金/氧化亞銅異質(zhì)球的制備及其可見光催化性能
商 旸 陳 陽 施湛斌 張東鳳 郭 林
(北京航空航天大學(xué)化學(xué)與環(huán)境學(xué)院,北京100191)
1 Introduction
The requirement of sustainable energy sources and reduction of environmental pollution has driven considerable research efforts on water splitting and photodegradation of organic pollutants by using the abundant solar energy.1,2Many photoexcited semiconductor metal oxides,such as Cu2O,Fe2O3,TiO2,ZnO,and WO3,were reported as the efficient photocatalyst in aqueous solutions.3-11Among them,Cu2O with bandgap energy of 2.1 eV is expected as one of the promising materials in visible-light photocatalytic degradation,12which stimulated the research effort on the controlled growth of Cu2O and the investigation of morphology-dependent photocatalytic activities.13-17However,the lower photocatalytic efficiency owing to the fast recombination of the photogenerated electron and hole(e-/h+)pairs was still the main barrier limiting the applications of Cu2O in photocatalysis.5
Previous reports indicated that the hybridation between noble metal with semiconductor could reduce the recombination of the photogenerated e-/h+pairs,and thus enhance the photocatalytic efficiency.18-24For one thing,the loaded noble metal nanoparticles(NPs)can serve as electron sink to promote the e-/h+pairs separation24,25andthus enhance the quantum yield.26For another thing,the surface plasmon resonance(SPR)effect,defined as the collective coherent oscillation of the free charges on noble metal NPs irradiated by visible-light,can enhance the local electric field in the neighborhood of the noble metal NPs,and accelerate the formation of e-/h+pairs in the near-surface region of the semiconductor.27-29In addition,metal oxide photocatalyst with high support surface areas and the uniform distributions of the loaded noble metal NPs are necessary to get high photocatalytic efficiency.30Unlike TiO2,which has been extensively studied for loading with noble metal NPs,24,30,31Cu2O support tends to react with the noble metal precursor due to the low standard reduction potential.3And larger noble metal NPs tend to grow on the surface of Cu2O,which may hinder the adsorption and degradation of organic pollutant.5
Our previous work reported the preparation of Cu2O mesoporous spheres(MPS),which exhibit excellent adsorption performance.32We believed that the higher specific surface area and short-range-ordered structure of Cu2O MPS are beneficial for the sufficient contact between the organic dye molecules and Cu2O.To further explore their photocatalytic activities,herein,we report the preparation of Au/Cu2O heterogeneous spheres(HGS)by in-situ growth of Au NPs on the surface of Cu2O MPS through a wet-chemical reduction process while keep the mesoporous structure intact.Furthermore,we investigate the visible light photocatalytic activities of Au/Cu2O HGS for the degradation of methylene blue(MB).
2 Experimental
2.1 Materials
All the reagents were used without further purification.Copper chloride(CuCl2·2H2O,AR,≥99.0%),absolute ethanol(AR,≥99.7%),and ammonia(NH3·H2O,25%-28%,mass fraction)were purchased from Beijing Chemical Works.Triblock copolymer Pluronic P123(EO20PO70EO20,MW 5800)was purchased from Sigma-Aldrich,USA.Ascorbic acid(AR,≥99.7%)was purchased from Xilong Chemical Industry Incorporated Co.Ltd.Chloroauric acid(HAuCl4·4H2O,AR,≥47.8%)was purchased from Shenyang Jinke reagent factory.Sodium borohydride(NaBH4,AR,≥98.0%)and L-cysteine(AR,≥99.5%)were purchased fromAladdin reagent.
