Synthesis of MgO-Al2O3/ZSM-5 by Solid State Reaction for Propane Dehydrogenation
Zhou Shijian; Zhou Yuming; Sheng Xiaoli; Zhang Yiwei; Zhang Zewu; Shi Junjun; Kong Jie
(School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189)
Synthesis of MgO-Al2O3/ZSM-5 by Solid State Reaction for Propane Dehydrogenation
Zhou Shijian; Zhou Yuming; Sheng Xiaoli; Zhang Yiwei; Zhang Zewu; Shi Junjun; Kong Jie
(School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189)
Solid-state grinding is a simple and effective method to introduce guest species into the channels of microporous materials through filling. The structure and the surface acidity of the materials were obtained from BET isotherms and NH3-TPD, respectively. XRD, UV-vis, UV diffuse-reflectance, and TEM were used to characterize the phases, and the morphology, respectively. The clustered layers of MgO-Al2O3phases were formed in the internal pore surface and were highly dispersed inside the channels of the ZSM-5 host. So the volume of MgO-Al2O3/ZSM-5 composite was larger than the ZSM-5 zeolite itself and some mesoporous channels appeared when Mg/Al species entered the channels. Meanwhile, new acid sites emerged in MgO-Al2O3/ZSM-5 composite and the acid amount of the sample changed. The improved Pt dispersion and the increased acid content would cause the increase of propane conversion and the modification of selectivity during the reaction.
MgO-Al2O3; solid state reaction; PtSn; propane dehydrogenation
1 Introduction
Propene as a kind of important chemical raw materials has gained more and more attention in chemical processing. It has found wide application in the production of polypropene, polyacrylontrile, acrolein, glycerine, isopropanol, and other chemicals. Extensive studies have been made on supported Pt or Cr catalysts[1]. However, the catalyst deactivation due to coke formation is inevitable because of the rigorous reaction conditions. Therefore, it is the key to develop a new catalyst possessing high activity, stability and selectivity to promote the propene yield. The ZSM-5 zeolite, which is one of the crystalline microporous catalysts, is widely used in various hydrocarbon processing thanks to its strong catalytic performance and excellent thermal, hydrothermal and chemical stabilities[2-7]. Kumar, et al.[8]investigated the influence of the pore geometry of catalyst supports on the Pt catalytic properties for propane dehydrogenation and pointed out that the microporous ZSM-5 zeolite was a better catalytic support than the mesoporous SBA-15 material. In our previous study, many metal species were introduced in the Pt/ZSM-5 catalyst by the impregnation method[9-12]. It is confirmed that Sn not only reduces coke formation by suppressing side reactions to increase catalyst stability, but also increases the dispersion of Pt by its geometrical effect to enhance the catalyst activity. And the addition of Na, La, and Mg species can neutralize the strong acid sites on the surface of the catalysts and improve the relationship between the metals and the support.
Solid-state grinding is a complementary means to the conventional technique and is applied in producing crystalline microporous and mesoporous materials[13-14]. Many metal species had been introduced in the channels of the materials by this method[14-17]. Li, et al.[18]claimed that the Mo/ZSM-5 catalysts which were synthesized by solid-state reaction showed that the Mo species were well dispersed in the internal and external surface of the ZSM-5 zeolite, while the framework of ZSM-5 zeolite was retained. Wang, et al.[19]confirmed that solid-state grinding not only is a simple and effective method to introduce guest species into the channels of ordered mesporous materials through filling in different degrees, but also leads to guests with similar ordered mesostructures to the hosts. Wang, et al.[17]found out that CuO was well dispersed in the SBA-15 material, which had taken into account two additional factors. The first is the increasednumber of Cu—O—Si interaction sites due to the lack of surface silanol groups, the second is the confined space between the template aggregate and the silica wall where the guest is inserted into the channels of the support. The space limitation in SBA-15 inhibits aggregation of guest species. Zhang, et al.[9,20]reported that Na+(0.196 nm), La3+(0.119 nm) and Sn4+(0.14 nm) could enter the main channels of ZSM-5 zeolite during catalyst preparation which is contributed to the fact that the average diameter of these values was less than the pore mouth diameter of the ZSM-5 zeolite. It is known that the average diameter of Mg2+and Al3+is 0.072 nm and 0.045 nm, respectively, which would make the ions enter the channels of the zeolite. Solid-state salt inclusion is a rapid and efficient route to fabricate a metal species with good dispersion in the porous materials. As shown in the Scheme 1, the adhesion energy of the wall and the template in materials strongly affects the dispersion of the guest species in the channels. The weak interaction between the wall and the template provides a real possibility for the guest precursor to infiltrate into the occluded pores and form a highly dispersed layer of guest species. Mg/Al species are introduced into the channels to modify thereby the structural properties of the ZSM-5 samples, resulting in the emergence of some new acid sites. The prepared samples were characterized by XRD, BET isotherm, TEM, NH3-TPD, and UV-vis. Besides, the application of these materials as catalyst supports for propane dehydrogenation was analyzed. The performance of these catalysts in the propane dehydrogenation is illustrated hereinafter.
