Al-Fe-P三元系的熱力學(xué)優(yōu)化
曹戰(zhàn)民 謝 偉 王昆鵬 牛春菊 杜廣巍 喬芝郁
(1北京科技大學(xué)鋼鐵冶金新技術(shù)國家重點(diǎn)實(shí)驗(yàn)室,冶金與生態(tài)工程學(xué)院,北京 100083;2北京科技大學(xué)材料科學(xué)與工程學(xué)院,北京 100083)
1 Introduction
Phosphorus has known to be an essential metalloid element in forming an amorphous phase exhibiting useful engineering properties,such as,the Invar effect,1corrosion resistance,2and catalysis.3The Al-Fe-P ternary system has drawn more and more attention due to that amorphous phase formation has been found in the Al-Fe-P system by using a melt-spinning technique.4The evaluation of the glass-forming ability(GFA)of alloys is of great importance by judging in advance whether amorphous phases could be formed at given conditions.The successful evaluation5-7of GFA of some alloys indicates that the expressions of the Gibbs energies of the phases are very powerful in qualitatively analyzing the composition dependency of the GFA.The Al-Fe-P system is also a fundamental ternary system of the Fe-based and P-containing multi-component alloys,which are very important for the advanced metallurgy and materials.Knowledge of the thermodynamic properties of the Al-Fe-P system is significant for the development of Fe-based and P-containing multi-component alloys by providing information regarding the phases that are present at service temperature,their compositions and volume fractions and so on.Therefore,the interest in this system is increasing,as shown by a recent publication,8in which,however,the researchers only gave a description of the Fe rich side and merely accounted for one isothermal section at 723 K during the calculation.
Thus,our study was aimed to give a thermodynamic optimization of the Al-Fe-P system over the whole composition range based on all available experimental information in the literature by using CALPHAD(CALculation of PHAse Diagram)approach with Thermo-Calc software package.9The optimized self-consistent thermodynamic parameters of the Al-Fe-P system are expected to be helpful for both the better understanding of the composition dependency of the GFA in the Al-Fe-P system and the development of the Fe-based and P-containing multi-component alloy thermodynamic database.
2 Information on the binary and the ternary systems
2.1 Al-P system
The Al-P system was extensively investigated by many researchers.White et al.10released the existence of only one intermediate phase,AlP,which was further confirmed by Panish and Ilegems11,12during the study of the Ga-rich corner of the Ga-Al-P ternary system.Tu et al.13confirmed the existence of AlP again in the investigation of the 723 K isothermal section of the Al-P-Zn ternary system with the help of combined techniques of optical microscopy,scanning electron microscopy coupled with energy dispersive X-ray spectroscopy(SEMEDS),and X-ray diffraction(XRD).Kischio14reported the melting point of AlP to be(2803±50)K without experimental detail.Czochrallski15established a possible upper limit to the solubility of P in γ-Al of 0.0007 at mole fraction of P.
Several researchers experimentally measured the heat of formation of AlP using different methods and large difference existed among the experimental results.Kischio14obtained ΔfH295K(AlP)=(-82630±1050)J·mol-1·atom-1from the heat of dissolution of AlP in aqueous HCl and the known formation of the products.Wang and Zaheervuddin16measured the heats of combustion of AlP and equal mol mixtures of Al and red P.Adjusting their results to white P as reference state,ΔfH298K(AlP)=(-69500±4600)J·mol-1·atom-1was obtained.Maria et al.17measured the partial pressures of Al and P2vapor equilibrium with AlP,from 1270 to 1800 K using the Knudsen effusion method in combination with mass spectrometry,and ΔfH298K(AlP)=(-59000±6700)J·mol-1·atom-1was obtained.A second law fit to Maria′s experimental results17was carried out by McAlister18and ΔfH298K(AlP)=(-57600±17700)J·mol-1·atom-1with statistical error was yielded.Martosudirdjo and Pratt19reported ΔfH(AlP)=(-74000±900)J·mol-1·atom-1using precipitation calorimetry in liquid Sn at 582 K.