2.2 Synthesis
2.2.1 Synthesis of Cu2O mesoporous spheres
The Cu2O MPS were fabricated according to our previous works with minor modification.32Typically,0.612 g P123 was firstly dissolved in the mixture of 28.0 mL deionized water and 2.0 mL ethanol at 18°C under constant stirring.And given volume of NH3·H2O(14 mol·L-1)was added into the CuCl2aqueous solution(0.20 mol·L-1)to make the molar ratio of NH3to Cu2+kept as 10:1.The dark blue color of the solution indicated the formation of the Cu(NH3)2+4.Then,2.25 mL Cu(NH3)2+4solution was poured into the solution of P123 under constant stirring.After 30 min,5.0 mL ascorbic acid(AA,0.60 mol·L-1)was added dropwise into the above mixture.All the procedures were kept in water bath at 18°C(calibrated by Lauda Ecoline staredition RE 106).The reaction mixture underwent a series of color change from deep blue,light blue,limpid,white turbid,and finally to bright yellow turbid.The solution was kept stirring for another 10 min,and the resulting bright yellow precipitate was collected by centrifugation,washed with ethanol for several times to remove the P123,and then dried under vacuum at 60°C for 4 h.
2.2.2 Synthesis of Au/Cu2O heterogeneous spheres
In a typical synthesis,0.015 g Cu2O MPS was dispersed in 15.0 mL ethanol followed by the addition of 0.20 mL of L-cysteine aqueous solution(0.010 mol·L-1).After subjected to sonication for 30 min,1.0 mL HAuCl4aqueous solution(5.0 mmol·L-1)was added into the Cu2O dispersing.After the solution was vigorously stirred for another 30 min,0.25 mL NaBH4aqueous solution(0.030 mol·L-1)was quickly added into the solution.The mixture was aged for 0.5 h.All the procedures were kept in water bath at 10°C(calibrated by Lauda Ecoline staredition RE 106).The resulting precipitate was collected by centrifugation and decanting,followed by washing with distilled water for 3 times and absolute ethanol twice,respectively.Then,the products were dried under vacuum at 60°C for 4 h for the final characterization.
2.3 Characterization
The structure of the products was characterized by the powder X-ray diffraction(XRD)using a Rigaku Rotaflex Dmax 2200(Japan)diffractometer with Cu Kαradiation(λ=0.15406 nm).Scanning electron microscopy(SEM)images of the samples were obtained using Hitachi S-4800(Japan)with an accelerating voltage of 10 kV.Transmission electron microscopy(TEM)and High-resolution transmission electron microscopy(HRTEM)images were recorded by JEOL JEM-2100F(Japan)with an accelerating voltage of 200 kV.Elemental composition data were collected by EDAX equipped within the JEOL JEM-2100F.Specific surface areas were measured by using at least 0.1 g sample at-196°C through Brunauer-Emmett-Teller(BET)nitrogen adsorption-desorption(NOVA 2200e,Quanthachrome,USA).Before the measurements,all samples were degassed in vacuum at 150°C in the port of the adsorption analyzer for 4 h.The Brunauer-Emmett-Teller method was utilized to calculate the specific surface area(SBET).The pore size distribution(PSD)was derived from the adsorption branch using the Barrett-Joyner-Halenda(BJH)theory.Absorption spectra were recorded on a UV-3600 UV-Vis-NIR spectrophotometer made in Shimadzu,Japan.X-ray photoelectron spectroscopy(XPS)measurements were carried out on an Axis Ultra spectrometer(UK)under ultrahigh vacuum conditions with a standard Al Kαexcitation source(1486.6 eV).The charging effect was corrected by adjusting the binding energy of the main C 1s peak to 284.6 eV.
2.4 Photocatalytic activity measurement
The photocatalytic activities of the Cu2O and Au/Cu2O were evaluated by the degradation of methylene blue(50 mL,5 mg·L-1)containing 0.015 g as-obtained sample placed in a 200 mL cylindrical quartz vessel under 300 W Xe lamp with UV cutoff filter(providing visible light with λ larger than 400 nm).Before the light was turned on,the solution was stirred in the dark for 30 min to ensure adsorption-desorption equilibrium between the Cu2O and dyes.Under constant stirring in the dark,about 3 mL of the mixture solution was taken out at different intervals.After centrifugation,the UV-Vis spectrum of the supernatant was recorded to monitor the adsorption behavior.
