Effects of creep feeding and supplemental glutamine or glutamine plus glutamate(Aminogut)on pre-and post-weaning growth performance and intestinal health of piglets
Rafael A Cabrera,James L Usry,Consuelo Arrellano,Eduardo T Nogueira,Marianne Kutschenko,Adam J Moeserand Jack Odle
Background
After pigs are weaned from their dams,morphological and functional changes occur in their small intestine.Pluske et al.[1]reported decreased villi height and increased crypt dept.Because newly-weaned pigs are transitioned from milk to dry feed,the pig’s intestinal tract is unable to fully digest and absorb the more complex plant-based macronutrients in the feed.Various researchers[2,3]have reported that this accumulation of undigested and unabsorbed feed creates the perfect medium for opportunistic bacteria such as haemolytic E.coli to grow.The normal weaning process stimulates pancreatic development and its enzymatic output;however there is a delay until the different enzymes reach sufficient levels[4].This in turn can cause post-weaning diarrhea.Creep feeding is deemed to be very important during the suckling period for swine practitioners because it(a)increases weaning weight when offered in small and frequent quantities and(b)eases the transition period for the piglets from sow’s milk to the dry feed.The latter has a physiological implication in order to avoid digestive upset such as post-weaning diarrhea and poor growth.Some argue[5,6]that the use of creep feed during the suckling period could potentially trigger hypersensitivity to feed antigens that can stimulate postweaning diarrhea.Barnett et al.[7]observed antibody titers in the blood of weaned piglets and confirmed that feed antigens can induce an immune reaction in creep-fed pigs.The reduction in feed intake associated with weaning has been known to affect intestinal integrity and potentially cause pathological disorders.Klasing[8]argued that dietary supplementation of some nutrients or immune modulators can rectify the intestinal impairment and modulate the immune function of animals contributing to improvements in overall health and performance.Nutrition can regulate the type of immune response by a number of mechanisms[8].Swine nutritionists have traditionally focused on those amino acids that cannot be synthesized by the animals with little attention given to those that can be synthesize by the animals and yet have a great impact on regulating nutrient metabolism and the immune responses[9,10].These amino acids include arginine,glutamine,glutamate,proline,leucine,cysteine and tryptophan.Recent studies indicate that these amino acids serve important regulatory functions in nutrient metabolism,protein turnover,and immune function,thereby enhancing growth and feed efficiency in pigs.The underlying mechanisms include activation of nitric oxide,mammalian target of rapamycin(mTOR),gaseous signaling,and AMP-activated protein kinase pathways as well as anti-oxidative[11].
Glutamine is a major metabolic fuel for rapidly dividing cells,including enterocytes and lymphocytes,as well as a key regulator of gene expression and cell signaling pathways[12].Schrock and Goldstein[13]reported that glutamine serves as precursor for the increased renal ammoniagenesis during chronic metabolic acidosis.The amide nitrogen of glutamine is essential for purine and pyrimidine biosynthesis.
Glutamine has important and unique metabolic functions,and it is considered a conditionally essential amino acid in some species under inflammatory conditions[14]and disease states[15,16].Souba and others[17]have indicated that the provision of GLN-enriched diets in various stress states associated with bacterial translocation decreases the incidence of translocation of bacteria by decreasing the adherence of bacteria to enterocytes.Reeds and others[18]argued that the high metabolic rate of the intestinal mucosa is very unique when compared to the others organs in the body.First,the enterocytes are specialized in absorbing nutrients from the lumen to the basal lamina.Second,mucosa cells are presented with high quantities of substrates from both the intestinal lumen and the mesenteric arterial circulation.Accordingly,under fed conditions,the quantification of substrate used by the gut can be challenging to quantify given the dual supply from both diet and the arterial circulation.Finally,GLN is the only amino acid in arterial blood that is taken up by the small intestine in swine[19].The small intestine(even though only represents 3 to 4%of the body weight)utilizes 30%of the arterial GLN and 67%of dietary GLN in swine.For comparison,95 to 97%of dietary glutamate is extracted by the pig small intestine in first pass[20,21]but only 50%is metabolized to CO2[18].
Because the gastrointestinal tract has an obligatory requirement for L-GLN[18]and availability of L-GLN from endogenous tissue production may not be sufficient for the maintenance of the structural and functional integrity of the intestinal mucosa[22,23],We hypothesized that creep feeding of suckling piglets and adding L-glutamine or the combination of L-glutamine and L-glutamate to pre and/or post-weaning diets would alleviate villi atrophy,reduce post-weaning diarrhea and improve post-weaning growth.
The objective of the study was to evaluate the effects of L-GLN and AminoGut(containing L-GLN and L-glutamate)on intestinal histology,intestinal absorptive capacity,enzymatic activity,and growth performance in a commercial swine operation.The effects of these supplements on piglet growth performance have not been evaluated during the whole nursery period following supplementation during the pre-weaning period.
Methods
All protocols were under the supervision of licensed veterinarians.Standard operating procedures for animal care were in accord with published guidelines for animal care[24].The experimental animals were not subjected to prolonged constraint or surgical procedures and were humanely treated throughout the experiment.The study was conducted during the summer 2010 on a 4800-sow farm in Maple Hill,NC(Murphy-Brown,LLC;Rose Hill,NC).One-hundred and twenty litters were randomly allotted to one of eight dietary treatment scenarios(Figure 1).At one week prior to weaning,four creep-feed treatments were initiated:A)No creep feed;B)Creep feed,control diet;C)Creep feed containing 1%GLN;D)Creep feed containing 0.88%AminoGut.AminoGut is a commercial dietary supplement produced by Ajinomoto do Brazil(S?o Paulo,Brazil)containing a mixture of L-glutamine(min 10%)and L-glutamate(min 10%).The pelleted creep feed was a phase 1 nursery diet(Table 1),manufactured at the North Carolina State University feed mill.Litters were offered fresh creep feed at 4-h intervals from 8 am to 4 pm each day.Litters weights were recorded at birth(WayPig model 252,Raytec Manufacturing,Ephrata,PA)and weaning(Avery Weight-Tronix model 640,www.agscales.com,Fairmont,MN).Pigs were weaned at an average of 21 d and were transported to the Site 2 nursery(~300 meters from farrowing Site 1),and litters from preweaning treatments A and B were each divided among the following nursery diets:1)Control diet,2)GLN diet,and 3)AminoGut diet(Figure 1,Table 1).Litters from preweaning treatments C and D were continued on similar diets post-weaning(e.g.,GLN and AminoGut respectively).Additional litters were allowed to nurse the sow(without creep feed)until 4 week of age to provide agematched controls for invasive measures of intestinal health(n=7).The experimental design is illustrated in Figure 1,showing abbreviations used for each dietary treatment scenario.After weaning,phase 1 diet(Table 1)was budgeted at 2.72 kg/pig,Phase 2 diet was budgeted at 5.45 kg/pig and Phase 3 diets was budgeted at 18.16 kg/pig.At 3 and 6 week postweaning,the pigs and feeders were weighed for growth and feed conversion calculations.
At one week post weaning,one pig per pen was fasted overnight and then intra-gastrically gavaged with a D-xylose/mannitol solution as follows.A solution containing 0.2 g/L of D-xylose(Pfizer,N.Y.,NY)and 0.3 g/L of mannitol(Sigma,Saint Louis,MO)was prepared and was given to the pigs on an average of 9.5 h after fasting.The selected dose was 6.5 mL/kg of body weight.Pigs were individually weighed(Berkley FS-50 hanging scale,Somers Point,NJ).At precisely two h post gavage,pigs were bled via jugular venipuncture.The time of bleeding was selected based upon work by Doerfler et al.[25].After pigs were bled they were humanly euthanized for collection of intestinal tissues.Jejunum samples(25 cm from the stomach)were collected for both light and scanning electron microscopy(SEM).The portion cut specifically for SEM,was cut open and laid flat in a small cartridge in order to obtain a better picture of the intestinal villi.A separate jejunum tissue sample was cut and the intestinal mucosa was scrapped for maltase activity analysis.Blood was centrifuged after 24 h and the serum stored at-20°C for further analysis.Performance data were statistically analyzed using the PROC GLIMMIX of SAS with birth weight and weaning age as covariates.Intestinal and serum metabolite data were analyzed using the Mixed Procedure of SAS with the body weight(one week post-weaning in the nursery)used as covariate.