Tu et al.13gave a description of the Al-P binary system during the thermodynamic analysis of the Al-P-Zn ternary system.Unfortunately,their description could not reflect the phase relation of AlP with other phases correctly when extrapolating the parameters into the Al-Fe-P ternary system.Therefore,the thermodynamic parameters of the Al-P system were re-optimized in this work.
2.2 Al-Fe and Fe-P binary systems
In the Al-Fe system the equilibrium phases are the liquid,the α-Fe solid solution based on body-centered cubic(bcc)Fe,the γ-Fe solid solution based on face-centered cubic(fcc)Fe,the AlFe formed through ordering of α-Fe,Al13Fe4,Al5Fe2,Al2Fe,Al5Fe4,and the γ-Al based on fcc Al.Many researchers,including Kaufman and Nesor,20Saunders and Rivlin21and Seierstein22,optimized the Al-Fe binary system.The assessment by Seierstein22is the first consistent description of the Al-Fe phase diagram and has been successfully extrapolated to the related ternary system by many researchers.23-25Thus,the parameters optimized by Seierstein22have been adopted in present assessment,except that the thermodynamic parameters of Al2Fe phase have been slightly modified to ensure the Al2Fe phase be stable in the Al-Fe-P ternary system.The optimized parameters are shown in Table 1,and the calculated Al-Fe phase diagram is shown in Fig.1(a).
The Fe-P system is composed of the liquid,the α-Fe solid solution based on bcc Fe,the γ-Fe solid solution based on fcc Fe,Fe3P,Fe2P,FeP,FeP2,FeP4,and P(red and white P).A critical assessment of the Fe-P system has been carried out by Okamoto,26Ohtani27and Tokunaga28et al.However,in all these evaluations the valuable thermodynamic properties in the Fe-P system measured by Zaitsev et al.29using differential scanning calorimetry and Knudsen effusion method with mass-spectrometric analysis of the gaseous phase were omitted.Most recently,a thermodynamic re-optimization of the Fe-P system has been carried out by Cao et al.,30and satisfactorily reproduced most experimental phase diagram and thermodynamic properties.Hence,the parameters assessed by Cao et al.30with minor modification were used in present work.The modified parameters and calculated phase diagram of Fe-P system are shown in Table 1 and Fig.1b,respectively.
2.3 Al-Fe-P ternary system
Because of the high volatilization of P,the Al-Fe-P ternary phase diagram has been studied only for the Fe rich corner of this system.Vogel and Klose31studied the phase equilibria in the Fe-Fe2P-AlP-Fe50Al50region of the Al-Fe-P ternary system using differential thermal analysis(DTA)and metallographyanalysis.The vertical sections of w(P)=6%,9%(mass fraction)and w(Al)=10%,25%were determined.The isothermal section at room temperature and the liquidus surface in the Fe-Fe2PAlP-Fe50Al50region were also investigated experimentally.There was found that the AlP-Fe2P and AlP-Fe50Al50sections are the quasi-binaries of the simple eutectic type.Kaneko et al.32investigated the phase relationships between phosphide-phase and iron-phase in the Al-Fe-P system by chemical and X-ray examinations and the obtained results agree with those reported by Vogel and Klose.31Limited thermodynamic properties relevant to the Al-Fe-P system are reported.Yamada and Kato33,34determined the activity interaction coefficients of phosphorus in the Fe-P-i system at 1873 K using a Knudsen cell-mass spectrometer combination with the computation from the ratio of the intensities of P+and Fe+peaks,and=4.6±0.7 was obtained.Based on the Miedema model and Toop equation Ding et al.35thermodynamically predicted the activity interaction coefficients between alloying elements and P in liquid Fe at 1873 K and the value of predictedis 8.78,which is much larger than the measured mentioned above.On the basis of the above mentioned information,the phase equilibria in the Al-Fe-P ternary system were reviewed in references.36-38