3 Results and discussion
3.1 Characterization of Au/Cu2O HGS
The phase and purity of the samples were verified by X-ray diffraction(XRD)characterizations.Fig.1a illustrated the typical XRD pattern of the as-prepared pure Cu2O MPS.All the diffraction peaks could be well indexed to cuprite Cu2O(JCPDS No.05-0667).No peaks from impurities such as CuO and Cu can be identified.Fig.1b showed a typical XRD pattern of the as-prepared Au/Cu2O HGS.Besides the four diffraction peaks originated from cuprite Cu2O,there appears a peak at 2θ=38.2°,which can be indexed to(111)crystal plane of cube phase Au(JCPDS No.04-0784).The weak diffraction peak of Au indicated the low content ofAu in the sample.

Fig.1 XRD patterns of(a)pure Cu2O MPS and(b)Au/Cu2O HGS
Fig.2 presented typical scanning electron microscopy and transmission electron microscopy of the pure Cu2O MPS and Au/Cu2O HGS.The size of the pure Cu2O MPS was 150 to 350 nm(Fig.2a),and the magnified SEM image of a representative spheres illustrated its mesoporous structure feature with the pore diameter of~8 nm(inset of Fig.2a).Fig.2b was the typical SEM image of Au/Cu2O HGS,which demonstrated that no obvious size change of the spheres was observed after Au loading except for the observation of some small attachments.TEM observations as shown in Fig.2d revealed that the spheres kept the mesoporous structure and small particles of~4 nm could be identified on the surface of the mesoporous structures.The small particles on the surface tended to aggregate,corresponding to the attachment in the SEM image.The energy dispersive X-ray(EDX analysis as shown in Fig.2c)gave signals of Au and Cu,which confirmed that the small particles observed in TEM were Au NPs.To learn more structure information,high-resolution transmission electron microscopy was employed.The typical TEM images of Au/Cu2O HGS were shown in Figs.2d and 2e.From the HRTEM image(Fig.2f)recorded from the edge of the sphere(as indicated by the framed area in Fig.2e),lattice fringes with interplanar spacing of 0.246 and 0.235 nm can be identified.The former can be ascribed to the(111)crystal plane of the cubic Cu2O,while the latter can be ascribed to the(111)crystal plane of the cubic Au.The result revealed that the Cu2O mesoporous structure was good supports for the dispersedness of the Au NPs to construct novel catalytically nanoreactors.
Contrast experiment was carried out to optimize the ratio of Au/Cu2O by changing the amount of the added HAuCl-4solution.According to the experiment,the added HAuCl4/Cu2O ratio is 5%,and the EDX analysis illustrated that the ratio of the loaded Au NPs on Cu2O MPS was 2.4%.If increased the HAuCl4aqueous solution to 1.5 mL,the EDX analysis(Fig.S1(Supporting Information))showed that the ratio of the loaded Au NPs on Cu2O MPS was increased to 2.5%.It means that the amount of Au loaded on Cu2O does not obviously increase with the amount of HAuCl4.To optimize use ratio of the expensive HAuCl4,1.0 mL HAuCl4was added to react with Cu2O MPS.
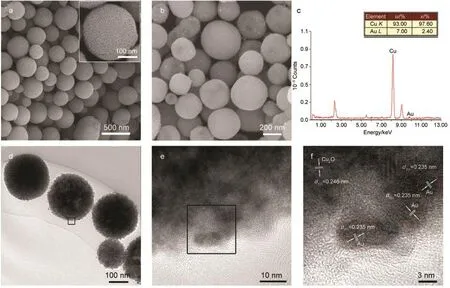
Fig.2 SEM images of(a)pure Cu2O MPS and(b)Au/Cu2O HGS;(c)EDX spectrum of theAu/Cu2O HGS;(d)TEM image ofAu/Cu2O HGS;(e)magnified TEM image recorded on the framed area in(d);(f)HRTEM image recorded on the framed area in(e)
The Brunauer-Emmett-Teller N2adsorption-desorption isotherms of the Cu2O and Au/Cu2O HGS(Fig.3)exhibited type IV hysteresis loops at relative pressures of p/p0=0.45-0.98,providing another evidence for the intact mesoporous structure.The measured surface area of Au/Cu2O HGS is 45.22 m2·g-1,similar with that of the pure Cu2O MPS(48.04 m2·g-1).There are obvious strong and narrow peaks at about 7.4 nm calculated by Barrett-Joyner-Halenda(BJH)analysis using the adsorption branch of the isotherm,proving the narrow pore size distributions and the unchanged Cu2O mesoporous structures after loadedAu NPs.