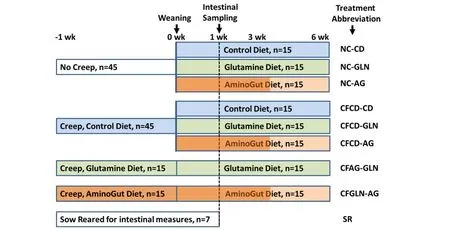
Figure 1 Experimental design showing four pre-weaning creep diet groups and eight post-weaning diet groups together with sowreared control pigs.Creep feed was initiated 1 week prior to weaning and consisted of pelleted Phase 1 diets.Post-weaning diets consisted of either control basal diet(see Table 1)with additions of either 1%L-GLN(in all feed Phases 1–3)or 0.88%AminoGut in Phases 1 and 2 and 0.66%in Phase 3.Pigs were weighted at birth,weaning,(some at 1 wk post-weaning),3 wk and 6 wk post-weaning.Selected pigs were euthanized(n=7/trt)at 1 wk post-weaning for intestinal health measurements.Various treatment abbreviations are also summarized.
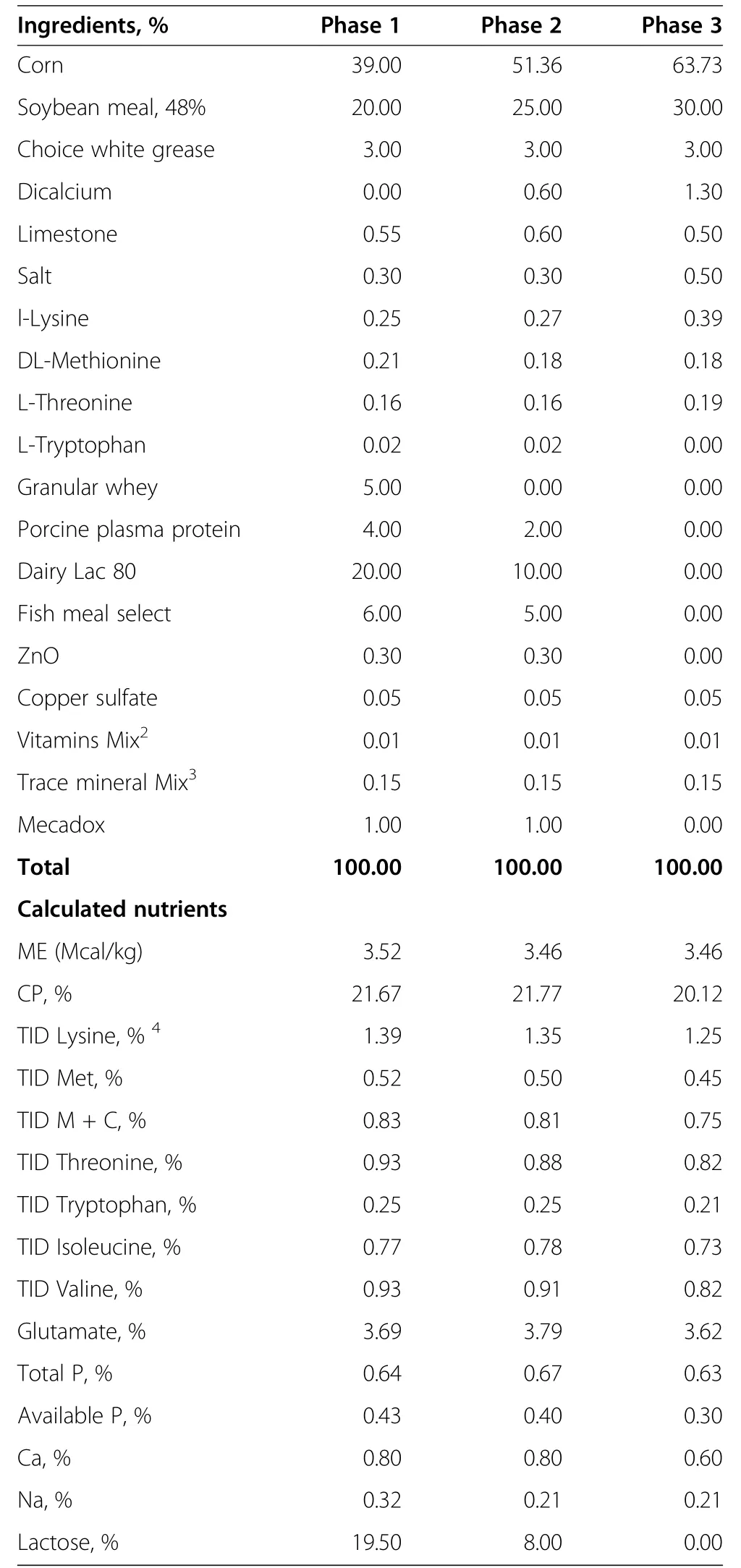
Table 1 Ingredients and nutrient composition of the basal diets(Phase 1,2 and 3)1
Scanning electron microscopy
Jejunum samples were collected from four-week old piglets(1 wk post-weaning)and immersed in 4 F:1G fixative containing 4%formaldehyde and 1%glutaraldehyde in a phosphate buffer,with an osmolarity of 176 mOsM and a pH of 7.2-7.4[26].Samples were cut to be between 2–3 mm in order to minimize chances of bulk charging.Samples were rinsed in 0.1 mol/L phosphate buffer and dehydrated in an ethanolic series to 100%ethanol before subjection to critical point drying after being stored for approximately 7 wk in the 4 F:1G fixative.Samples were then mounted on SEM stubs with carbon tape and sputter coated with gold-palladium before being viewed with a JEOL JSM-6360LV scanning electron microscope(JEOL,Peabody,MA).This microscope is a fully digital instrument that can view specimens by secondary electron imaging(SEI),backscatter electron imaging(BEI),at high vacuum,or at low vacuum.
Hematoxylin&eosin staining
Jejunum samples were collected(25 cm from the stomach)and preserved in a formalin solution and stored in room temperature for histology analysis.Tissues were trimmed into five millimeter thick sections and placed in processing cassettes.The tissues were processed in a Tissue-Tek VIP5 tissue processor(Sakura Finetek,Torrance,CA)using a standard overnight processing schedule.Tissues were embedded in paraffin and five micron sections were mounted on glass slides.The slides were stained on a DRS-601 slide stainer(Sakura Finetek,Torrance,CA)with hematoxylin and eosin,cleared and mounted with a permanent media.The stained tissues on glass slides were examined using an Olympus AH-2 Vanox-S microscope(Ultrasonic Power Corporation,Freeport,IL)and measured using SPOT?software(SPOT?Imaging Solutions,Sterling Heights,MI).
PCNA Staining
Five micron jejunal slices were mounted on glass slides.A primary mouse monoclonal antibody(PC10)was used for the as a proliferation marker.This antibody is specific for proliferating cell nuclear antigen,PCNA,p36 protein expressed at high levels in proliferating cells.It was diluted at 1:1,500 and incubated for 30 min.The remaining steps were completed using the Dako EnVision Mouse kit(Dako,Denmark).Intensively stained and the total number of enterocytes were counted in 8 consecutive well-orientated crypts(those that extended to the muscularis mucosa).