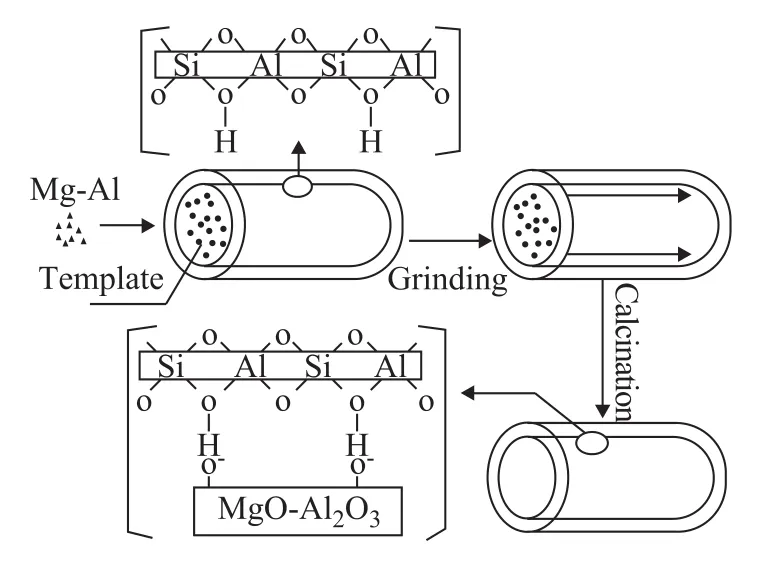
Scheme 1 Schematic diagram of the described solid-state procedure for loading oxide species
2 Experimental
2.1 Synthesis of the zeolite
ZSM-5 zeolite with template was hydrothermally synthesized according to Zhang and co-workers[21]. MgOAl2O3/ZSM-5 composite was synthesized by adding Mg(NO3)2·6H2O and Al(NO3)3·9H2O into the channels. Mg(NO3)2·6H2O and Al(NO3)3·9H2O at an Al/Mg molar ratio of 2 were ground with ZSM-5 powder thoroughly in the mortar. The obtained mixture was calcined at 600 ℃and then cooled down to room temperature.
2.2 Catalyst preparation
PtSn/MgO-Al2O3/ZSM-5 catalyst was prepared by a coimpregnation method on the support. The nominal composition of this catalyst covered 0.5 wt% of Pt and 1.0 wt% of Sn. Afterwards, the catalyst was calcined at 500 ℃ for 5 h, and then reduced under H2flow at 500 ℃ for 8 h.
2.3 Catalysts characterization
X-ray diffraction (XRD) patterns of different samples were obtained on a XD-3A X-ray powder diffractometer coupled to a copper anode tube. The Kα radiation was selected with a diffracted beam monochromator. An angular range 2θfrom 5° to 70° was recorded using step scanning and long counting times to determine the positions of the ZSM-5 peaks.
The nitrogen adsorption-desorption isotherms were measured at -196 ℃ on a Micromeritics ASAP2000 apparatus. Before measurements, the samples were degassed at 300 ℃and 1×10-3torr. The pore size distribution curve came from the analysis of the desorption branch of the isotherm. The specific surface area was obtained using the BET method. The micropore volume, mesopore volume, and external surface area were calculated by means of the t-plot method. The pore size was reported as the average pore diameter.