Fig.3 Nitrogen adsorption-desorption isotherms and the corresponding BJH pore size distribution curve(inset)of the Cu2O MPS andAu/Cu2O HGS
Fig.4 showed the UV-Vis absorption spectra of pure Cu2O MPS and Au/Cu2O HGS.The absorption spectrum of Cu2O MPS displayed an adsorption peak centered at about 450 nm(Fig.4b).By contrast,the Au/Cu2O HGS exhibited increased light absorption intensity with a much broader absorption peak,which centred at about 510 nm(Fig.4c).As a noble metal,Au NPs often exhibit strong SPR due to the collective oscillation of conduction electrons when exposed to an external electromagnetic field.33It is well documented that the transverse SPR(TSPR)absorption band of Au NPs with 3-5 nm in solution is about 520 nm(Fig.4a).5,34Due to the low amount of Au loaded on the Cu2O MPS,the plasmon resonance of Au NPs is weaker than the absorption of Cu2O,and the hardly observed adsorption peak of Au SPR might be overlapped with the absorption peak of Cu2O and lead a broaden spectrum feature of Au/Cu2O HGS.
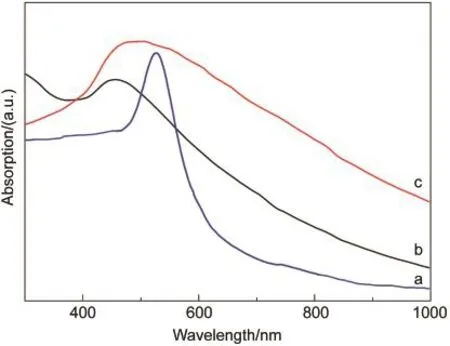
Fig.4 UV-Vis absorption spectra of(a)Au NPs with~4 nm,(b)Cu2O MPS,and(c)Au/Cu2O HGS

Fig.5 XPS results of theAu/Cu2O HGS
The element analysis of the Au/Cu2O HGS examined by XPS was shown in Fig.5.The XPS spectra gave strong signals at around 932.1,952.0,83.9,and 87.6 eV,the former two correspond to the Cu 2p3/2and Cu 2p1/2of Cu+,35while the latter two can be assigned to metallic Au0.36Although the signals related to Cu2+states were also observed(satellite peaks at 934.7,943.8,and 954.6 eV),they are very weak.According to the previous reports,it may due to the adsorption of ambient CO2and/or hydration during the sample handling(e.g.,surface CuCO3and/or Cu(OH)2),which is always not detectable by XRD due to the small amount and the poor crystalline.33The predominant pure-metallic Au0component observed herein excludes the presence of any Au-Cu alloys(e.g.,Cu3Au).33Thus,the XPS study also reconfirms the formation of binary nanocomposites of theAu NPs and Cu2O.
3.2 Formation mechanism
Due to the quite different standard reduction potential=+0.15 V),Cu2O inclined to be oxidized by HAuCl4.To keep the Cu2O mesoporous structure intact with Au NPs loading,the key factor is to ensure the redox action betweenions and Cu2O not to occur before the introduction of NaBH4.Therefore,the L-cysteine molecules with bi-functional groups(-NH2and -COOH)act as a linker for in-situ growth of Au NPs on Cu2O MPS.The strong coordination ability between-COOH group and Cu atoms in Cu2O MPS makes the L-cysteine bind to the Cu2O.WhenAuCl-4is introduced,the bonding between Au3+and-NH2group promote the adsorption ofonto the surface of the pores of the Cu2O MPS,which ensured the in-situ reduction of AuCl-4and the formation of theAu/Cu2O heterogeneous structures.
When distilled water was used as the reaction solvent,the solution color simultaneously transformed into dark green just after HAuCl4solution was added.This rapid process without using the reducing agent NaBH4could be expressed by Eq.(1).