Analysis of mannitol
Samples of serum were frozen,thawed at room temperature and vortexed to mix.Samples were then filtered by centrifugation using Ultrafiltration Spin Columns(0.45 μm,Millipore,Temecula,CA).An aliquot of 200 μL of sample was transferred to HPLC autosampler vials containing 250 μL inserts.An internal standard solution of myoinositol was added(2 μL).Analysis was done using High Performance Liquid Chromatography(HPLC).The extracts were analyzed using a Dionex BioLC(Dionex Corporation,Sunnyvale,CA)at a controlled temperature of 30°C.The system consisted of a gradient pump,an autosampler,and a pulsed amperometric detector.The mobile phase was 52 mmol/L NaOH(Thermo-Fisher Chemical Corp.Pittsburgh,PA)at an isocratic flow rate of 1.0 mL/min.The column used was a Dionex PA-10,250 mm length and 4 mm i.d.,fitted with Dionex PA-10 and borate guard columns.The detector was programmed to run a quadruple waveform as recommended by the manufacturer.A shift in the detector range was 1 μC.The injection volume was 10 μL.The mannitol was calculated using an authentic standard of d-mannitol and myo-inositol as an internal standard.All the reference standards were purchased from Sigma Chemical Corp(St.Louis,MO).
Analysis of xylose
The collected pig serum(20 μL)was subjected to a modified micro method[27,28]first described by Eberts et al.[29]for determination of plasma D-xylose.To each 20 μL plasma sample,2 mL of phloroglucinol(Sigma Chemical Co.,Saint Louis,MO 63178–9916)color reagent was added and heated for 4 min at 100°C.The samples were allowed to cool at room temperature in a water bath.After cooling,the absorbance of each sample was read on a Gilford UV–vis spectrophotometer(Thermo Fisher Scientific,Inc.;Waltham,MA)set at 554 nm.
Maltase enzyme activity
The maltase assay was performed as described by Dahlqvist[30].Maltase activity(U/g of protein)was expressed as units,with 1 unit defined as the amount of enzyme transforming 1.0 μmol of substrate per min at 25°C.
Results
Resultsfor pre-weaning performance are summarized in Table 2.We found weaning age to be significant(P=0.001)among the pre-wean treatments.For subsequent comparisons,this variable was used as covariate.Pigs/litter,sow parity,birth weight,weaning weight,and mortality were not different among the treatments.Creep feed consumption also did not differ for those treatments receiving creep feed.The average creep feed consumptions for the control diet and those supplemented with either glutamine or AminoGut were 49.44,45.57 and 48.44 g/pig respectively.We did not find an effect of creep feeding on post-weaning performance(Tables 3&4).A longer(>1 wk)creep feed period needs to be examined.Feed conversion(feed/gain)showed means among treatments close to significance(P=0.056)and Tukey’s test for pairwise mean comparisons showed that Pigs in the CFGLN-GLN group had the best feed conversion(feed/gain)in the first three-week period post-weaning,exceeding(P=0.044)controls(CFCD-CD)by 34%.All others variables were not significant during this post-weaning period among the treatments(Table 3).The NC-AG group had(P=0.02)the greatest feed intake among all treatments in the last three week of the study(Table 4),exceeding controls(CFCD-CD)by 12%.All others variables were not significant during this post-weaning period among the treatments.CFCD-GLN,Sow-Reared and CFGLN-GLN groups had the greatest(P=0.049)villi height exceeding those which were creep fed with a control diet and later supplemented with AminoGut(CFCD-AG)by 20%,19%and 18%respectively(Table 5).The Sow-Reared group was added as a point of reference against the other treatments.All tissue samples for all treatments were taken at 28 d of age.We also found that pigs creep fed with a diet supplemented with AminoGut and fed a post-weaning diet supplemented with AminoGut(CFAG-AG)had the deepest(P=0.001)crypts among all the treatments.Sow-Reared,CFCD-GLN and CFGLN-GLN,and groups had the greatest(P=0.001)number of cells proliferating(PCNA),exceeding those which did not receive creep feed and later receiving a control diet(NC-CD)by 63%,54%and 43%respectively.We found a correlation between villi height and PNCA:the taller the villi height,the greater the number of proliferating cells.Sow-Reared pigs showed the greatest(P=0.001)intestinal absorption capacity for xylose and mannitol when compared with the others treatments.The levels of xylose and mannitol found in the sow reared pigs blood exceeded the average of the levels found in the other treatments by 3.2 and 7.4 folds respectively.This is consistent with the architecture of the villi of the sow reared pigs when compared to the other treatments(see qualitative SEM images,Figure 2).There was no significance difference among the other treatments on the absorption of these sugars.We found the levels of xylose in the blood to be higher than those of mannitol even though a higher amount of mannitol was diluted in the final solution(0.2 g/L vs.0.3 g/L).We found no significant differences among the treatments in maltase activityalthough there was a tendency(P=0.18)for creep fed treatments to be numerically different than those which did not receive creep feed(260 vs.214 μmoles/min.g of protein respectively).
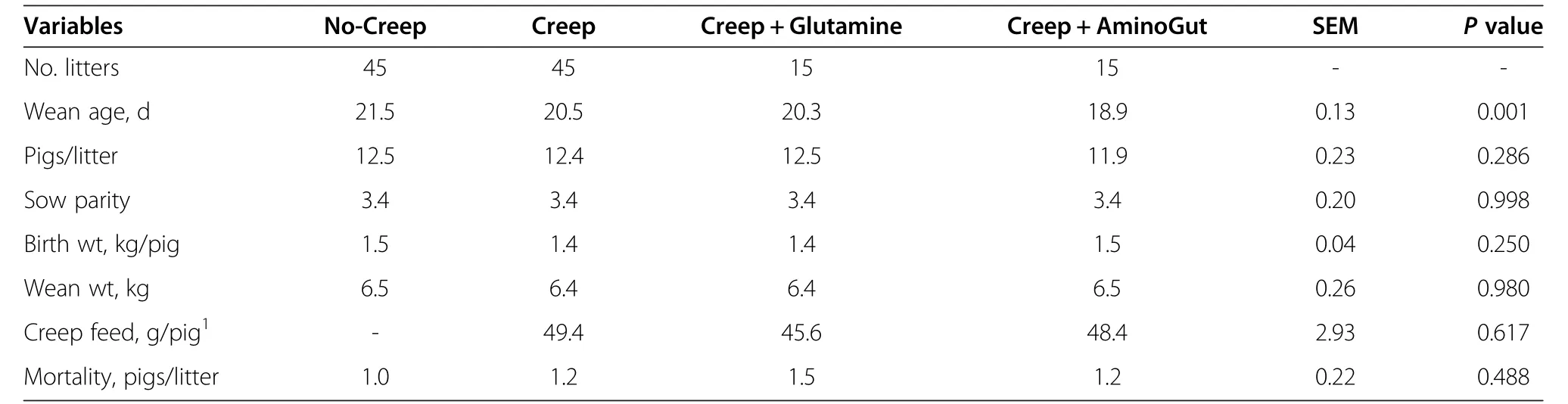
Table 2 Pre-weaning performance of creep-fed piglets
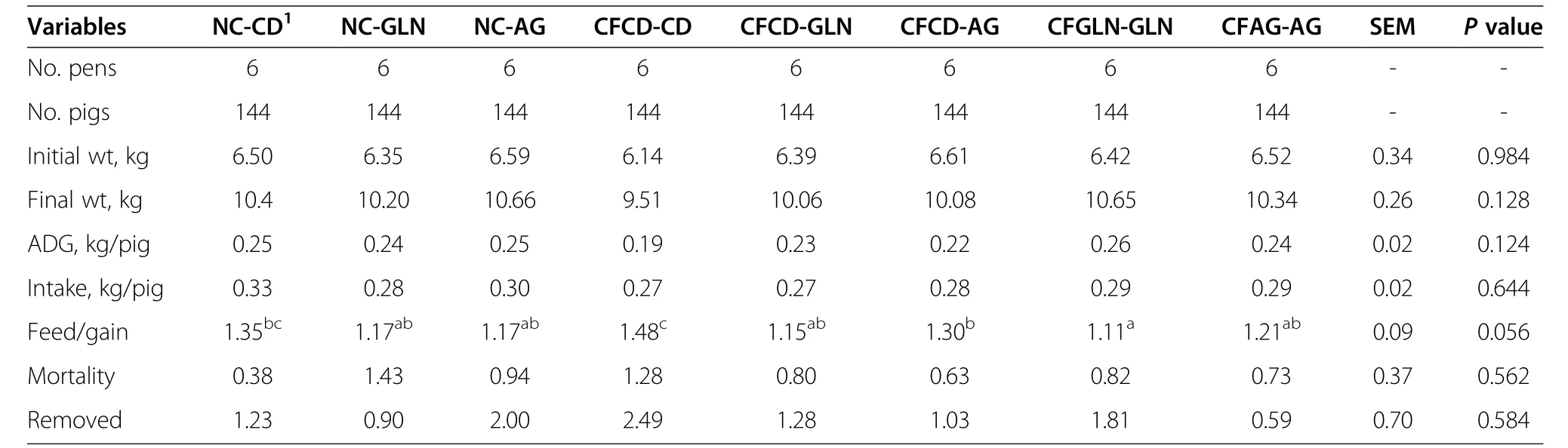
Table 3 Pig performance from week 1 to 3 post-weaning
Discussion
Windmueller and Spaeth[31]determined that in the adult rat small intestine,CO2,lactate,alanine and glucose account for 56–64,16–20,4–8,and 2-10%of the total catabolized carbons of luminal glutamine,glutamate and aspartate,respectively.These results and others showed that amino acids(glutamine,glutamate and aspartate),rather than glucose,are the major fuels for the small intestine mucosa,responsible for providing energy required for intestinal ATP-dependent metabolic processes[32].Although there seems little doubt that glutamine plays an important,but remarkably poorly characterized role in the metabolism of many proliferating cells,much of the more recent literature on intestinal metabolism has ignored two observations made by Windmueller and Spaeth[33].Those are,first,that the metabolism of luminal glutamatewas even more extensive than that of arterial glutamine;and second,that the presence of high concentrations of glutamate in the intestinal lumen had only a small(less than 25%)effect on intestinal utilization of glutamine.This perhaps suggests that these two closely related amino acids may have different functional roles in the intestinal mucosa.
