配位化合物Zn(Met)3(NO3)2·H2O(s)(Met=L-α-蛋氨酸)的低溫?zé)崛菁皹?biāo)準(zhǔn)摩爾生成焓
張國春 周春生 高勝利
(商洛學(xué)院化學(xué)與化學(xué)工程系,陜西商洛 726000)
1 Introduction
It is well known that zinc as one of the trace elements plays an important role in the physical growth of human beings,especially in intelligence development.Many diseases result from deficiency of zinc in the human body,such as dwarfism,hypertension,rheumatoid arthritis,sugar diabetes,and cardiovascular diseases,etc.The L-α-amino acids are indispensable nutrients in organisms and the basic units of the proteins necessary for life activity.Zinc compounds with L-α-amino acids are considered to be one of the most efficient zinc tonics because they have a higher absorption ratio in the human body than traditionally inorganic and organic weak-acid zinc tonics.Such zinc coordination compounds showed wide applications in medicine,foodstuff,animal feed,and cosmetic as nutrient additive.1-3Gao et al.4has investigated the phase chemistry of the coordination behavior of zinc salts with L-α-methionine by the method of semi-micro phase equilibrium.The corresponding equilibrium phase diagram and refractive index diagram have been constructed from the compositions of saturated solutions and the data on refractive indices in ternary systems of Zn-Met-H2O.The compound,Zn(Met)3(NO3)2·H2O(s),was synthesized in water and acetone under the guidance of equilibrium phase diagram,characterized by means of Fourier transform infrared(FTIR)spectroscopy,X-ray diffraction(XRD),thermogravimetric analysis/differential thermal analysis(TG/DTA),and the initial dehydration temperature(TD)of the solid compound was reported to be 323.15 K by thermogravimetry.
However,other important thermodynamic properties of the compound have not been reported in the literature so far.In continuation of our studies on the compounds of zinc with L-αamino acid,the low temperature heat capacities over the temperature range 78-371 K and the standard molar enthalpy of formation of the title compound at T=298.15 K have been measured by adiabatic calorimetry and isoperibol solution-reaction calorimetry,respectively.The resulting data would provide more thermodynamic supports for the practical application of the compound.
2 Experimental
2.1 Synthesis and characterization of the compound
ZnSO4·7H2O(s),NaNO3(s),L-α-methionine(s),α-Al2O3(s),and HCl(aq)obtained from Shanghai Reagent Factory,China,were of analytical grade with purity prior to 99.50%.KCl(s)with 99.90%purity as Standard Reference Material 1655 was obtained from the National Institute of Standards and Technology.The solid compound,Zn(Met)3(NO3)2·H2O(s)as white lumpy crystals,was prepared by a semi-micro phase equilibrium method.4,5The solid compound obtained was kept in a desiccator containing P4O10.The results of chemical and elemental analyses,IR,TG/DTG,and XRD indicated that the compound was formulated as Zn(Met)3(NO3)2·H2O(s).The purity of the compound was higher than 99.90%mass fraction(analytical uncertainty in mass fraction≤0.20%).
2.2 Adiabatic calorimetry
A high-precision automatic adiabatic calorimeter was used to measure the heat capacities over the temperature range 78 K≤T≤371 K.The calorimeter was fabricated in the Thermochemistry Laboratory of Dalian Institute of Chemical Physics,Chinese Academy of Sciences.The principle and structure of the adiabatic calorimeter were described in detail elsewhere.6,7Briefly,the calorimeter comprised a sample cell,a platinum resistance thermometer,an electric heater,inner and outer adiabatic shields,two sets of six-junction chromel-constant thermopiles installed between the calorimetric cell and the inner shield and between the inner and outer shields,respectively,and a high vacuum can.The miniature platinum resistance thermometer(IPRT No.2,Shanghai Institute of Industrial Automatic Meters,16 mm length,1.6 mm diameter,and a nominal resistance of 100 Ω)was used to measure the temperature of the sample.The thermometer was calibrated on the basis of ITS-90 by the Station of Low Temperature Metrology and Measurements,Academia Sinica,China.The electrical energy introduced into the sample cell and the equilibrium temperature of the cell after the energy input were automatically recorded with the Data Acquisition/Switch Unit(model 34970A,Agilent,USA),and processed online by a computer.To verify the accuracy of the calorimeter,the heat capacity of the reference standard material(α-Al2O3)was measured over the temperature range 78 K≤T≤373 K.The sample mass used was 1.6382 g,which was equivalent to 0.0161 mol based on its molar mass,M(Al2O3)=101.9613 g·mol-1.The experimental molar heat capacities of α-Al2O3were fitted by the least-squares method to a polynomial.Deviations of the experimental results from those of the smoothed curve lie within±0.2%,while the uncertainty is within 0.3%,when compared with the values given by the former National Bureau of Standards8over the entire temperature range.Heat capacity measurements were continuously and automatically carried out by means of the standard method of intermittently heating the sample and alternately measuring the temperature.The heating rate and temperature increments were controlled at 0.1-0.4 K·min-1and 1-3 K,respectively.The heating duration was 10 min,and the temperature drift rate of the sample cell measured during an equilibrium period was always kept within 10-3-10-4K·min-1for acquisition of the heat capacity data.The data of heat capacities and corresponding equilibrium temperature have been corrected for heat exchange of the sample cell with its surroundings.6,7The sample mass of Zn(Met)3(NO3)2·H2O(s)used for the calorimetric measurement is 3.8993 g,which is equivalent to 0.00595 mol in terms of its molar mass,M=655.0560 g·mol-1.