From the corresponding SEM image as shown in Fig.6a,some sheet-like structures were obvious in the products,which is the characteristic structure feature of CuO.37The EDX analysis(Fig.S2c)confirms that the sheet-like structures are composed of Cu and O,and the Cu/O molar ratio of the product is 51/49 that can be ascribed to CuO.From the HRTEM image(Fig.S2b)recorded from the edge of the structures(as indicated by the framed area in Fig.S2a),lattice fringes with interplanar spacing of 0.231 nm can be ascribed to the(111)crystal plane of the CuO.The CuO sheet-like structures may be the by-products when the Eq.(1)was happened that Cu2O MPS was oxidized into CuO by oxygen in the air.Furthermore,some larger Au NPs can be found independent with the Cu2O MPS.The larger Au NPs may result from the fast reduction of Au seeds and the subsequent quick seeded growth process.38It is well documented that the additional ethanol in deionized water can slow down the formation rate of inorganic nanocrystals.39When the solvent changed into the mixture of 10.0 mL distilled water and 5.0 mL ethanol,it can be seen that the sheet structures are decreased(Fig.6b).This result illustrates that the degree of oxidation for Cu2O is declined by the addition of ethanol.Furthermore,some of hollow spheres may cause by the corrosion of the production of HCl.When increasing the amount of ethanol to 10.0 mL,the sheet structures significantly reduced.Therefore,the use of ethanol retarded the redox be-tween AuCl-4and Cu2O and facilitated the absorption of AuCl-4onto the surface of the pores of the Cu2O MPS.After the strong reducing agent NaBH4was added,a rapid redox reaction between HAuCl4and NaBH4could happen as shown in Eq.(2).9H2O+3NaBH4+8HAuCl4=8Au+3NaCl+29HCl+8H3BO3(2)The fast reduction is beneficial for the fast nucleation of Au,which produces small sizedAu NPs.

Fig.6 SEM images of theAu/Cu2O HGS obtained with different solution of(a)15.0 mLdeionized water,(b)10.0 mLdeionized water with 5.0 mLethanol,and(c)5.0 mLdeionized water with 10.0 mLethanol
3.3 Photocatalytic degradation of MB
To avoid the adsorption of the negatively charged dyes such as methyl orange,32the photocatalytic activities of the as-prepared Cu2O HGS were investigated on the decomposition of positively charged methylene blue(MB)under visible-light irradiation.Fig.7A summarized the activities of the photocatalyst toward MB degradation through monitoring the adsorption intensity at 664 nm versus time.Before visible-light irradiation,the mixed solution containing the catalyst and MB was stirred in the dark for 30 min to ensure that MB was adsorbed to saturation on the surface of catalysts.In the blank test(without catalyst),Ct/C0(relative concentration)of MB was degraded by only 4%after visible-light irradiation for 120 min(curve a in Fig.7A).However,the degradation rate was significantly improved in the presence of the Cu2O MPS with a slight adsorption capacity in the range of 0.04-0.05 of Ct/C0.Under the same conditions,Au/Cu2O HGS illustrated a higher photocatalytic activity(curve c in Fig.7A)than Cu2O MPS.MB degraded to 62%by the Cu2O MPS(curve b in Fig.7A)after 120 min;by contrast,Au/Cu2O HGS decomposed the MB to 85%after 120 min.Therefore,the photocatalytic activity was enhanced afterAu NPs loaded on the Cu2O MPS.

Fig.7 (A)Curves of the photocatalytic activities on the decomposition of MB concentration and(B)apparent reaction rate constant versus visible irradiation time in the presence of different Cu2O catalysts
The photodegradation of MB could be described as a firstorder reaction by using a simplified Langmuir-Hinshelwood model,40-42when C0is very small:ln(Ct/C0)=-kt,where k is the apparent first-order reaction rate constant.Fig.7B shows the linear relationship represented by the ln(Ct/C0)versus reaction time t for different catalysts employed in this work.As all these plots match the first-order reaction kinetics very well,the apparent reaction rate constant(k)can be calculated from the rate equation ln(Ct/C0)=-kt(Fig.7B).The reaction rate constants for the photodegradation of MB were 4×10-4,8.2×10-3,and 1.43×10-2min-1for the blank experiment,in the presence of Cu2O and Au/Cu2O,respectively.The kinetic reaction constants k of MB photodegradation in the presence of Au/Cu2O were 1.74 times that of the reaction in the presence of pure Cu2O MPS.