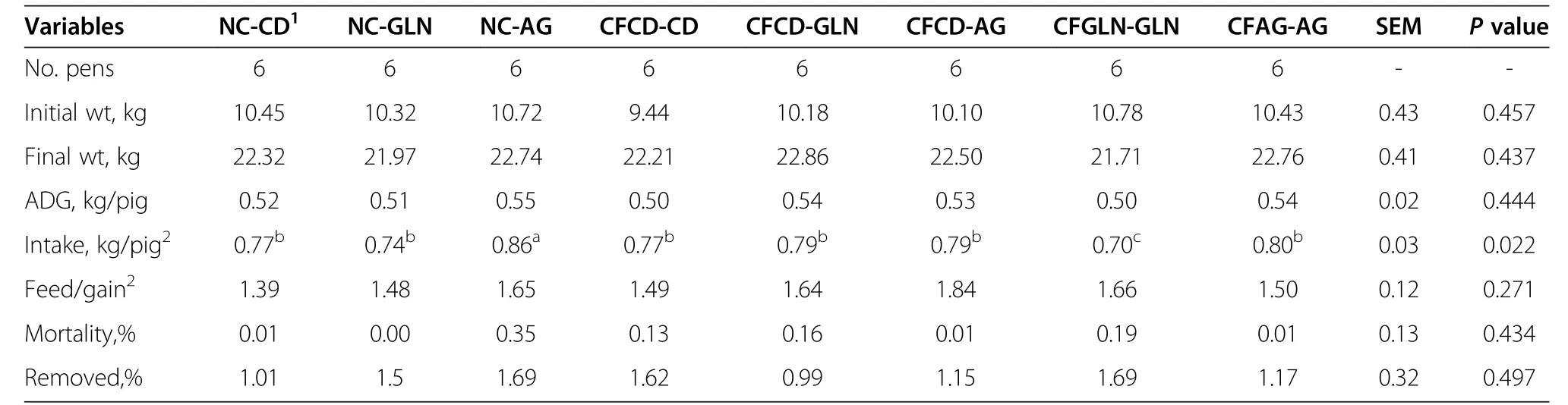
Table 4 Pig performance from week 3 to 6 post-weaning
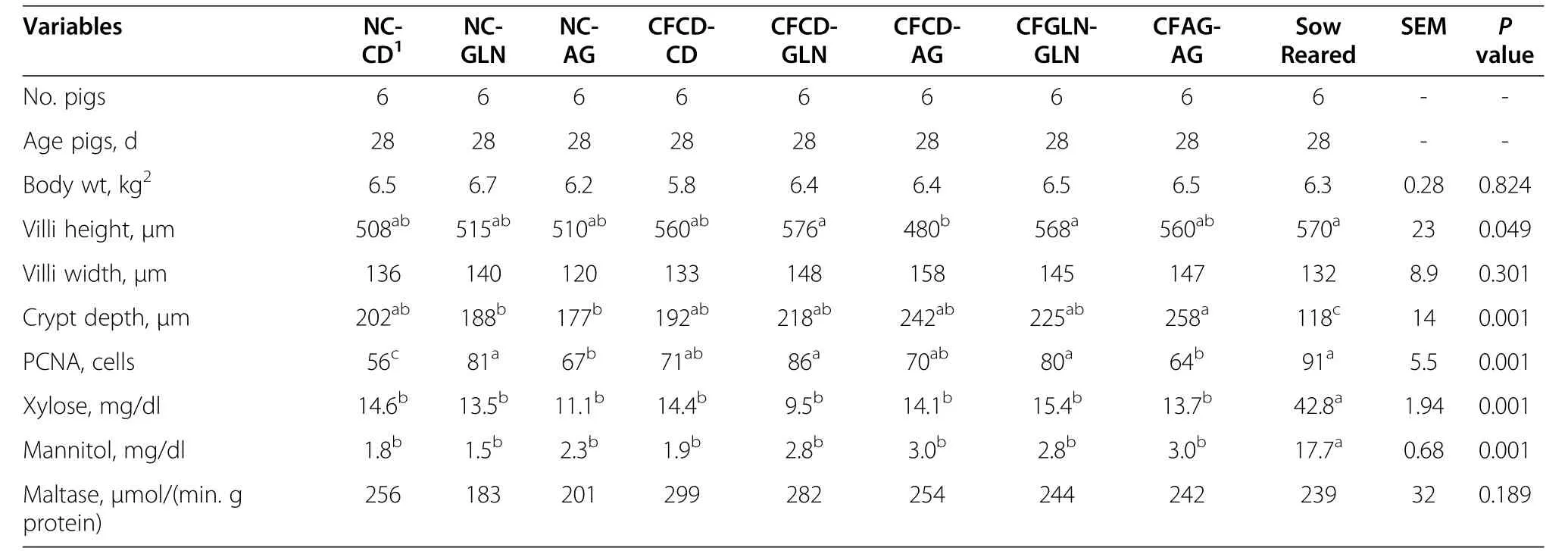
Table 5 Intestinal morphology,PCNA staining,and maltase activity,and serum xylose and mannitol following oral gavage of pigs 1-wk post-weaning
It seems that glutamate can partially substitute for Gln in several pathways,including ATP production and synthesis of arginine,alanine,proline and aspartate[34].Glutamate plays a significant role on avoiding Gln degradation by mitochondrial phosphate-activated glutaminase in extra hepatic tissues and cells yielding a sparing effect on the use of Gln as a metabolic fuel[35]and its availability in cells[36].Wu[10]rightly notes that that key functions of Gln(syntheses of Gln-tRNA,aminosugars,carbamoylphosphate,NAD,NADP,as well as purines and pyrimidines;renal ammoniagenesis;and regulation of ornithine decarboxylase expression)cannot be supplied by glutamate.Wu and others[37]argued that although both Gln and glutamate provided in the enteral diet are extensively catabolized by the small intestine,this organ takes up Gln,but not glutamate,from the arterial blood.They suggested that due to the complex compartmentalization of cellular metabolism,extracellular glutamate may channel preferentially into the cytoplasm rather than into the mitochondria and,therefore,have different effects than the glutamate generated from Gln in mitochondria.