Table 1 Optimized thermodynamic parameters for the binary and ternary systems
Most recently,Wu et al.8investigated the phase equilibrium in the Al-Fe-P system when the P content was below 20%(mass fraction)with SEM-EDS and XRD and released an isothermal section at 723 K of the Al-Fe-P system at low phosphorus contents.And up to now,no ternary phase has been found in this ternary system.
3 Thermodynamic models
The thermodynamic models adopted here for the phases of the Al-Fe-P system are summarized in Table 1 and briefly introduced below.
3.1 Pure elements

3.2 Solution phases
The solution phases are modeled by the substitutional solution model and their Gibbs energies are described by the following expression:

where the three terms assume different forms according to the nature of the phase.Substitutional solutions are represented by only one sublattice where all the atoms mix randomly.In the binary case(A,B)1,the three contributions to the Gibbs energy are:
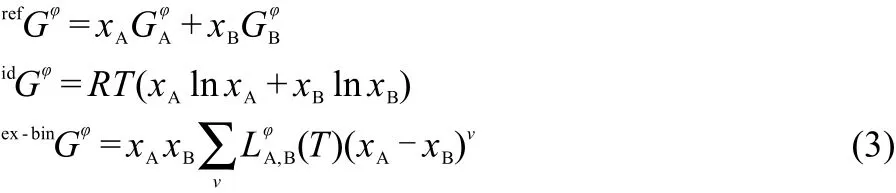

Fig.1 Calculated binary phase diagrams

In the case of ternary solutions,the expressions ofrefGφandare easily derived from the binary ones.As for the excess Gibbs energy,it is obtained by combing the binary excess Gibbs energies according to the Muggianu extrapolation formula40and adding a ternary excess term:

and the L functions have the form shown in Eq.(4).
This model has been adopted for the following phases:liquid,α,γ,and Al5Fe4(see Table 1).
3.3 Ordered/disordered phases
The ordered AlFe phase with bcc_B2 structure is modeled as(Al,Fe)0.5(Al,Fe)0.5in the Al-Fe system.22In order to represent the Gibbs energy of the ordered/disordered transitions using a single function,the disordered α-Fe phase with bcc_A2 structure is described by(Al,Fe)1.Ansara et al.41,42have derived an equation which allows the thermodynamic properties of the disordered phase to be evaluated independently.This is done by resolving the Gibbs energy into three terms as following:

in which yi′is the site fraction of i(i=Al and Fe)in the first sublattice,and yi″in the second one.(xi)is the Gibbs energy of the disordered α-Fe phase.The second term(yi′,yi″)is described by the sublattice model and implicitly contains a contribution from the disordered state.The last term(xi)represents the contribution from the disordered state to the ordered one.When the site fractions are equal,i.e.yi′=yi″,the last two terms cancel each other.The ordered AlFe phase with bcc_B2 structure and the disordered α-Fe phase with bcc_A2 structure are modeled as(Al,Fe,P)0.5(Al,Fe,P)0.5and(Al,Fe,P)1in the Al-Fe-P ternary system,respectively.Due to lack of the experimental information on the ordered/disordered transition in the Al-Fe-P ternary system the ternary interaction parameters are assumed to be zero.
3.4 Intermetallic compounds
Stoichiometric compounds are represented with as many sublattices as the number of component elements,with only one atom type in each sublattice.In the binary case the model is(A)u(B)1-uand the Gibbs energies are given by

The Eq.(7)was adopted to describe the Gibbs energies of the stoichiometric compounds that were lack of heat capacity data:Al5Fe2,Al2Fe,FeP2,FeP4,and AlP(see Table 1).
For Fe3P,Fe2P,and FeP phases,the heat capacity data are available in a wide temperature range29and their Gibbs energies are given by

Table 2 Lattice stability parameters used in the present optimization39

where ΔfH298Kand S298Kare the enthalpy of formation and the entropy at 298 K,and Cpis the heat capacities at constant pressure.
In Seierstein′s study,22non-stoichiometric compound,Al13Fe4,was modeled with three sublattices:(Al)0.6275(Fe)0.235(Al,Va)0.1375in order to account for its binary solubility range.The Gibbs energy is given by

