strikingly reduce the extent of atherosclerosis; CMIAS examination showed that arecoline treatments ameliorated the injury of vessel wall in the endothelial disrupted region, obviously decreased atherosclerotic plaque areas, fraction of lesion areas, intimal plaque areas,and intima/media thicknesses ratio. Based upon these observations in our animal model, we expect that arecoline also has antiatherosclerotic effects in human.
A series of studies indicate that endothelial dysfunction plays a pivotal role in initiating and exacerbating progression of vascular inflammation[4,5,28]. Endothelial cells response to multiple risk factors can lead to endothelial cells activation (or endothelial dysfunction) and subsequent leukocyte-endothelium interactions.These changes contain up-regulation of specific adhesion molecules (ICAM-1, VCAM-1) that promote monocyte adhesion and transmigration,as well as the secretion of MCP-1[4]. Our previous studies in vitro had demonstrated that arecoline could inhibit the mRNA excess expression of adhesive molecules in RAECs injured by various atherogenic risk factors, such as ox-LDL, high concentration of D-glucose, and homocysteine[16,17,18]. In current study, we also find that arecoline systemic administration can suppress the expressions of ICAM-1 and VCAM-1 in the aortic tissues from the hypercholesterolemic ApoE-/-mice, which is consistent with our previous studies.
It was well documented that NO, a signal molecule within cells and between adjacent cells, opposed atherogenic processes. Of note, NO selectively reduces endothelial expression of adhesion molecules and pro-inflammatory cytokines, especially in regulating the expression of MCP-1[29,30]. As a pivotal chemotactic factor, MCP-1 attracts monocytes to adhere to endothelial cells and migrate into the intima of the arterial wall. The ox-LDL also induces local vascular cells to produce MCP-1,which causes monocytes recruitment, and enhances the progression of the atherosclerotic lesions[23].