The vast majority of research showing the benefits of supplementing Gln in the diet can be found in research with swine.Wu and co-workers[38]reported that among all the amino acids,uterine and umbilical uptake of Gln was the greatest in pregnant gilts,implicating an important role for Gln in fetal growth and development.They fed 1%Gln in the diet of gestating gilts between 90 and 114 d of gestation and found that it significantly increased average birth weight.They also found that the number of intrauterine growth retarded piglets,variation in birth weight and pre-weaning mortality were reduced by 39,33,and 46%,respectively,when compared with the control group.
Kim and Wu[39]reported that lactating sows have a high requirement for Gln and the uptake of Gln by the porcine mammary glands may be inadequate for the synthesis of milk proteins.By d 10 during the lactating period,the mammary glands uptake 16 g Gln/d from the arterial circulation[40],however Haynes and others[41]reported that at that point in time,36 g Gln/d is being secreted.Wu and colleagues[38]fed 1%Gln from d1 to d 20 to lactating sows and found an increase of Gln concentrations in the plasma,skeletal muscle and whole milk of the sows,as well as piglet growth and survival.
Haynes et al.,[41]evaluated the effectiveness of Gln or L-alanyl-L-glutamine(Ala-Gln)in vivowith 7-d-old piglets challenged with a single intraperitoneal injection LPS(0.1 mg/kg body weight).Administration of Gln or Ala-Gln to LPS challenged piglets increased Gln concentrations in small intestinal lumen and plasma,reduced intestinal expression of TLR-4,active caspase-3 and NFkB,ameliorated intestinal injury,decreased rectal temperature and enhanced growth performance.These results demonstrate a protective effect of Gln or Ala-Gln against LPS-induced enterocytes death.They also reported that the Gln supplementation stimulated the growth of sow-reared piglets by 12%.
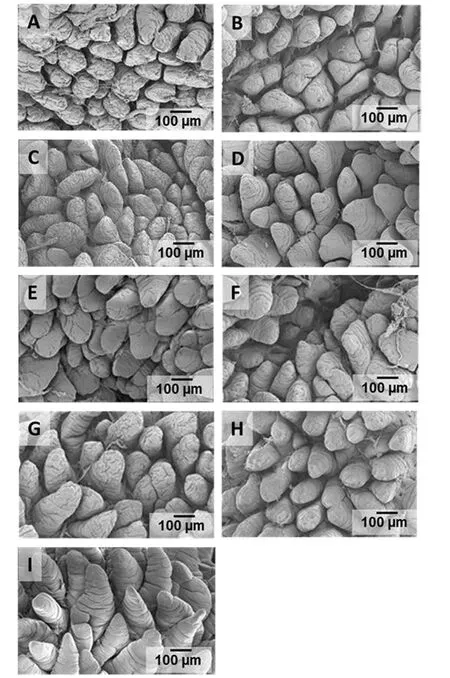
Figure 2 Scanning electron micrographs of jejunal villi of pigs at four wk of age.A.No creep Control Diet(NC-CD).B.No creep Glutamine(NC-GLN).C.No creep AminoGut(NC-AG).D.Creep Fed Control Diet-Control Diet(CFCD-CD).E.Creep Fed Control Diet-Glutamine(CFCD-GLN).F.Creep Fed Control-AminoGut(CFCD-AG).G.Creep Fed Glutamine-Glutamine(CFGLN-GLN).H.Creep Fed AminoGut-AminoGut(CFAG-AG).I.Sow-Reared Control.
Yi et al.[42]found that feeding glutamine had beneficial effects in alleviating growth depression of E.coli K88+-challenged pigs,mainly via maintaining intestinal morphology and function,and/or possible modulating the somatotrophic axis.Jiang and others[43]reported similar results.Wu et al.[44]orally administered Gln(0.5 g/kg BW/d)to low-birth weight piglets from 0 to 21 d of age and found that their growth were improved by 16%and their pre-wean mortality by 48%.
Our results are most consistent with those reported by Wu et al.,[45].They found a 29%improvement in feed conversion (21d post-weaning)when supplementing 1%glutamine.Glutamine(Gln)supplementation(1%)prevented jejunal atrophy(measured as villus height)during the first week post-weaning and increased feed:gain ratio(indicator of growth performance)by 25%during the second week post-weaning.It also increased plasma concentration of aspartate,glutamate and alanine and also reduced the extent to which plasma taurine concentration fell in post-weaning pigs.The prevention of villi atrophy during the first week post-weaning also has been reported by Wang and co-workers[46].
Liu and others[47]reported similar results than those reported by Wu et al.[45].They fed 1%L-glutamine or 1%L-glutamate to weaned pigs from 28-d to 42-d of age.Jejunal atrophy was prevented during the first week for the groups fed either L-GLN or L-glutamate when compared to the control group.Again these results provide an experimental basis for the use of glutamine and glutamate to improve piglet intestinal health and to support improved growth performance.
D-Xylose absorption test has been used as a tool for the assessment of the effect of anticoccidials on the intestinal absorptive capacity of broilers during experimental coccidiosis[48]and malabsorption in poult enteritis and mortality syndrome[25].D-xylose,a poorly metabolized pentose sugar,is well absorbed from the small intestine of chickens and readily excreted in the urine[25].Blood D-xylose concentrations are expected to peak at 30–60 min after intake in poultry[25,48]and 60 min in pigs[49,50].
Mannitol has been clinically applied in diagnostic and therapeutic doses for 1)the determination of extracellular fluid volume and glomerular filtration rate,2)testing intestinal absorption and mucosal integrity,3)as a diuretic and 4)as a radical scavenger and osmotically active component of infusions.
There are few studies using these two sugars as markers of gastrointestinal in vivo permeability in pigs in a commercial setting.In this study,the uptake of xylose was greater than mannitol regardless of their molecular weight(150 and 182 g/mol respectively)and the amount administered(mannitol higher than xylose).Xylose can be metabolized in the gut by bacteria,and then absorbed whereas mannitol cannot.Therefore we would expect xylose to be absorbed more rapidly than mannitol.Mannitol is partially metabolized,the remainder being excreted in the urine.Nasrallah and Iber[51]administered orally doses of 20 to 100 g of14C-mannitol to five humans with cirrhosis of the liver and to five subjects who had normal liver function.They found that at least one-sixth of orally ingested mannitol is absorbed and about one-third is metabolized.
The lack of significant differences in performance among the treatments for the entire 6-wk period correlates well with the lack of significant differences among the treatments for the levels xylose and mannitol absorbed and found in their blood.We were not surprised by the high levels of intestinal absorptive capacity shown by the sow reared pigs when compared to the other treatments.
These tests of small intestine permeability to lowmolecular-weightcarbohydrateshaveaconsiderable application in the study of small intestine diseases such as celiac disease in humans[52],diagnosing food allergy and assessing the effectiveness of anti-allergic agents such as sodium cromoglycate[53].
In young animals,lactase activity prevails,however as it gets older then maltase activity(as well amylases,lipases proteases)increases.Low concentration of maltase in the surface of epithelial cells may be an indication of villus atrophy due to disease or malnutrition[54].We were unable to find any significant differences among the treatments in maltase activity.