2.3 Isoperibol solution-reaction calorimetry
The isoperibol solution-reaction calorimeter is primarily consisted of a precision temperature controlling system,an electric energy calibration system,a calorimetric body,an electric stirring system,a thermostatic bath made from transparent silicate glass,a precision temperature measuring system and a data acquisition system.The principle and structure of the calorimeter have been described in detail elsewhere.9The reliability of the calorimeter was verified initially by measuring the dissolution enthalpies of KCl(calorimetrical primary standard)in doubly distilled water at T=298.15 K.9The mean dissolution enthalpy was(17.547±0.013)kJ·mol-1for KCl,which compares well with the corresponding published data,(17.536±0.003)kJ·mol-1.10In all dissolution experiments,100 mL of 2 mol·L-1HCl was chosen as the calorimetric solvent for measuring the dissolution enthalpies of the mixtures{ZnSO4·7H2O(s)+2NaNO3(s)+3Met(s)}and{Zn(Met)3(NO3)2·H2O(s)+Na2SO4(s)}.The solid samples,ZnSO4·7H2O(s),NaNO3(s),and Met(s),were ground in an agate mortar into a fine powder.The solid compounds were weighed on parchment paper separately to avoid mixing before dissolution.The mixture of~0.288 g of ZnSO4·7H2O(s),~0.171 g of NaNO3(s),and ~0.450 g of Met(s)at a mole ratio of n(ZnSO4·7H2O):n(NaNO3):n(Met)=1:2:3 was dissolved in 100 mL of 2 mol·L-1HCl solution at T=298.15 K.The final solution obtained was designated as solution A.The solids,Zn(Met)3(NO3)2·H2O(s)and Na2SO4(s),were ground into a fine powder and dried in a vacuum drying oven in order to remove additional adsorbed water.The solid compounds were weighed on parchment paper separately to avoid mixing before dissolution.The dissolution enthalpy of the mixture of~0.65 g of Zn(Met)3(NO3)2·H2O(s)and ~0.14 g of Na2SO4(s)at a mole ratio of n(Zn(Met)3(NO3)2·H2O):n(Na2SO4)=1:1 in 100 mL of 2 mol·L-1HCl solution was determined under the same conditions as mentioned above.The final solution obtained was named as solution A′.Finally,the UV/Vis spectra and the data of the refractive indices were used to confirm whether solution A was in the same thermodynamic state as that of solution A′.The results indicate that chemical components and physicochemical properties of solution A are consistent with those of solution A′.
3 Results and discussion
3.1 Low-temperature heat capacities
The structure of the coordination compound is shown to be stable over the temperature range 78-325 K,as shown in Table 1 and Fig.1.No phase change,association nor thermal decomposition is seen to occur.However,at temperatures above 325 K,the heat capacity curve rises steeply.The phenomenon is associated with the dehydration or thermal decomposition of the compound,as shown by TG analysis.4In addition,the initial dehydration temperature(TD)has been obtained by analysis of the heat capacity curve.Two sections of the heat capacity curves,i.e.,78-325 K and 326-371 K are extrapolated linearly.The intersection point of these two straight lines is the initial decomposition temperature,TD=325.10 K(Fig.1).TG analysis shows the initial dehydration temperature of 323.15 K,the final dehydration temperature of 458.15 K and the mass loss of 2.79%.The mass loss is identical with the percentage of the water molecule in the coordination compound(the theoretical mass loss during dehydration is 2.75%).The initial dehydration temperature obtained from TG analysis is in agreement with that from the heat capacity curve.The main reason for the difference(1.90 K)between the two results is that the sample surface adsorbed moisture from the air during TG measurement.The surface adsorbed water removed prior to crystal water in the sample,which reduces the initial dehydration temperature and increases the actual mass loss of the sample.Howev-er,during the adiabatic calorimetric measurements,when the sample is put into the sample cell,the air pressure within the sample cell is vacuumed to below 100 Pa with a vacuum pump to prevent moisture from being adsorbed on the surface of the sample.Then,about 100 kPa of high purity helium is filled in the sample cell to increase the rate of heat conduction of sample cell during low temperature heat capacity measurements and also to reduce the time to reach thermal equilibrium.The sample cell was sealed by soldering.In the temperature range of 78-325 K,the rise in heat capacity curve is steady.However,at 326-371 K,the curve rises very steeply,indicating that the compound is decomposed into another substance.The experimental points in the temperature range of 78-325 K are fitted by the least-squares method,and a polynomial equation of experimental molar heat capacities(Cp,m)/(J·K-1·mol-1)versus the reduced temperature(X),X=f(T)=[T-(T1+T2)/2]/[(T1-T2)/2]where T1=325 K and T2=78 K,is obtained(Eq.(1)).