そerefore, it is reasonable to postulate that arecoline administration may affect the expression of chemoattractants via modulating the NO release in endothelial cells. Our results in vivo identified that arecoline treatment down-regulated the gene and protein expressions of MCP-1 in the aortic tissues and obviously increased serum NO level in ApoE-/-mice fed on a high fat diet. In in vitro experiments,we found that arecoline pretreatment significantly increased the NO production, decreased MCP-1 secretion and suppressed the adherence of monocytic U937 cells to ox-LDL-activated endothelial cells,which were all abolished by atropine, a nonspecific muscarinic receptor antagonist. そese results indicate that the inhibition of MCP-1 by arecoline is due to the activation of NNMR in endothelial cells.
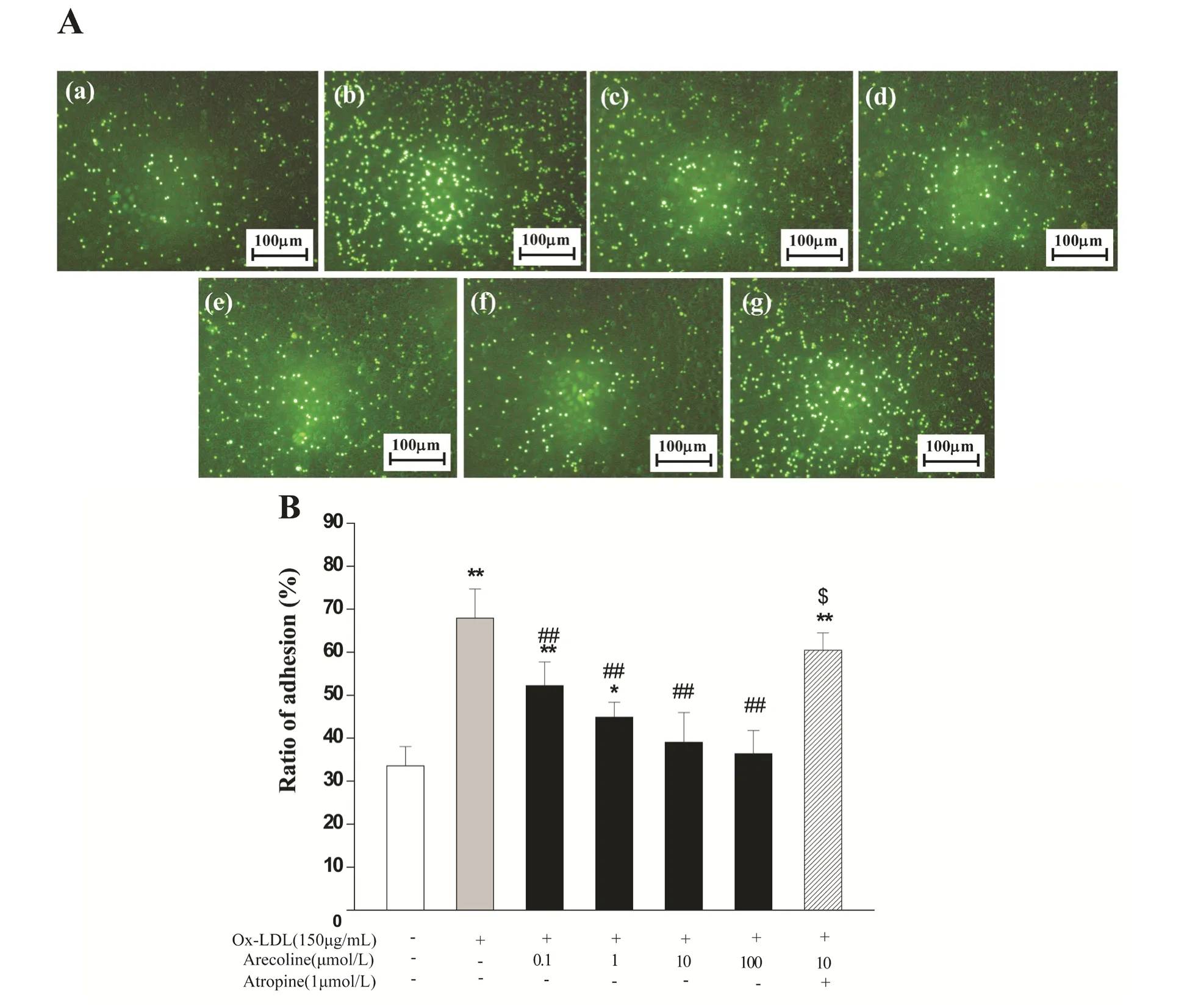
Fig. 5 Effects of arecoline on the adhesion of fluorescein-labeled U937 cells to ox-LDL-activated HUVECs in the absence or presence of atropine. A: HUVECs were seeded in the bottom chamber of 24-well transwell plates to reach confluent monolayers, and preincubated with arecoline for 6 hours and then incubated with ox-LDL at 150 μg/ml for 18 hours. BCECFAM labeled U937 monocytes were seeded in the upper chamber and allowed to migration for 3 hours. (a) control; (b) ox-LDL;(c) ox-LDL + arecoline 0.1 μmol/L; (d) ox-LDL + arecoline 1 μmol/L; (e) ox-LDL + arecoline 10 μmol/L; (f) ox-LDL + arecoline 100 μmol/L; (g) ox-LDL + arecoline 10 μmol/L + atropine 1.0 μmol/L. B: Adhesion of U937 monocytes to HUVECs was detected by measuring fluorescence with excitation at 488 nm and emission at 535 nm using a bottom reading fluorescent plate reader. Data were the mean±SD from 6 separate experiments. *P<0.05, **P<0.01 vs control group; #P<0.05, ##P<0.01 vs ox-LDL treatment; $P<0.05 vs the data obtained in the absence of atropine.
Moreover, the results showed that the inhibition of MCP-1 by arecoline could also be abolished by L-NAME, an eNOS inhibitor. This result indicates that the protection against atherosclerosis by arecoline may be dependent of endothelium-eNOSNO signal pathway.