The enhanced photocatalytic activities are attributed to the loaded Au NPs on Cu2O,which may act as electron sink to slow down the recombination of the photogenerated e-/h+pairs in Cu2O so as to improve the separation on its surfaces.The Fermi level of Au is-5.1 eV,43which is lower than that of Cu2O.As the Au/Cu2O heterojunction formed,an interfacial charge equilibrium will be established through electrons transfer from Au to Cu2O(Scheme 1a).When the as-obtained Au/Cu2O is excited by visible light,electrons in the conduction band(VB)are excited to the valence band(CB);meanwhile,holes are simultaneously generated in the VB.Since the bottom of the CB of Cu2O is higher than the new equilibrium Fermi level of Au/Cu2O,the photo-generated electrons will transfer from the CB of Cu2O to the Au NPs until new interfacial charge equilibrium is reached(Scheme 1b).Due to the Schottky barrier formed at the metal-semiconductor interface,Au NPs will act as electron sink that enhances the separation of photogenerated e-/h+pairs,and prolong their lifetime.The photogenerated holes in the VB can be trapped by OH-,resulting in generation of hydroxyl radical(·OH).Furthermore,the exposed{111}surface of Cu2O MPS possess active“Cu”atoms which tend to adsorb O2that will capture photogenerated electrons,and then lead to the formation of superoxide anion radical(·O-2).Finally,the·O-2radicals are all reduced to·OH radicals.Due to the strong oxidization of·OH free radicals(the reduction potential of·OH is about 2.8 V),those radicals attack organic dye and cause dye molecules photodegradation under the irradiation of visible light.
Furthermore,the SPR of Au NPs anchored on Cu2O MPS may also enhance the visible-light photocatalytic efficiency of the Au/Cu2O HGS.It has been demonstrated that SPR-induced local electric field enhancement in the neighborhood of metal NPs could accelerate the generation of e-/h+pairs in the semiconductor.23,44Under visible-light illumination,electric fields are spatially inhomogeneous and intensive in the vicinity of Au NPs,24which would induce rapid formation of e-/h+pairs on the Cu2O surface region near the Au NPs.Therefore,more photoelectrons are generated and could produce more·OH radicals which attack organic dye and cause dye molecules photodegradation under the irradiation of visible-light.

Scheme 1 Schematic illustration of(a)energy level diagram ofAu/Cu2O interface before visible-light irradiation and(b)charge separation process and photocatalytic mechanism of theAu/Cu2O GHS under visible-light irradiation
4 Conclusions
In summary,Au/Cu2O GHS have been successfully prepared through in-situ growth of Au NPs on the surfaces of Cu2O MPS by using a facile wet-chemical reduction.The Au/Cu2O exhibits higher visible-light photocatalytic activities than pure Cu2O counterpart for the degradation of MB.Au NPs are believed to work in two ways to improve the photocatalytic activities.On the one hand,Au NPs served as an electron sink to allow the quick separation of photogenerated electrons and holes and prolong their lifetime to have sufficient time to participate the overall photocatalytic reactions;On the other hand,the SPR-induced local electric field enhancement on Au NPs may increase the generation of electrons and holes of Cu2O under visible-light irradiation,and thus improve the photocatalytic efficiency of the Au/Cu2O HGS.We envisage that this strategy of the heterostructure synthesis involving loaded noble metal NPs on semiconductor surface may have important impact on the future development of highly efficient visible light photocatalyst for organic pollutant degradation.