Table 1 Experimental molar heat capacities of the compound Zn(Met)3(NO3)2·H2O(s)(molar mass M=655.056 g·mol-1)

Fig.1 Plot of heat capacity(Cp,m)against temperature(T)for Zn(Met)3(NO3)2·H2O(s)

where X=(T-201.5)/123.5.The standard deviations of experimental molar heat capacities from the smoothed heat capacities calculated by the polynomial equation are within±0.3%except for some points around the lower and upper temperature.In addition,Fig.1 shows that a slow process of dehydration occurs after 325 K rather than an obvious peak of dehydration.There are two possible reasons for this phenomenon.On the one hand,the crystal water possesses a large percentage in the compound;while on the other hand,the mass of the sample is large.Since the sample cell is sealed during the entire calorimetric experiment,once the dehydration occurs,the speed of dehydration should accelerate with the temperature rising.Meanwhile,the rate of the dehydration is also inhibited as a result of the rapid increase of vapor pressure of the water in the sample cell.

Table 2 Smoothed heat capacities and thermodynamic functions of the compound Zn(Met)3(NO3)2·H2O(s)
3.2 Smoothed heat capacities and thermodynamic functions of the sample
The smoothed values of molar heat capacities and thermodynamic functions of the compound,Zn(Met)3(NO3)2·H2O(s)are derived according to the following thermodynamic equations(Eqs.(2)-(4)).