Scanning electron microscopy(SEM)allows observation of the surface of the epithelium in three dimensions and gives a fresh dimension in the investigation of gut mucosa[55].The visual assessment of the SEM graphs showed that pigs which were not creep fed during the suckling period had a rough villi surface with numerous cells shedding(apoptosis)along the entire length of the villi(Figure 2A).They also showed deep transversal furrows in most(if not all)the epithelial cells(Figure 2A,B).Those treatments creep fed either with a control diet or supplemented with glutamine or Aminogut showed longer villus than those treatments which were not creep fed(552 microns vs.511 microns respectively)(Figure 2 D,E&F).The CFGLN-GLN treatment showed elongated,well defined and high villus(Figure 2G).Increased villus height could increase total luminal villus absorption area and could result in adequate digestive enzyme concentration and/or increased transport of nutrient at the villus surface.Gln has been shown to enhance epithelial repair in several models of intestinal injury and to stimulate epithelial proliferation and protein synthesis or reduce apoptosis in cell culture[56,57].Increased uptake of Gln in the crypts not only could promote a compensatory increase in Na+absorption but also would place this nutrient in the ideal location to promote crypt cell production and restoration of the villus architecture.The CGAG-AG treatment showed deep and wide crypts.This could be explain by the fact that glutamine is donating an amide group for the biosynthesis of purines(adenine and guanine)and pyrimidines(thymine and cytosine)which are the nucleotides bases to support nucleic acid production(DNA)for rapidly dividing cells in the crypts.In RNA,the complement of adenine is uracil instead of thymine.The sow reared pigs showed what may be the perfect villi structure:healthy,well defined villus,no signs of apoptotic cells and sufficient mucin production(Figure 2I).Mucins are a family of high molecularweight,heavilyglycosylated proteins produced by epithelial tissues(specifically by the goblet cells)in most metazoans.Two noticeable jejunal villi structure characteristics in all treatments for 28 d pigs were 1)transversal furrows that were present along the entire length of the villi and 2)the shape of the villi were not finger-like but rather wide and tongue-like in shape.It was evident that the small intestinal mucosa undergoes profound structural and developmental changes during the first 4 week of the pig’s life and these changes are manifested in shape,size and density of the villi.guiding us on the design of the research protocol and the inclusion rate of both Glutamine and AminoGut.Both products are being evaluated but not currently being used in the US.
Conclusion
The supplementation of glutamine and glutamine plus glutamate(AminoGut)in pre-and post-weaning diets improved feed conversion in the first three week postweaning when compared to CFCD-CD treatment.These findings are in the agreement with those reporting a reduction in villi atrophy when supplementing glutamine at 1%in diets during the first week post-weaning.Sow reared pigs showed the best intestinal absorptive capacity and villi architecture.More research is needed at the field level to justify the economical feasibility of adding either glutamine or AminoGut in current commercial livestock diets and the European model of weaning pigs at 28 d of age.Consideration should also be given to potential supplementation of the sow to enrich milk concentrations[58].
The existing vast knowledge of the roles of functional AA’s such as glutamine and others(arginine,glutamate,proline,leucine,cysteine and tryptophan)provides the scientific basis for nutritionists to revise current nutrient requirements for livestock especially weaned pigs.These findings indicate that strong consideration must be given to GLN and glutamate as nutritionally essential amino acids for post-weaning pigs diets.
Authors’contributions
RAC as the lead author was in charge of designing the research protocol,obtaining all materials needed for it,executing it,carrying out all the lab work,data collection and analysis and finally writing the manuscript.JLU was instrumental on guiding the lead author on the design of the research protocol.CA provided the author with statistical guidance on the data analysis.ETN and MK provided significant literature and previous work references about glutamine and its biological functions as a non-essential amino acid.They were also involved in the design of the research protocol.JO as a co-advisor to the lead author,he was involved in the design,execution and interpretation of the results.He also assisted with blood and tissues collection and with the statistical analysis.AJM as a co-advisor to the lead author,he was involved in the design and interpretation of the results.He helped design our in vivo method for determining gut barrier function.All authors read and approved the final version of the manuscript.
Abbreviations
mTOR:Mammalian target of Rapamycin;IgA:Immunoglobulin A;GLN or Gln:Glutamine;AG:AminoGut;NC:Non-Creep fed;CFCD:Creep Fed Control Diet;CFGLN:Creep Fed Glutamine;CFAG:Creep Fed AminoGut;NCCD:Non-Creep Fed and later receiving a Control Diet;CFCD-CD:Creep Fed Control Diet and later receiving a Control Diet;NC-GLN:Non-Creep Fed and later receiving a diet supplemented with Glutamine.;CFDC-GLN:Creep Fed Control Diet and later receiving a diet supplemented with Glutamine.;NCAG:Non-Creep Fed and later receiving a diet supplemented with AminoGut;CFCD-AG:Creep Fed Control Diet and later receiving a diet supplemented with AminoGut.;CFGLN-GLN:Creep Fed Glutamine and later receiving a diet supplemented with Glutamine.;CFAG-AG:Creep Fed AminoGut and later receiving a diet supplemented with AminoGut;SR:Sow reared pigs;PCNA:Proliferating cells nuclei antigen;SBM:Soy bean meal;AA:Amino acids;C:Celsius;CO2:Carbon dioxide;SEM:Scanning electron microscopy;HPLC:High performance liquid Chromatography;PAD:Pulsed amperometric detector;BW:Body weight;ADG:Average daily gain;mM:Millimolar;mL:Milliliters;nm:Nanometer;min:Minutes;μL:Microliters;ATP:Adenosine triphosphate;g:Gram;L:Liter;tRNA:Transfer ribonucleic acid;NAD:Nicotinamide adenine dinucleotide;NADP:Nicotinamide adenine dinucleotide phosphate;d:Day;mg:Milligram;kg:Kilogram;LPS:Lipopolysaccharides;TLR:Toll like receptors;NF?B:Necrotic factor kappa B;CWG:Choice white grease;ZnO:Zinc oxide;NRC:National research council;ME:Metabolizable energy;CP:Crude protein;TID:Total ileal digestible.
Competing interests
Full financial support for this trial was provided by Ajinomoto Heartland Lysine and Ajinomoto do Brazil.Dr.James L.Usry served as Swine Technical Director for Ajinomoto Heartland Lysine(currently serves as Swine Technical Director for Micronutrients),and both Marianne Kutschenko and Dr.Eduardo T.Nogueira serve as Technical Managers for Ajinomoto do Brazil.All three are authors in this manuscript.However their assistance was limited to
Authors’information
RC holds a PhD in Animal Nutrition from North Carolina State University.His area of research is neonatal survival,nutrient digestibility and gastrointestinal health of swine.In 2001,he was awarded the“Innovative Award Applied Research”by National Pork Producer Council at the Midwest Animal Science Meeting in Des Moines,Iowa.He is a member of the North Carolina Pork Council and the American Society of Animal Science.He currently serves as Director of Swine Technical Services for Huvepharma USA,Inc.JLU holds a PhD in Agricultural Engineering from the University of Kentucky in animal growth modeling.He spent 21 years at Ajinomoto Heartland where he became VP of Nutritional Services and currently is employed at Micronutrients as Director of Swine Nutrition.Most of his career has centered on amino acid research and development.CA holds a PhD in Statistics from North Carolina State University.Her research interests include experimental design applied to life sciences,statistical modeling,and discrete data analysis.She is interested in statistical consulting,research methodology and creative learning and teaching.She is a Research Assistant professor in the Department of Statistics at NCSU and a member of American Statistical Society.ETN holds a PhD in Animal Nutrition from Vi?osa Federal University(UFV,Brazil)/University of Western Australia(UWA,Australia).His area of research is amino acids nutrition.He currently serves as Latin America Technical General Manager for Ajinomoto do Brazil/Ajinomoto Animal Nutrition.MK holds an MSc in Animal Nutrition from Maringa State University(UEM,Brazil).Her area of research is amino acid nutrition.She currently serves as Latin America Technical Manager for Ajinomoto do Brazil/Ajinomoto Animal Nutrition.