It is well known that adhesion molecules and MCP-1 in endothelial cells are mostly under the control of nuclear transcription factor NF-κB, a common denominator of inflammatory stimulants[25,26,31]. Therefore, we investigated whether NF-κB activity was involved in the inhibitory mechanism of arecoline on adhesion molecules and MCP-1 expressions. As expected, arecoline could increase IκB-α degradation and inhibit P65 phosphorylation and translocation in aortic tissues from ApoE-/-mice, which demonstrates that arecoline reduces the inflammatory responses and ameliorates atherosclerosis, at least in part, via modulating NF-κB activity in the vascular endothelium. そese results provide a possible molecular mechanism which is through increasing endothelial NO production,inhibiting the activity of NF-κB as well as expressions of adhesion molecules, by which the activation of endothelial NNMR attenuates atherosclerosis.
In present study, we also showed that arecoline treatments had no effect on serum total cholesterol levels. Therefore, the antiatherosclerotic effect of arecoline appears to be a direct consequence of the inhibition on endothelial cells activation in ApoE-/-mice fed on a high cholesterol diet.
In summary, our results in current study indicate that the anti-inflammatory effects of arecoline are mainly attributed to the stimulation of NNMR in endothelial cells. These results also provide a possible explanation for the anti-atherogenic properties observed following the treatment of acetylcholinesterase inhibitors in ApoE-/-mice[15].Based on these observations, we conclude that inhibition of endothelial cells activation by stimulation of endothelial non-neuronal muscarinic receptors is an importantly alternative strategy to treat patients with atherosclerosis.
Acknowledgements
そe present study was supported by grants from the State Key Research Project of China (AWS11J003)and Tianjin Key Technologies Research and Development Program (05ZHGCGX01300). There are no conflicts of interest.
1. Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis[J]. Annu Rev Immunol, 2009, 27: 165-197.
2. Hansson GK, Libby P. そe immune response in atherosclerosis: a double-edged sword[J]. Nat Rev Immunol, 2006, 6: 508-519.
3. Libby P. Inflammation in atherosclerosis[J]. Nature, 2002, 420: 868-874.
4. Tesfamariam B, DeFelice AF. Endothelial injury in the initiation and progression of vascular disorders[J]. Vascul Pharmacol, 2007,46(4): 229-237.
5. Sitia S, Tomasoni L, Atzeni F, et al. From endothelial dysfunction to atherosclerosis[J]. Autoimmun Rev, 2010, 9(12): 830-834.
6. Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis[J]. J Atheroscler そromb, 2003, 10(2): 63-71.
7. Burke-Gaあney A, Brooks AV, Bogle RG. Regulation of chemokine expression in atherosclerosis[J]. Vascul Pharmacol, 2002, 38(5):283-292.
8. Schade D, Kotthaus J, Clement B. Modulating the NO generating system from a medicinal chemistry perspective: current trends and therapeutic options in cardiovascular disease[J]. Pharmacol そer,2010, 126(3): 279-300.
9. Tomasoni L, Sitia S, Borghi C, et al. Eあects of treatment strategy on endothelial function[J]. Autoimmun Rev, 2010, 9(12): 840-844.
10. Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the nonneuronal cholinergic system in humans[J]. Br J Pharmacol, 2008,154(8): 1558-1571.
11. Kawashima K, Fujii T. Basic and clinical aspects of non-neuronal acetylcholine: overview of non-neuronal cholinergic systems and their biological significance[J]. J Pharmacol Sci, 2008, 106(2): 167-173.
12. Kirkpatrick CJ, Bittinger F, Nozadze K, et al. Expression and function of the non-neuronal cholinergic system in endothelial cells[J]. Life Sci, 2003, 72(18-19): 2111-2116.
13. Eglen RM. Muscarinic receptor subtypes in neuronal and nonneuronal cholinergic function[J]. Auton Autacoid Pharmacol, 2006,26(3): 219-233.
14. Razani-Boroujerdi S, Behl M, Hahn FF, et al. Role of muscarinic receptors in the regulation of immune and inflammatory responses[J]. J Neuroimmunol, 2008, 194(1-2): 83-88.
15. Inanaga K, Ichiki T, Miyazaki R, et al. Acetylcholinesterase inhibitors attenuate atherogenesis in apolipoprotein E-knockout mice[J]. Atherosclerosis, 2010, 213(1): 52-58.
16. Shi CG, Hu G, Wang H. Protective eあect of arecoline on expression of inflammatory molecules in endothelial cells injuried by oxLDL[J]. Chin J Cardiol, 2004, 32:650.