where the superscript?denotes the third sublattice of the presented model,is the site fraction of i in the third sublattice,Va is the vacancy in the third sublattice.andare the Gibbs energy of the two end members(Al)0.6275(Fe)0.235(Al)0.1375and(Al)0.6275(Fe)0.235(Va)0.1375.
No ternary intermetallic compounds have been reported in previous study and all binary intermetallic phases were treated to have zero ternary solubility in the Al-Fe-P system in present work.
4 Optimization results and discussion
The binary and ternary interaction parameters have been optimized using the PARROT module included in the Thermo-Calc software package.9The phase diagram data and experimental thermodynamic information were used as the input to the program.Each datum value was given a certain weight by our personal evaluation of the datum source and considering the consistency between the phase diagram and the thermodynamic properties.The interaction parameters have been evaluated by trial and error method during the course of the optimization until most of the selected experimental information is reproduced within the expected uncertainty limits.As a result,a complete list of the thermodynamic parameters describing the Al-Fe-P system is summarized in Table 1.Computed phase equilibria are compared to the selected experimental information reviewed in Sections 2.1 and 2.3.And a brief discussion is given as below.
4.1 Al-P binary system
The calculated phase diagram of Al-P system is shown in Fig.1(c).The evaluated melting point of AlP is 2803 K,which agrees well with the experimental value(2803±50)K.14As mentioned in Section 2.1,there are large differences among the experimental values of enthalpy formation of AlP determined by different researchers.The calculated ΔfH298K(AlP)=-74.0 kJ·mol-1·atom-1,is close to the value measured by Wang and Zaheervuddin.16
4.2 Al-Fe-P ternary system
By using the present optimized parameters of the Al-P system along with the reported parameters of the Al-Fe22and Fe-P30systems,and based on the vertical section and isothermal section diagram information,the Al-Fe-P ternary system is further optimized.The parameters of the Al-Fe and Fe-P system were slightly modified in order to fit the experimental data better.

Fig.2 (a)Isothermal section diagram of the Al-Fe-P system at room temperature determined by Vogel and Klose31 and redrawn by Schmid-Fetzer and Tomashik38 and(b)calculated isothermal section diagram at room temperature in this work

Fig.3 Calculated vertical section diagrams compared with the experimental data31
The experimental and calculated isothermal section diagrams at room temperature are shown in Fig.2.It is easy to see that most of the phase relations have been well reproduced.The representative calculated vertical section diagrams with experimental data are illustrated in Fig.3.Although there is,in general,a satisfactory agreement between the experimental data reported by Vogel and Klose31for the phase diagram,some differences still exist,e.g.a relative discrepancy on the liquidus line between the calculated and the experimental data was observed.Actually,great care has been taken to reduce these discrepancies in our assessment,but it was found that it is difficult to fit these experimental data very well.It is still necessary to get more new experimental data to solve this problem.Additionally,some of the equilibria with respect to the bcc phase are inconsistent with the measured vertical sections by Vogel and Klose,31e.g.in our assessment,bcc phase is respectively in equilibrium with Fe2P and liq+Fe2P in the dashed regions,as shown in Fig.3(d),rather than AlP and liq+AlP reported by Vogel and Klose.31
It was worthy to point out that we calculated the isothermal section diagram of the Al-Fe-P system at 723 K and compared with the latest experimental data,8which was published after the present assessment had been finished,and a good agreement between them was obtained,as shown in Fig.4.It proved that the thermodynamic parameters gained in our optimization are reliable.

Fig.4 Calculated isothermal section diagram of the Al-Fe-P system at 723 K compared with the experimental data8

Fig.5 Calculated liquidus projection of the Al-Fe-P system

Table 3 Present calculated values for the invariant reactions in the Al-Fe-P system with the experimental data31
Taking into account the available information on the binary and ternary systems,the complete liquidus projection for the whole Al-Fe-P system are illustrated in Fig.5.The calculated and experimental invariant temperatures and the corresponding compositions for the various invariant reactions are summarized in Table 3.Although the temperatures are generally in good agreement with the experimental values,there are still some differences in the compositions.Invariant reactions E7 and E8 are very near to the binary subsystems,so these two invariant reactions cannot be visible in Fig.5.Necessary experiments are also needed to validate the liquidus projection in the Al-Fe-P system.
5 Explanation on the glass-forming ability