AJM holds a MS in Swine Nutrition,a PhD in Gastrointestinal Physiology and a Doctor of Veterinary Medicine(DVM)all from NCSU.His main area of research is to study basic mechanisms of stress-induced intestinal dysfunction.Stress is an important contributing factor to enteric disorders of veterinary species and humans however,the mechanisms are poorly understood.His work has focused on the role of mucosal mast cells in psychological stress-induced disturbances in intestinal mucosal barrier function.He believes that this work will have important implications in the understanding of stress-related gut disorders such as infectious diarrhea,Inflammatory Bowel Disease,and Irritable Bowel Syndrome,and will facilitate the design of novel preventative and treatment strategies for veterinary and human patients suffering from these disorders.He is an assistant professor of GI physiology and swine medicine at NC State College of Veterinary Medicine.He is member of several professional societies including the American Physiological Society,American Association of Swine Veterinarians,and American Gastroenterological Association.JO has a PhD in Nutritional Biochemistry from the University of Wisconsin.As a Williams Neal Reynolds Professor in the Department of Animal Science at NCSU,his research interests are molecular and metabolic regulation of lipid digestion and metabolism;neonatal nutrition;intestinal growth and metabolism in normal and pathophysiological states.His program is focused on using the young piglet as a model for the human infant in nutrition and digestive physiology.His most recent awards include“Williams Neal Reynolds Distinguished Professor”and “The Outstanding Graduate Instructor”both given by the College of Agriculture and Life Science at NCSU,the“Animal Growth and Development Research”given by the American Society of Animal Science.He was a member of the National Research Council(NRC)committee which recently published the new 2012 Nutrient Requirements of Swine.He is an Associate Editor in Advances in Nutrition(American Society for Nutrition)and the Journal of Animal Science and Biotechnology.
Acknowledgements
Research was conducted at the Maple Hill sow farm,NC(Murphy-Brown,LLC;Rose Hill,NC).The authors would like to thank Ajinomoto Heartland Lysine and Ajinomoto do Brazil for the financial support to carry out this trial.The authors would also like to thank Dr.Jeff Hansen,Dr.Ana de Souza and Dr.Dustin Kendall from Murphy-Brown LLC for coordinating this trial with the farm staff.The authors recognize the assistance provided by Indira Gomez,Wilmer Pacheco and Santa Maria Mendoza with the daily feeding,care of the animals,and blood and tissues collection.We are extremely grateful to Lauren Lanier who agreed to make this project her internship assignment with Murphy Brown LLC.We appreciate the technical assistance provided from Dr.Lisa Dean(Food Science department,NCSU)with the xylose assay,Dr.Michael Dykstra(College of Veterinary Medicine,NCSU)with the SEM guidance,Mrs.Debbie Ort(Poultry Science department,NCSU)with the mannitol assay and Dr.Sheila Jacobi(Laboratory of Developmental Nutrition,Department of Animal Science,NCSU)with the maltase assay and the sow reared pigs’blood and tissue collection.
Author details
1Laboratory of Developmental Nutrition,Department of Animal Science,North Carolina State University,101 Polk Hall,North Carolina State University,Raleigh,NC 27695,USA.2Ajinomoto Heartland Lysine,Chicago,IL 60631,USA.3Department of Statistics,North Carolina State University,Raleigh,NC 27695-8203,USA.4Ajinomoto do Brasil.Ajinomoto Animal Nutrition,S?o Paulo,SP 04015-001,Brazil.5Department of Population Health and Pathobiology,College of Veterinary Medicine,North Carolina State University,Raleigh,NC 27695,USA.6Author current employment:Huvepharma USA,525 West Park Drive Suite 230,Peachtree City,GA 30269,USA.7Author current employment:Micronutrients,1550 Research Way,Indianapolis,IN 46231,USA.
Published∶3 August 2013
1. Plunske JR,Hampson DJ,Williams IH:Factors influencing the structure and function of the small intestine in the weaned pig.A review.Livest Prod Sci 1997,1997(51):215–236.
2. Kenworthy R,Crabb WE:The intestinal flora of young pigs with particular reference to early weaning,E,Coli and scours.J Comp Path 1963,73∶215–228.
3. Palmer NC,Hulland TK:Factors predisposing to the development of coliform gastroenteritis in weaned pigs.Can Vet J 1965,6∶310–316.
4. Cera KR,Mahan DC,Reinhart GA:Effect of weaning,week postweaning and diet composition on pancreatic and small intestinal luminal lipase response in young swine.J Anim Sci 1990,68∶384–391.
5. Newby TJ,Miller BG,Stokes CR,Bourne FJ:Hypersensitivity to dietary antigens as the predisposing factor in post-weaning diarrhea.Pig Vet Soc Proc 1983,10∶50.
6. Miller BG,Newby TJ,Stokes CR,Bourne FJ:Influence of diet on postweaning malabsorption and diarrhea in the pig.Res Vet Sci 1984,36∶187.
7. Barnett KL,Kornegay ET,Risley CR,Lindemann MD,Schurig GG:Characterization of creep feed consumption and its subsequent effects on immune response,scouring index and performance of weanling pigs.J Anim Sci 1989,67∶2698–2708.
8. Klasing KC:Nutrition and the immune system.Br Poul Sci 2007,48∶525–537.
9. Li P,Yin YL,Li DF,Kim SW,Wu G:Amino acids and immune function.Br J Nutr 2007,98∶237–252.
10.Wu G:Amino acids∶metabolism,functions,and nutrition.Amino acids 2009,37∶1–17.
11.Wu G:Recent advances in swine amino acid nutrition.J Anim Sci Biotech 2010,1∶118–130.
12.Rhoads JM,Wu G:Glutamine,arginine,and leucine signaling in the intestine.Amino acids 2009,37∶111–122.
13.Schrock H,Goldstein L:Inter-organ relationships for glutamine metabolism in normal and acidotic rats.Am J Physiol 1981,240∶E519–E525.
14.Newsholme P:Why is L-glutamine metabolism important to cells of the immune system in health,post injury,surgery or infection?J Nutr 2001,131∶2515S–2522S.
15.Nappert G,Zello GA,Naylor JM:Intestinal metabolism of glutamine and potential use of glutamine as a therapeutic agent in diarrheic calves.J Am Vet Med Assoc 1997,211∶547–553.
16.Van de Poll MC,Soeters PB,Deutz NE,Fearon KC,Dejong CH:Renal metabolism of amino acids∶Its role in interogan amino acid exchange.Am J Clin Nutr 2004,79∶185–197.
17.Souba WW,Herskowitz K,Salloum RM,Chena MK,Augsten TR:Gut glutamine metabolism.J Parenter Enter Nutr 1990,14(Suppl.4):45S–50S.
18.Reeds PJ,Burrin DG,Stoll B,Jahoor F:Intestinal glutamate metabolism.J Nutr 2000,130∶978S–982S.
19.Wu G,Knabe DA,Flynn NE:Synthesis of citrulline from glutamine in pig enterocytes.Biochem J 1994,299∶115–121.
20.Stoll B,Burrin DG:Measuring splanchnic amino acid metabolism in vivo using stable isotopic tracers.J Anim Sci 2006,84∶E60–E72.
21.Wu G:Recent advances in swine amino acid nutrition.J Anim Sci Biotech 2010,1∶49–61.
22.Van der Hulst RRWJ,Van Kreel BK,Von Meyenfeldt MF,Brummer RK,Arends JW,Deutz NE,Soeters PB:Glutamine and the preservation of gut integrity.Lancet 1993,431∶1363–1365.
23.James LA,Lunn PG,Elia M:Glutamine metabolism in the gastrointestinal tract of the rat assessed by the relative activities of glutaminase and glutamine synthetase.Br J Nutr 1998,79∶365–372.
24.FASS:Guide for the care and use of agricultural animals in agricultural research and teaching.1 revth edition.Savoy,IL:Fed.Anim.Sci.Soc;1999.
25.Doerfler RE,Cain LD,Edens FW,Parkhurst CR,Qureshi MA,Havenstein GB:D-Xylose absorption as a measurement of malabsorption in poult enteritis and mortality syndrome.Poult Sci 2000,79∶656–660.