The fitted polynomial values of the molar heat capacities and fundamental thermodynamic functions of the sample relative to the standard reference temperature,298.15 K at an interval of 5 K,are given in Table 2.
3.3 Dissolution enthalpies of the mixtures{ZnSO4·7H2O(s)+2NaNO3(s)+3Met(s)}and{Zn(Met)3(NO3)2·H2O(s)+Na2SO4(s)}
The compound,Zn(Met)3(NO3)2·H2O(s),is one of the products in the following reaction(Eq.(5)).
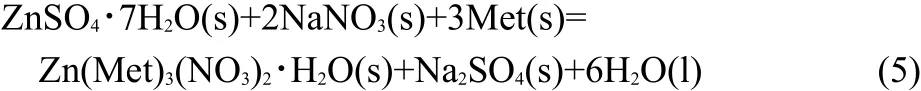
The enthalpy change of the proposed reaction above and standard molar enthalpy of formation of the compound Zn(Met)3(NO3)2·H2O(s)are determined by a designed thermochemical cycle using the experimental data of isoperibol calorimetry and other thermodynamic data.
If“s”=calorimetric solvent,2 mol·L-1HCl(aq),the dissolution process of the mixture of reactants in reaction(5)is expressed as{ZnSO4·7H2O(s)+2NaNO3(s)+3Met(s)}+“s”to give solution A.The experimental results of the process are listed in Table 3.The dissolution process of the products,{Zn(Met)3(NO3)2·H2O(s)+Na2SO4(s)},in reaction(5)may be expressed as{Zn(Met)3(NO3)2·H2O(s)+Na2SO4(s)}+“s”to give solution A′.The results of the dissolution experiments are shown in Table 4.solution A′+{6H2O(l)}=solution A.The enthalpy of dissolution of{6H2O(l)}(ΔdHm,3)as one of the products in reaction(5)in the solvent is within the range of experimental error,and may be omitted since the amount of H2O(l)is very small according to the stoichiometric number of H2O(l)in reaction(5),i.e.,ΔdHm,3=0 kJ·mol-1.The enthalpy change of reaction(5),ΔrHm,5,can be calculated in accordance with the designed thermochemical cycle above and experimental results are listed in Tables 3 and 4 by the following equation:ΔrHm,10=)=(16.936±0.100)kJ·mol-1.

Table 3 Dissolution enthalpy of{ZnSO4·7H2O(s)+2NaNO3(s)+3Met(s)}mixture at a mole ratio of n(ZnSO4·7H2O)∶n(NaNO3)∶n(Met)=1∶2∶3 in 100 mL 2 mol·L-1 HCl solution at 298.15 K

Table 4 Dissolution enthalpy of{Zn(Met)3(NO3)2·H2O(s)+Na2SO4(s)}mixture at a mole ratio of n(Zn(Met)3(NO3)2·H2O)∶n(Na2SO4)=1∶1 in 100 mL 2 mol·L-1 HCl solution at 298.15 K

Table 5 Reaction scheme used to determine the standard molar enthalpy of formation of Zn(Met)3(NO3)2·H2O(s)at 298.15 K
In order to obtain the standard molar enthalpy of formation of the compound Zn(Met)3(NO3)2·H2O(s),a reaction scheme is arranged and given in Table 5.The enthalpy change of reaction(5)obtained from experimental values of the dissolution enthalpies of{ZnSO4·7H2O(s)+2NaNO3(s)+3Met(s)}and{Zn(Met)3(NO3)2·H2O(s)+Na2SO4(s)}in 100 mL of 2 mol·L-1hydrochloric acid combined with some auxiliary thermodynamic data:11-14Δf[Na2SO4,s]=-1383.16 kJ·mol-1,11Δf[NaNO3,s]=-466.24 kJ·mol-1,12Δf[Met,s]=-(577.50±0.70)kJ·mol-1,13Δf[H2O,l,298.15 K]=-(285.83±0.04)kJ mol-1,14have been used to calculate the standard molar enthalpy of formation of the compound Zn(Met)3(NO3)2·H2O(s)as follows:
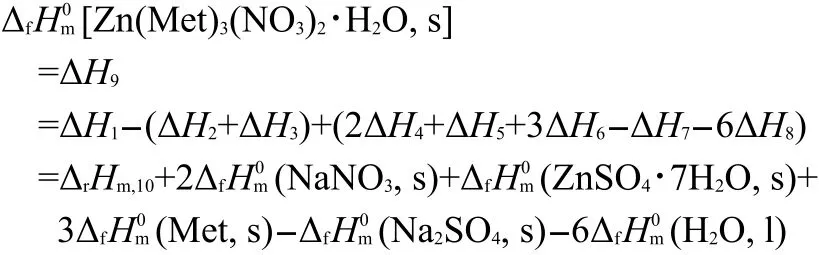