Supporting Information: available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1) Fujishima,A.;Honda,K.Nature 1972,238,37.doi:10.1038/238037a0
(2) Zou,Z.G.;Ye,J.H.;Sayama,K.;Arakawa,H.Nature 2001,414,625.doi:10.1038/414625a
(3)Wang,Z.H.;Zhao,S.P.,Zhu,S.Y.;Sun Y.L.;Fang,M.CrystEngComm 2011,13,2262.doi:10.1039/c0ce00681e
(4) Fan,H.B.;Zhang,D.F.;Guo,L.Acta Phys.-Chim.Sin.2012,28,2214.[范海濱,張東鳳,郭 林.物理化學(xué)學(xué)報(bào),2012,28,2214.]doi:10.3866/PKU.WHXB201206122
(5) Pan,Y.L.;Deng,S.Z.;Polavarapu,L.;Gao,N.Y.;Yuan,P.Y.;Sow,C.H.;Xu,Q.H.Langmuir 2012,28,12304.doi:10.1021/la301813v
(6)Kochuveedu,S.T.;Oh,J.H.;Do,Y.R.;Kim,D.H.Chem.Eur.J.2012,18,7467.
(7) Shang,Y.;Sun,D.;Shao,Y.M.;Zhang,D.F.;Guo,L.;Yang,S.H.Chem.Eur.J.2012,18,14261.doi:10.1002/chem.v18.45
(8)Subramanian,V.;Wolf,E.E.;Kamat,P.V.J.Am.Chem.Soc.2004,126,4943.doi:10.1021/ja0315199
(9)Tong,G.X.;Guan J.G.;Xiao,Z.D.;Huang,X.;Guan,Y.J.Nanopart.Res.2010,12,3025.doi:10.1007/s11051-010-9897-2
(10)Tong,G.X.;Guan J.G.;Zhang,Q.J.Mater.Chem.Phys.2011,127,371.doi:10.1016/j.matchemphys.2011.02.021
(11)Wei,S.Q.;Ma,Y.Y.;Chen,Y.Y.;Liu,L.;Liu,Y.;Shao,Z.C.J.Hazard.Mater.2011,194,243.doi:10.1016/j.jhazmat.2011.07.096
(12) Hara,M.;Kondo,T.;Komoda,M.;Ikeda,S.;Shinohara,K.;Tanaka,A.;Kondo J.N.;Domen,K.Chem.Commun.1998,357.
(13)Zhou,W.W.;Yan,B.;Cheng,C.W.;Cong,C.X.;Hu,H.L.;Fan,H.J.;Yu,T.CrystEngComm 2009,11,2291.doi:10.1039/b912034n
(14) Cao,Y.B.;Fan,J.M.;Bai,L.Y.;Yuan,F.L.;Chen,Y.F.Cryst.Growth Des.2010,10,232.doi:10.1021/cg9008637
(15) Li,H.;Ni,Y.H.;Cai,Y.F.;Zhang,L.;Zhou,J.Z.;Hong,J.M.;Wei,X.W.J.Mater.Chem.2009,19,594.doi:10.1039/b818574c
(16)Xu,H.L.;Wang,W.Z.;Zhu,W.J.Phys.Chem.B 2006,110,13829.doi:10.1021/jp061934y
(17) Sun,S.D.;Zhang,H.;Song,X.P.;Liang,S.H.;Kong,C.C.;Yang,Z.M.CrystEngComm 2011,13,6040.doi:10.1039/c1ce05597f
(18) Deo,M.;Shinde,D.;Yengantiwar,A.;Jog,J.;Hannoyer,B.;Sauvage,X.;Moreb,M.;Ogale,S.J.Mater.Chem.2012,22,17055.doi:10.1039/c2jm32660d
(19)Wang,Y.B.;Zhang,Y.N.;Zhao,G.H.;Tian,H.Y.;Shi,H.J.;Zhou,T.C.ACS Appl.Mater.Interfaces 2012,4,3965.doi:10.1021/am300795w
(20) Cao,S.W.;Yin,Z.;Barber,J.;Boey,F.Y.C.;Loo,S.C.J.;Xue,C.ACS Appl.Mater.Interfaces 2012,4,418.doi:10.1021/am201481b
(21) Georgekutty,R.;Seery,M.K.;Pillai,S.C.J.Phys.Chem.C 2008,112,13563.doi:10.1021/jp802729a
(22)Wang,P.;Huang,B.B.;Qin,X.Y.;Zhang,X.Y.;Dai,Y.;Wei,J.Y.;Whangbo,M.H.Angew.Chem.Int.Edit.2008,47,7931.doi:10.1002/anie.v47:41
(23) Jiang,J.;Zhang,L.Z.Chem.Eur.J.2012,18,6360.doi:10.1002/chem.201102606
(24)Wang,H.;You,T.T.;Shi,W.W.;Li,J.H.;Guo,L.J.Phys.Chem.C 2012,116,6490.doi:10.1021/jp212303q
(25) Li,X.Z.;Li,F.B.Environ.Sci.Technol.2001,35,2381.doi:10.1021/es001752w
(26)Zhang,H.;Wang,G.;Chen,D.;Lv,X.J.;Li,J.H.Chem.Mater.2008,20,6543.doi:10.1021/cm801796q
(27) Hou,W.B.;Cronin,S.B.Adv.Funct.Mater.2012,23,1612.