Surface acidity was measured by NH3-TPD using a Micromeritics ASAP2750 apparatus. The sample (0.15 g) was preheated at 500 ℃ for 1 h, and then cooled down to room temperature in flowing He. At this temperature, sufficient pulses of NH3were injected until adsorption saturation. TPD was carried out from 100 ℃ to 550 ℃ at a heating rate of 10 ℃/min using helium (at a flow rate of 30 ml/min)as the carrier gas.
UV-vis spectra were recorded, in different time intervals, between 200 and 800 nm using a 1-cm path length quartz cuvette and pure DMF was used as the reference on a UV-1100 spectrophotometer.
2.4 Catalytic evaluation
Propane dehydrogenation was carried out in a conventional quartz tubular micro-reactor. The catalyst (1.5 g) was placed into the center of the reactor. Reaction was conducted at: a reaction temperature of 590 ℃, a reaction pressure of 0.1 MPa, a H2/C3H8molar ratio of 0.25, and a propane weight hourly space velocity (WHSV) of 3.0 h-1. The reaction products were analyzed with an online GC-14C gas chromatograph equipped with an activated alumina packed column and a flame ionization detector (FID). The conversion of propane is defined as the percentage of propane converted to all different products. The selectivity to propene is defined as the amount of the obtained propene divided by the amount of reactant converted to all products.
3 Results and Discussion
3.1 Catalyst characterization
The XRD patterns of different samples are displayed in Figure 1. Apparently, it can be observed that all the samples have similar sharp intensity peaks in XRD patterns, implying that the structure of ZSM-5 zeolite is well preserved during the sample preparation. Compared with the XRD pattern of ZSM-5 zeolite (see Figure 1(1)), the characteristic peak intensities of other patterns (see Figure 1(2), 1(3), and 1(4)) below 10° decreased with the addition of Mg/Al species. It is well-known that the low-angle XRD intensities in the pattern of ZSM-5 zeolite are sensitive to the presence of any species inside the channels[18]. The decrease of peak intensity of low-angle in the XRD patterns of different samples also implies the entry of Mg/ Al species into the channels. It can be seen from Figure 1 that crystalline phases of magnesium oxides were detected in the MgO/ZSM-5 composite (at 2θ=43° and 62°), indicating that MgO species appeared on the surface of the ZSM-5 zeolite. Zhang, et al.[22]mentioned that Al species might migrate into the zeolite framework during calcination so as to produce some acid sites of moderate intensity, which consequently increased the acid amount of the catalyst. The fact that MgO-Al2O3/ZSM-5 sample has no crystalline phases of Mg/Al species is ascribed to the formation of clustered layers of MgO-Al2O3phase in the internal pore surface and the high dispersion of Mg/Al species inside the channels of the ZSM-5 host.