17. Duan ZB , Wang H. Regulation effect of arecoline on excess expression of adhesive molecules in endothelial cells injuried with high concentration of D-glucose[J]. Chin J Pharmacol そer, 2006,11: 27-32.
18. Duan ZB, Wang H. Protective eあect of compounds on endothelial cells injuried with homocysteine[J]. Chin Pharm Bull, 2006, 22:537-542.
19. Portugal LR, Fernandes LR, Pietra Pedroso VS, et al. Influence of low-density lipoprotein (LDL) receptor on lipid composition,inflammation and parasitism during Toxoplasma gondii infection[J]. Microbes Infect, 2008, 10(3): 276-284.
20. Long CL, Qin XC, Pan ZY, et al. Activation of ATP-sensitive potassium channels protects vascular endothelial cells from hypertension and renal injury induced by hyperuricemia[J]. J Hypertens, 2008, 26(12): 2326-2338.
21. Iwasaki M, Saito K, Sekikawa K, et al. Tumor necrosis factor-alpha from bone marrow-derived cells is not essential for the expression of adhesion molecules in lipopolysaccharide-induced nasal inflammation[J]. Cytokine, 2003, 21(3): 129-136.
22. Vaziri ND, Bai Y, Yuan J, et al. ApoA-1 mimetic peptide reverses uremia-induced upregulation of pro-atherogenic pathways in the aorta[J]. Am J Nephrol, 2010, 32(3): 201-211.
23. Majumdar S, Aggarwal BB. Methotrexate suppresses NF-kappa B activation through inhibition of I kappa B alpha phosphorylation and degradation[J]. J Immunol, 2001, 167(5): 2911-2920.
24. Wang L, Hao Q, Wang YD, et al. Protective effects of dehydroepiandrosterone on atherosclerosis in ovariectomized rabbits via alleviating inflammatory injury in endothelial cells[J].Atherosclerosis, 2011, 214: 47-57.
25. de Winther MP, Kanters E, Kraal G, et al. Nuclear factor kappa B signaling in atherogenesis[J]. Arterioscler そromb Vasc Biol, 2005,25(5): 904-914.
26. Gareus R, Kotsaki E, Xanthoulea S, et al. Endothelial cell-specific NF-kappa B inhibition protects mice from atherosclerosis[J]. Cell Metab, 2008, 8(5): 372-383.
27. Libby P, Okamoto Y, Rocha VZ, et al. Inflammation in atherosclerosis: transition from theory to practice[J]. Circ J, 2010,74(2): 213-220.
28. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis[J]. Circulation, 2004, 109(23 Suppl 1): III27-32.
29. De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines[J]. J Clin Invest, 1995, 96(1): 60-68.
30. Tsao PS, Wang B, Buitrago R, et al. Nitric oxide regulates monocyte chemotactic protein-1[J]. Circulation, 1997, 96(3): 934-940.
31. Zhou Z, Connell MC, MacEwan DJ. TNFR1-induced NF-kappa B,but not ERK, p38MAPK or JNK activation, mediates TNF-induced ICAM-1 and VCAM-1 expression on endothelial cells[J]. Cell Signal, 2007, 19(6): 1238-1248.
 中國(guó)應(yīng)用生理學(xué)雜志2014年6期
中國(guó)應(yīng)用生理學(xué)雜志2014年6期
- 中國(guó)應(yīng)用生理學(xué)雜志的其它文章
- Pathophysiological changes in mitochondria of mammalian exposed to hypoxia at high altitude
- A dysfunction of CD4+ T lymphocytes in peripheral immune system of Parkinson’s disease model mice
- Microvesicles derived from hypoxia/reoxygenation-treated human umbilical vein endothelial cells impair relaxation of rat thoracic aortic rings
- Effect of acclimation training on physiological changes in a randomized controlled trial in hot-humid environment
- Scene-trait coping style of military rescuers in Wenchuan earthquake
- Plasma endothelin-1 and nitric oxide correlate withligustrazine alleviation of pulmonary artery hypertension in patients of chronic cor pulmonale from high altitude plateau during acute exacerbation