Fig.6 Calculated driving force(DF)surface projection at 973 K superimposed with iso-DF lines and the experimental glass compositions denoted by Inoue4
The driving force(DF)criterion5,6is based on the assumption that the phase having the highest driving force is most like-ly to form first.Considering that the formation of an amorphous phase would be favored when the nucleation and growth of crystalline phases are retarded,then the composition with the highest glass-forming ability can be the one with the lowest driving force of formation of crystalline phases.In order to analyze the composition dependency of the glass-forming ability,the initial crystallization driving force,which can be regarded as a representation of the nucleation ability of the crystalline phases,was employed in this work.The initial driving forces of all crystalline phases under every specified liquid composition at 973 K were first calculated and then the maximal values under each composition were chosen to construct the DF surface projection,as shown in Fig.6.Iso-DF lines and the experimental glass compositions are superimposed on it.All the experimental data are in the region where the driving forces are lower than 0.9.This is in accordance with the driving force criterion:alloys having lower driving forces possess higher glassforming abilities.This proves that the driving force criterion can be used as an index to predict the composition field most likely having the best glass-forming ability before any experiments.
6 Conclusions
Based on the available experimental information on the Al-P and the Al-Fe-P systems as well as the published assessment of the Al-Fe and the Fe-P binary systems,a critical assessment of the Al-P binary and the Al-Fe-P ternary system was carried out and a consistent thermodynamic description for describing all the phases in the Al-Fe-P system was obtained.The phase diagrams of the Al-Fe-P system over the entire composition range,including the vertical sections for w(P)=6%,9%,and w(Al)=10%,25%,the isothermal section at room temperature as well as the liquidus projection was constructed from present thermodynamic calculation.Most reported compositions of amorphous phase lie in the regions with low initial driving forces for the crystalline phases,which soundly proves the reasonability and reliability of the present thermodynamic description.
(1)Fukamichi,K.;Kikuchi,M.;Hiroyoshi,H.;Masumoto,T.Anomalous Thermal Expansion,ΔE Effect,Invar and Elinvar Characteristics of Some Fe-based Amorphous Alloys.In Rapidly Quenched Metals III;Cantor,B.Ed.;The Metals Society:London,1978.
(2)Masumoto,T.;Hashimoto,K.;Naka,M.Corrosion Behavior of Amorphous Metals.In Ra pidly Quench ed Metals III;Cantor,B.Ed.;The Metals Society:London,1978.
(3)Yokoyama,A.;Komiyama,H.;Inoue,H.;Masumoto,T.;Kimura,H.M.S cripta Met.1981,15,365.
(4)Inoue,A.;Kitamura,A.;Masumoto,T.Mater.Sci.1983,18,753.doi:10.1007/BF00745573
(5)Kim,D.;Lee,B.J.;Kim,N.J.Intermetallics 2004,12,1103.doi:10.1016/j.intermet.2004.04.001
(6)Kim,D.;Lee,B.J.;Kim,N.J.Scripta Mater.2005,52,969.doi:10.1016/j.scriptamat.2005.01.038
(7)Bo,H.;Wang,J.;Jin,S.;Qi,H.Y.;Yuan,X.L.;Liu,L.B.;Jin,Z.P.Intermetallics 2010,18,2322.doi:10.1016/j.intermet.2010.08.002