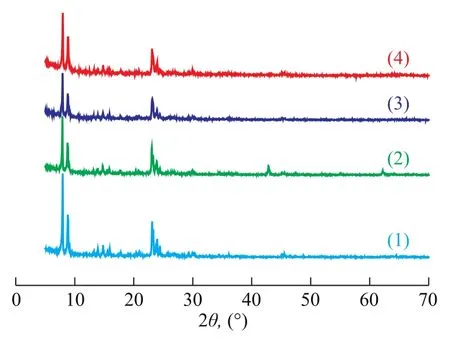
Figure 1 XRD patterns of different samples
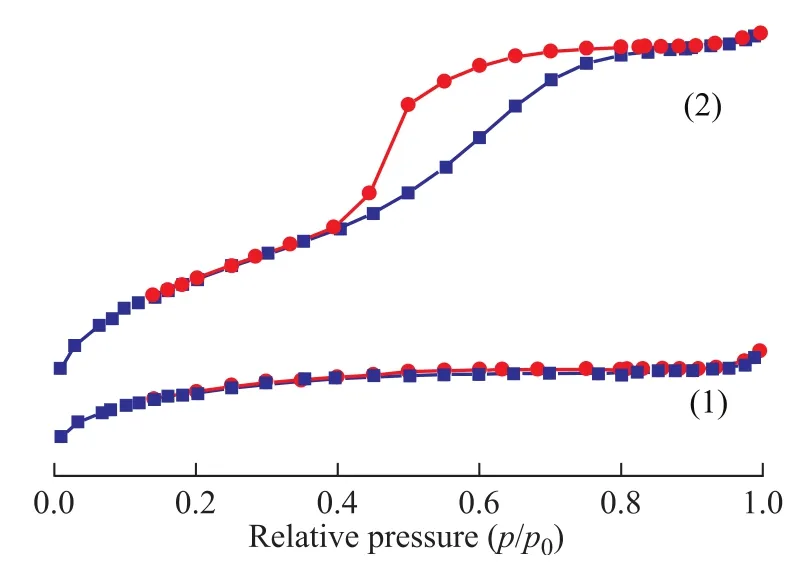
Figure 2 N2adsorption-desorption isotherms of different samples
As it is shown in Figure 2, the MgO-Al2O3/ZSM-5 composite demonstrates a type IV N2adsorption-desorption isotherm with H4 hysteresis capillary condensation at 0.2<(p/p0)<0.8 due to the presence of a number of mesopores (3.5—4.5 nm in size). However, the ZSM-5 zeolite shows a type I isotherm, which is characteristic of microporous materials[23]. The surface area of MgOAl2O3/ZSM-5 composite is 268.3 m2/g, which is much smaller than ZSM-5 zeolite (331.5 m2/g). But the volume of MgO-Al2O3/ZSM-5 composite is 0.123 cm3/g, which is much larger than ZSM-5 zeolite (0.025 cm3/g). These results suggest that Mg/Al species entered the channelsto make some pores plugged[7,24]and meanwhile some mesoporous channels appeared. Mesoporous aluminosilicate materials must be more appropriate for resolving diffusion and mass transfer limitations of microporous zeolites[3,25]. However, the nitrogen adsorption-desorption isotherm of MgO-Al2O3/ZSM-5 composite is also not so well, which must be attributed to the fine dispersion of Mg/Al species on the internal pore surfaces of ZSM-5 zeolite, because Mg/Al species can enhance the roughness of the pore surface and partially block the pore structure.
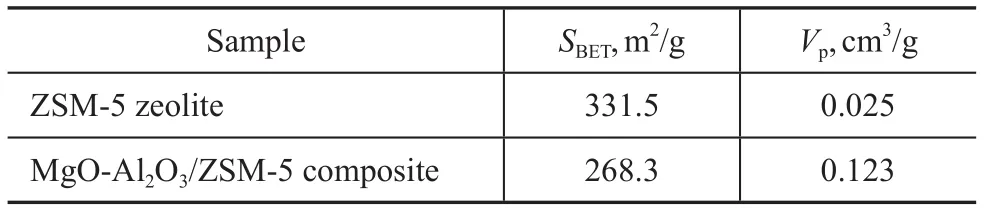
Table 1 Data on characterization of different samples
Figure 3 depicts the UV-vis diffuse reflectance spectra of different samples. In the prepared ZSM-5 sample, the weak interaction between the silica wall and the template aggregates provides a real possibility for the guest precursor to infiltrate into the occluded pores and form a highly dispersed layer of guest species. Mg/Al species are well dispersed inside the channels because the space limitation inhibits the aggregation of guest species. In Figure 3, new bands that appear at around 350 nm and 400 nm for MgO-Al2O3/ZSM-5 composite have no equivalent in the spectra of ZSM-5 zeolite, confirming the infiltration of precursor salt into the occluded pores of the as-prepared sample[17]. As a consequence, the weak interaction force between the silica wall and the template lead to a easy infiltration of the precursor salt into the confined space between them.