where ΔH1to ΔH9are the molar enthalpy changes of the corresponding reactions in Table 5.The results of UV/Vis spectrum and refractive index are important for checking the differences of the structure and composition for the two solutions.In the present study,all the reactants and products of reaction(5)can be easily dissolved in the selected solvent.The measured values of the refractive indices of solution A and solution A′are 1.3882±0.0004 and 1.3880±0.0005,respectively.The results of UV/Vis spectrum and the data of the refractive indices of solution A obtained are in agreement with those of solution A′.These results have demonstrated that the solutions A and A′are same,and the designed Hess thermochemical cycle can be used reliably to derive the standard molar enthalpy of formation of the compound Zn(Met)3(NO3)2·H2O(s).
4 Conclusions
In summary,as for the compound Zn(Met)3(NO3)2·H2O(s),low temperature heat capacities have been measured by a precision automated adiabatic calorimeter over the temperature range 78-371 K.The thermodynamic functions of dehydration,the enthalpy,entropy and Gibbs free energy are obtained.In addition,the standard molar enthalpy of formation of the compound is also determined from the enthalpies of dissolution of the reactants and products and other thermodynamic data by a Hess thermochemical cycle.
(1)Mahmoud,M.;Abdel-Monem,S.;Paul,M.Zinc Methionine Complex for Acne Treatment.US Pat.US 4039681.A,1977-08-02.
(2)Taguchi,S.;Inokuchi,M.;Nakajima,N.;Inomata,M.;Natitoh,Y.Antipruritic Drug and Antipruritic Composition.WO Pat.10178,1992-06-25.
(3)Harvey,H.;Ashmed,K.U.Preparation of Pharmaceutical Grade Amino Acid Chelates.US Pat.US 4830716,1989-05-16.
(4)Gao,S.L.;Hou,Y.D.;Liu,J.R.;Ji,M.;Shi,Q.Z.Acta Chim.Sin.2000,58(1),65.[高勝利,侯育冬,劉建睿,冀 棉,史啟禎.化學(xué)學(xué)報(bào),2000,58(1),65.]
(5)Jiang,H.Y.;Ren,D.H.;Xie,H.F.Ch in.J.Northwest Univ.(Nat.Sci.Ed.)1986,22,1.[蔣海盈,任德厚,薛洪福.西北大學(xué)學(xué)報(bào)(自然科學(xué)版),1986,22,1.]
(6)Tan,Z.C.;Sun,G.Y.;Sun,Y.J.T herm.Anal.1995,45,59.doi:10.1007/BF02548664
(7)Tan,Z.C.;Liu,B.P.;Zhang,J.B.;Sun,L.X.J.Comp.Appl.Chem.2003,20,265.
(8)Ditmars,D.A.;Ishihara,S.;Chang,S.S.;Bernstein,G.;West,E.D.J.R es.Natl.Bur.Stand.1982,87,159.doi:10.6028/jres.087.012
(9)Di,Y.Y.;Tan,Z.C.;Li,L.W.;Gao,S.L.;Sun,L.X.J.Ch em.Thermodyn.2006,38,884.doi:10.1016/j.jct.2005.09.006
(10)Rychly,R.;Pekarek,V.J.Chem.T hermodyn.1977,9,391.doi:10.1016/0021-9614(77)90060-X
(11)Di,Y.Y.;Tan,Z.C.;Gao,S.L.;Wang,S.X.J.Ch em.Eng.Data 2004,49,965.doi:10.1021/je034264n
(12)Waeman,D.D.;Evans,W.H.;Parker,V.B.;Schumm,R.H.;Halow,I.;Bailey,S.M.;Churney,K.L.;Nuttall,R.L.Phys.Chem.R ef.Data 1982,11,138.
(13)Huffman,H.M.;Fox,S.M.;Ellis,E.L.J.Am.Chem.Soc.1937,59,2144.doi:10.1021/ja01290a018
(14)Dean,J.A.Lange′s Handb ook of Chemistry;13th ed.;Science Press:Beijing,1991;p 1491;translated by Shang,J.F.,Cao,S.J.,Xin,W.M.,Zheng,F.Y.,Lu,X.M.,Lin,C.Q.[Dean,J.A.蘭氏化學(xué)手冊(cè).第13版.尚久方,操時(shí)杰,辛無名,鄭飛勇,陸曉明,林長青,譯.北京:科學(xué)出版社,1991:1491.]