(8)Wu,C.J.;Huang,W.M.;Su,X.P.;Peng,H.P.;Wang,J.H.;Liu,Y.CAL PH AD 2012,38,1.doi:10.1016/j.calphad.2012.03.005
(9)Sundman,B.;Jansson,B.;Andersson,J.O.CALPHAD 1985,9,153.doi:10.1016/0364-5916(85)90021-5
(10)White,W.E.;Bushey,A.H.J.Am.Chem.Soc.1944,66,1666.doi:10.1021/ja01238a018
(11)Panish,M.B.;Ilegems,M.Prog.S olid State Chem.1972,7,39.doi:10.1016/0079-6786(72)90004-0
(12)Ilegems,M.;Panish,M.B.Crys.Growth 1973,20,77.doi:10.1016/0022-0248(73)90117-6
(13)Tu,H.;Yin,F.C.;Su,X.P.;Liu,Y.;Wang,X.M.C ALP H AD 2009,33,755.doi:10.1016/j.calphad.2009.10.003
(15)Czochrallski,J.Z.Metallkd.1923,15,273.
(16)Wang,C.C.;Zaheervuddin,M.Inorg.Nucl.Ch em.1963,25,326.doi:10.1016/0022-1902(63)80071-8
(17)de Maria,G.;Gingerich,K.A.;Piacente,V.Chem.Phys.1968,49,4705.
(18)McAlister,A.J.Alloy P hase Diagrams 1985,6(3),222.doi:10.1007/BF02880402
(19)Martosudirdjo,S.;Pratt,J.N.T h ermochim.A cta 1974,10,23.doi:10.1016/0040-6031(74)85019-7
(20)Kaufman,L.;Nesor,H.CA LPHA D 1978,2,325.doi:10.1016/0364-5916(78)90020-2
(21)Saunders,N.;Rivlin,V.G.Z.Metallkd.1987,78,795.
(22)Seierstein,M.The Al-Fe System.In COST 507,T hermoch emical Database for Light Metal Alloys;Ansara,I.,Dinsdale,A.T.,Rand,M.H.Eds.;Office for Official Publications of the European Communities:Luxembourg,1998.
(23)Zhang,L.J.;Du,Y.CAL PHAD 2007,31,529.doi:10.1016/j.calphad.2007.03.003
(24)Du,Y.;Schuster,J.C.;Liu,Z.K.;Hu,R.X.;Nash,P.;Sun,W.H.;Zhang,W.W.;Wang,J.;Zhang,L.J.;Tang,C.Y.;Zhu,Z.J.;Liu,S.H.;Ouyang,Y.F.;Zhang,W.Q.;Krendelsberger,N.Intermetallics 2008,16(4),554.doi:10.1016/j.intermet.2008.01.003
(25)Guo,C.P.;Du,Z.M.;Li,C.R.;Zhang,B.L.;Tao,M.C ALP H AD 2008,32,637.doi:10.1016/j.calphad.2008.08.007
(26)Okamoto,H.B ull.Alloy Phase Diagrams 1990,11,404.doi:10.1007/BF02843320
(27)Ohtani,H.;Hanaya,N.;Hasebe,M.;Teraoka,S.;Abe,M.C ALP H AD 2006,30,147.doi:10.1016/j.calphad.2005.09.006
(28)Tokunaga,T.;Hanaya,N.;Ohtani,H.;Hasebe,M.ISIJ International 2009,49(7),947.doi:10.2355/isijinternational.49.947
(29)Zaitsev,A.I.;Dobrokhotova,Z.V.;Litvina,A.D.;Mogutnov,B.M.Chem.S oc.Faraday Trans.1995,91(4),703.doi:10.1039/ft9959100703
(30)Cao,Z.M.;Wang,K.P.;Qiao,Z.Y.;Du,G.W.A cta Phys.-Chim.Sin.2012,28(1),37.[曹戰(zhàn)民,王昆鵬,喬芝郁,杜廣巍.物理化學(xué)學(xué)報(bào),2012,28(1),37.]doi:10.3866/PKU.WHXB201111172
(31)Vogel,R.;Klose,H.Arch.Eisenhuttenwesen 1952,23(7),287.
(32)Kaneko,H.;Nishizawa,T.;Tamaki,K.Nippon Kinzoku Gakkai-shi 1965,29(2),159.
(33)Yamada,K.;Kato,E.Tetsu-to-Hagane(J.Iron Steel Inst.Jap.)1979,65(2),273.
(34)Yamada,K.;Kato,E.Trans.Iron Steel Inst.Jap.1983,23(1),51.doi:10.2355/isijinternational1966.23.51
(35)Ding,X.;Wang,W.;Han,Q.Acta Metall.S in.1993,29(12),B527.
(36)Raghavan,V.The Al-Fe-P System(Aluminium-Iron-Phosphorus).In Phase Diagrams of Ternary Iron Alloys,Part 3,Ternary Systems Containing Iron and P hosphorus;Indian Institute of Metals:Calcutta,1988.
(37)Raghavan,V.Alloy P hase Dia grams 1989,5(1),32.
(38)Schmid-Fetzer,R.;Tomashik,V.A.L andolt-B R nstein-Group IV P hysical Chemistry 2008,11D1(1),172.
(39)Dinsdale,A.T.CAL PHAD 1991,15,317.doi:10.1016/0364-5916(91)90030-N
(40)Muggianu,Y.M.;Gambino,M.;Bros,J.P.Chim.Ph ys.1975,72,83.
(41)Ansara,I.;Dupin,N.;Lukas,H.L.;Sundman,B.J.Alloy.Compd.1997,247,20.doi:10.1016/S0925-8388(96)02652-7
(42)Dupin,N.;Ansara,I.;Sundman,B.CAL PH AD 2001,25,279.doi:10.1016/S0364-5916(01)00049-9