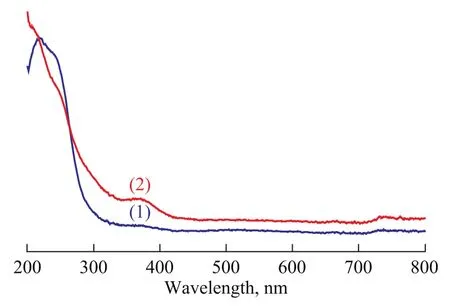
Figure 3 UV diffuse-reflectance spectra of different samples
The acid content on the surface of support was examined by NH3-TPD method, which was a widely used method for characterization of the acid strength and the amount of acid sites on ZSM-5 zeolite[26-27]. The low-temperature peak at 110 ℃ corresponds to weak Lewis acidity[28-29], and the low-temperature peak of MgO-Al2O3/ZSM-5 composite is much less than that of the ZSM-5 zeolite. This reveals that the amount of weak Lewis acidity of MgOAl2O3/ZSM-5 composite is less than that of the ZSM-5 zeolite. In comparison with the ZSM-5 zeolite, two other ammonia desorption peaks of MgO-Al2O3/ZSM-5 composite appeared at about 190 ℃ and 570 ℃. The hightemperature peak at 570 ℃ corresponds to the strong acid sites of the sample. Zhang, et al.[20]found out that soluble Al species migrating into the framework of the zeolite would produce some acid sites of moderate intensity. So the ammonia desorption peak at 190 ℃ suggests that some acid sites of moderate intensity emerge, which can be attributed to the combination of Mg/Al species with the Si-OH species of the zeolite. The above results show that incorporating Mg/Al species to the ZSM-5 zeolite could make new acid sites of the zeolite and increase the amount of acidity.
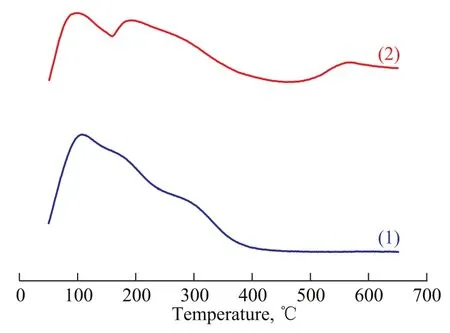
Figure 4 NH3-TPD profiles of different samples
Figure 5 represents the transmission electron micrographs of the PtSn catalysts. In Figure 5 the MgO/Al2O3particles cannot be seen distinctly, which is consistent with the XRD results. Furthermore, the electron micrograph clearly indicates that the structure of MgO-Al2O3/ZSM-5 composite is looser than that of the ZSM-5 zeolite, which might be ascribed to Mg/Al species that enter the channels. This outcome is in accord with the N2adsorption-desorption result, since the volume of MgO-Al2O3/ZSM-5 composite gets much larger. It should be noted that, in our experiments, the agglomeration process is accomplished after the metal incorporation. Therefore, the metal concentration on each sample will depend on the materials used. As shown in Figure 5, the metal dispersion of PtSn/ MgO-Al2O3/ZSM-5 catalyst is much better than the ZSM-5 zeolite. This result indicates that the zeolite after being modified should be beneficial to metal dispersion. This result is in accordance with the information of Bai and co-workers[25], which con firms that incorporation of Mg into the PtSnNa/ZSM-5 catalyst does more than just modifying the acid function of the support, but also modifies the structural characteristics of the metallic phase. Mg species would lead to a high extent of Pt-Sn interaction or improve the metallic particle distribution on the support.

Figure 5 Transmission electron micrographs of different catalysts
3.2 Catalytic performance
The catalytic behavior of different catalysts is depicted in Figure 6. It can be seen from Figure 6 that Mg/Al species have impacts on the catalytic conversion and selectivity of the catalyst. As expected, the increased acid content and metal dispersion lead to higher propane conversion on PtSn/MgO-Al2O3/ZSM-5 catalyst. The more the metal Pt species are dispersed on the surface of the catalyst, the higher the propane conversion would be observed. In consequence, a large amount of acid sites of moderate intensity can catalyze the hydrogenolysis reaction. The dehydrogenation and cracking of propane is assumed to proceed through carbonium-ion intermediates. The higher acid sites generally promote the subsequent cracking re-action of the initially formed C3+carbenium ions[30-31]. So it is obviously found that the propane conversion on PtSn/ MgO-Al2O3/ZSM-5 catalyst is much higher than on the PtSn/ZSM-5 catalyst. Meanwhile, the propane cracking reaction and the generation of carbocation are improved with the increase of acid sites. Therefore, the propene selectivity is decreased with increase in the propane conversion. Even so, the propene selectivity of the PtSn/MgOAl2O3/ZSM-5 catalyst still reaches up to 97%. In short, the PtSn/MgO-Al2O3/ZSM-5 catalyst shows a higher propane conversion with high selectivity to propene, which would have a promising prospect to be used as an industrial catalyst for propane dehydrogenation.