(28) Hirakawa,T.;Kamat,P.V.J.Am.Chem.Soc.2005,127,3928.doi:10.1021/ja042925a
(29) Costi,R.;Saunders,A.E.;Elmalem,E.;Salant,A.;Banin,U.Nano Lett.2008,8,637.doi:10.1021/nl0730514
(30)Jin,Z.;Xiao,M.D.;Bao,Z.H.;Wang,P.;Wang,J.F.Angew.Chem.Int.Edit.2012,51,6406.doi:10.1002/anie.201106948
(31)Li,C.C.;Zheng,Y.P.;Wang,T.H.J.Mater.Chem.2012,22,13216.doi:10.1039/c2jm16921e
(32) Shang,Y.;Zhang,D.F.;Guo,L.J.Mater.Chem.2012,22,856.doi:10.1039/c1jm14258e
(33)Pang,M.L.;Wang,Q.X.;Zeng,H.C.Chem.Eur.J.2012,46,14605.
(34) Zhang,D.F.;Niu,L.Y.;Jiang,L.;Yin,P.G.;Sun,L.D.;Zhang,H.;Zhang,R.;Guo,L.;Yan,C.H.J.Phys.Chem.C 2008,112,16011.doi:10.1021/jp803102h
(35) Zhang,D.F.;Zhang,H.;Shang,Y.;Guo,L.Cryst.Growth Des.2011,11,3748.doi:10.1021/cg101283w
(36)Zhang,J.;Liu,X.H.;Wang,L.W.;Yang,T.L.;Guo,X.Z.;Wu,S.H.;Wang,S.R.;Zhang,S.M.J.Phys.Chem.C 2011,115,5352.doi:10.1021/jp110421v
(37) Sun,D.;Yin,P.G.;Guo,L.Acta Phys.-Chim.Sin.2011,27,1543.[孫 都,殷鵬剛,郭 林.物理化學(xué)學(xué)報(bào),2011,27,1543.]doi:10.3866/PKU.WHXB20110619
(38)Gu,J.;Zhang,Y.W.;Tao,F.Chem.Soc.Rev.2012,41,8050.doi:10.1039/c2cs35184f
(39)Wang,Z.Y.;Luan,D.Y.;Boey,F.Y.C.;Lou,X.W.J.Am.Chem.Soc.2011,133,4738.doi:10.1021/ja2004329
(40) Peng,C.;Jiang,B.W.;Liu,Q.;Guo,Z.;Xu,Z.J.;Huang,Q.;Xu,H.J.;Tai,R.Z.;Fan,C.H.Energy Environ.Sci.2011,4,2035.doi:10.1039/c0ee00495b
(41)Zuo,X.L.;Peng,C.;Huang,Q.;Song,S.P.;Wang,L.H.;Li,D.;Fan,C.H.Nano Res.2009,2,617.doi:10.1007/s12274-009-9062-3
(42) Zhang,N.;Liu,S.Q.;Fu,X.Z.;Xu,Y.J.J.Phys.Chem.C 2011,115,9136.doi:10.1021/jp2009989
(43)Subramanian,V.;Wolf,E.E.;Kamat,P.V.J.Am.Chem.Soc.2004,126,4943.doi:10.1021/ja0315199
(44) Wu,J.L.;Chen,F.C.;Hsiao,Y.S.;Chien,F.C.;Chen,P.L.;Kuo,C.H.;Huang,M.H.;Hsu,C.S.ACS Nano 2011,5,959.doi:10.1021/nn102295p