26.McDowell EM,Trump BF:Histological fixatives suitable for diagnostic light and electron microscopy.Arch Pathol Lab Med 1976,100∶405.
27.Goodwin MA,Latimer KS,Fletcher OJ:Quantification of intestinal D-xylose absorption in normal turkeys.Poult Sci 1984,63∶1742–1747.
28.Goodwin MA,Latimer LS,Nersessian BN:Quantification of intestinal D-xylose absorption in normal and reovirus-inoculated turkeys.Avian Dis 1984,28∶959–967.
29.Eberts TJ,Sample RHB,Glick MR,Ellis GH:A simplified,colorimetric micro method for xylose in serum or urine,with phloroglucinol.Clin Chem 1979,25∶1440–1443.
30.Dahlqvist A:Assay of intestinal disaccharidases.Scand J Clin Lab Invest 1984,44∶169–172.
31.Windmueller HG,Spaeth AE:Metabolism of absorbed aspartate,asparagine and arginine by rat small intestine in vivo.Arch Biochem Biophys 1976,175∶670–676.
32.Burrin DG,Reeds PJ:Alternative fuels in the gastrointestinal tract.Curr Opin Gastroenterol 1997,13∶165–170.
33.Windmueller HG,Spaeth AE:Intestinal metabolism of glutamine and glutamate from the lumen when compared to glutamine from the blood.Arch Biochem Biophys 1975,171∶662–672.
34.Reeds PJ,Burrin DG,Stoll B,Jahoor F,Wykes L,Henry J,Frazer ME:Enteral glutamate is the preferential source for mucosal glutathione synthesis in fed piglets.Am J Physiol 1997,273∶E408–E415.
35.Yin YL,Huang RL,Li TJ,Ruan Z,Xie MY,Deng ZY,Hou YQ,Wu G:Amino acid metabolism in the portal-drained viscera of young pigs∶effects of dietary supplementation with chitosan and pea hull.Amino acids 2010,39∶1581–1587.
36.Boutry C,Bos C,Matsumoto H,Even P,Azzout-Marniche D,Tome D,Blachier F:Effects of monosodium glutamate supplementation on glutamine metabolism in rats.Front Biosci 2011,E3∶279–290.
37.Wu G,Borbolla AG,Knabe DA:The uptake of glutamine and release of arginine,citrulline and proline by the small intestine of developing pigs.J Nutr 1994,124∶2437–2444.
38.Wu G,Ott TL,Knabe DA,Bazer FW:Amino acid composition of the fetal pig.J Nutr 1999,129∶1031–1038.
39.Kim SW,Wu G:Regulatory role of amino acids in mammary gland growth and milk synthesis.Amino acids 2009,37∶89–95.
40.Trottier NL,Shipley CF,Easter RA:Plasma amino acid uptake by the mammary gland of the lactating sow.J Anim Sci 1997,75∶1266–1278.
41.Haynes TE,Li P,Li X,Shimotori K,Sato H:L-Glutamine or L-alanyl-L-glutamine prevents oxidant or endotoxin-induced death of neonatal enterocytes.Amino Acids 2009,37∶131–142.
42.Yi GF,Carroll JA,Allee GL,Gaines AM,Kendall DC,Usry J,Toride Y,Izuru S:Effect of glutamine and spray-dried plasma on growth performance,small intestine morphology,and immune responses of Escherichia coli K88+-challenged weaned pigs.J Anim Sci 2005,83∶634–643.
43.Jiang ZY,Sun LH,Lin YC,Ma XY,Zheng CT,Zhou GL,Chen F,Zou ST:Effects of dietary glycyl-glutamine on growth performance,small intestinal integrity,and immune responses of weaning piglets challenged with lipopolysaccharide.J Anim Sci 2009,87∶4050–4056.
44.Wu G:Functional amino acids in growth,reproduction and health.Adv Nutr 2010,1∶31–37.
45.Wu G,Meier SA,Knabe DA:Dietary glutamine supplementation prevents jejunal atrophy in waned pigs.J Nutr 1996,126∶2578–2584.
46.Wang JJ,Chen X,Li P,Li XL,Zhou HJ,Wang FL,Li DF,Yin YL,Wu G:Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation.J Nutr 2008,138∶1025–1032.
47.Liu T,Peng J,Xiong Y,Zhou S,Cheng X:Effects of dietary glutamine and glutamate supplementation on small intestine structure,active absorption and DNA,RNA concentrations in skeletal muscle tissue of weaned piglets during d 28 to 42 d of age.Asian-Aust J Anim Sci 2001,15∶238–242.
48.Mansoori B,Nodeh H,Modirsanei M,Rahbari S,Aparnak P:D-xylose absorption test∶A tool for the assessment of the effect of anticoccidials on the intestinal absorptive capacity of broilers during experimental coccidiosis.Anim Feed Sci Technol 2009,148∶301–308.
49.You-Sheng L,Jie-Shou L,Ninj L,Zhi-Wei J,Yun-Zhao Z,Nan-Yun L,Fang-Nan L:Evaluation of various solutions for small bowel graft preservation.World J Gastr 1998,4∶140–143.
50.Chang RW,David PJ,Oh J-T,Andreoli S,Kim HB,Fauza D,Jaksic T:Serial transverse enteroplasty enhances intestinal function in a model of short bowel syndrome.Ann Surg 2006,243∶223–228.
51.Nasrallah SM,Iber FL:Mannitol absorption and metabolism in man.Am J Med Sci 1969,258∶80–88.
52.Cox MA,Lewis KO,Cooper BT:Measurement of small intestinal permeability markers,lactulose and mannitol in serum.Dig Dis Sci 1999,44∶402–406.
53.Andre C,Andre F,Colin L,Cavagna S:Measurement of intestinal permeability to mannitol and lactulose as a means of diagnosing food allergy and evaluating therapeutic effectiveness of disodium cromoglycate.Ann Allergy 1987,59∶127–130.
54.Kidder DE,Manners MJ:Carbohydrases in the small intestine mucosa of sow-reared and 3-wk weaned pigs.Proc Nutr Soc 1978,37∶51A.
55.Skrzypek T,Valverde JL,Skrzypek H,Wolinski J,Kazimierczak W,Szymanczyk S,Pawlowska M,Zabielski R:Light and scanning electron microscopy evaluation of the postnatal small intestinal mucosa development in pigs.J Physiol Pharmacol 2005,56∶71–87.
56.Blikslager A,Hunt E,Guerrant R,Rhoads M,Argenzio A:Glutamine transporter in crypts compensates for loss of villus absorption in bovine cryptosporidiosis.Am J Physiol Gastrointest Liver Physiol 2001,281∶G645–G653.
57.Xi P,Jiang Z,Dai Z,Li X,Yao K,Zheng C,Lin Y,Wang J,Wu G:Regulation of protein turnover by L-glutamine in porcine intestinal epithelial cells.J Nutr Biochem 2012,23∶1012–1017.
58.Manso HE,Filho HC,de Carvalho LE,Kutschenko M,Nogueira ET,Watford M:Glutamine and glutamate supplementation raise milk glutamine concentrations in lactating gilts.J Anim Sci Biotech 2012,3∶2.
 Journal of Animal Science and Biotechnology2013年3期
Journal of Animal Science and Biotechnology2013年3期
- Journal of Animal Science and Biotechnology的其它文章
- Development of feeding systems and strategies of supplementation to enhance rumen fermentation and ruminant production in the tropics
- Use of a post-production fractionation process improves the nutritional value of wheat distillers grains with solubles for young broiler chicks
- Hypoxia promotes cell proliferation by modulating E2F1 in chicken pulmonary arterial smooth muscle cells
- Pregnancy recognition signaling mechanisms in ruminants and pigs
- Forage and breed effects on behavior and temperament of pregnant beef heifers
- Improving efficiency of sow productivity:nutrition and health