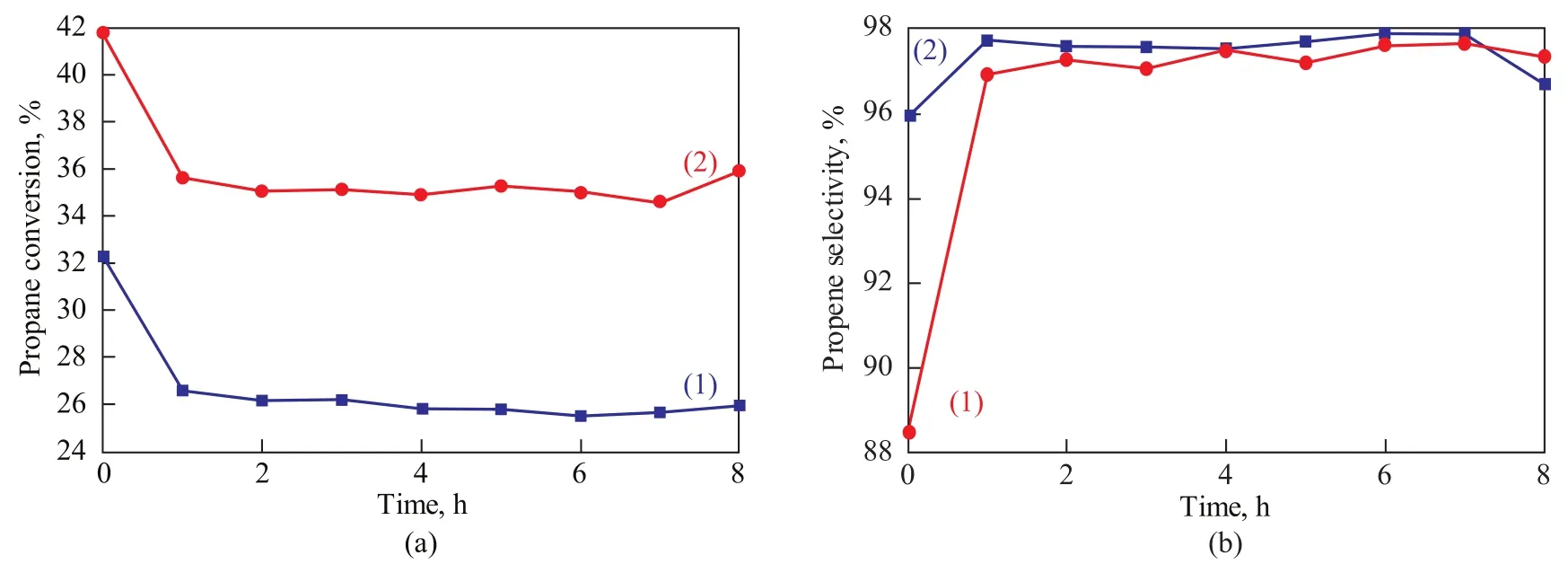
Figure 6 (a) Propane conversion and (b) propene selectivity of different catalysts for propane dehydrogenation
4 Conclusions
The acid properties, Pt particles dispersion, structural properties and catalytic performance of PtSn/MgO-Al2O3/ ZSM-5 catalysts in the propane dehydrogenation reaction were studied. When the Mg/Al species were introduced into the ZSM-5 zeolite, they formed the clustered layers of MgO-Al2O3phase in the internal pore surface and were highly dispersed inside the channels of the ZSM-5 host. After this process, some mesoporous channels would emerge and new acid sites appeared. This result is benef icial to the improvement of the dispersion of Pt particles. Thanks to these effects, a high propane conversion and propene selectivity on the PtSn/MgO-Al2O3/ZSM-5 catalyst could be achieved.
Acknowledgments:The authors are grateful to the financial supports of the National Natural Science Foundation of China (Grant No. 21376051, 21106017, 21306023 and 51077013), the Natural Science Foundation of Jiangsu (Grant No. BK20131288), the Fund Project for Transformation of Scientific and Technological Achievements of Jiangsu Province of China (Grant No. BA2011086), the Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20100092120047), the Key Program for the Scientific Research Guiding Fund of Basic Scientific Research Operation Expenditure of Southeast University (Grant No. 3207043101) and Instrumental Analysis Fund of Southeast University.
[1] Bhasin M M, McCain J H, Vora B V, et al. Dehydrogenation and oxydehydrogenation of paraffins to olefins [J]. Appl Catal A: Gen, 2001, 221(1/2): 397-419
[2] Corma A. From microporous to mesoporous molecular sieve materials and their use in catalysis [J]. Chem Rev, 1997, 97(6): 2373-2420
[3] Tao Y, Kanoh H, Abrams L, Kaneko K. Mesopore-modified zeolites: Preparation, characterization, and applications [J]. Chem Rev, 2006, 106(3): 896-910
[4] Degnan T F, Chitnis G K, Schipper P H. History of ZSM-5 fluid catalytic cracking additive development at Mobil [J]. Microporous Mesoporous Mater, 2000, 35: 245-252
[5] Jorge M, Auerbach S M, Monson P A. Modeling spontaneous formation of precursor nanoparticles in clear-solution zeolite synthesis [J]. J Am Chem Soc, 2005, 127(41): 14388-14400
[6] Bai L Y, Zhou Y M, Zhang Y W, et al. Influence of calcium addition on catalytic properties of PtSn/ZSM-5 catalyst for propane dehydrogenation [J]. Catal Lett, 2009, 129(3/4): 449-456
[7] Zhang Peixin, Zhou Yuming, Duan Yongzheng, et al. Influence of alumina content on catalytic performance of PtSn/ Al2O3/SBA-15 catalysts for propane dehydrogenation[J]. China Petroleum Processing and Petrochemical Technology, 2012, 14(4): 9-16
[8] Kumar M S, Holmen A, Chen D. The influence of pore geometry of Pt containing ZSM- 5, Beta and SBA-15 catalysts on dehydrogenation of propane [J]. Microporous Mesoporous Mater, 2009, 126(1/2): 152-158
[9] Zhang Y W, Zhou Y M, Liu H, et al. Effect of La addition on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation [J]. Appl Catal A: Gen, 2007, 333(2): 202-210
[10] Liu Xuan, Zhou Yuming, Zhang Yiwei, et al. Effect of Ga addition on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation[J]. China Petroleum Processing and Petrochemical Technology, 2011, 13(4): 45-52
[11] Zhang Y W, Zhou Y M, Qiu A D, et al. Effect of Na addition on catalytic performance of PtSn/ZSM-5 catalyst for propane dehydrogenation [J]. Acta Phys-Chim Sin, 2006, 22(6): 672-678
[12] Bai L Y, Zhou Y M, Zhang Y W, et al. Effect of calcination atmosphere on the catalytic properties of PtSnNaMg/ZSM-5 for propane dehydrogenation [J]. Catal Commun, 2009, 10(15): 2013-2017
[13] Xie Y C, Tang Y Q. Spontaneous monolayer dispersion of oxides and salts onto surfaces of supports: Applications to heterogeneous catalysis [J]. Adv Catal, 1990, 37: 1-43
[14] Wang Y M, Wu Z Y, Wang H J, et al. Fabrication of metal oxides occluded in ordered mesoporous hosts via a solidstate grinding route: The influence of host–guest interactions [J]. Adv Funct Mater, 2006, 16(18): 2374-2386
[15] Yue W B, Zhou W Z. Synthesis of porous crystals of metal oxides via a solid-liquid route [J]. Chem Mater, 2007, 19(9): 2359-2363
[16] Medina-Mendoza A K, Cortés-Jácome M A, Toledo-Antonio J A, et al. Highly dispersed uniformly sized Pt nanoparticles on mesoporous Al-SBA-15 by solid state impregnation [J]. Appl Catal B: Environ, 2011, 106(1/2): 14-25
[17] Wang Y M, Wu Z Y, Shi L Y, et al. Rapid functionalization of mesoporous materials: Directly dispersing metal oxides into as-prepared SBA-15 occluded with template [J]. Adv Mater, 2005, 17(3): 323-326
[18] Li B, Li S J, Li N, et al. Structure and acidity of Mo/ZSM-5 synthesized by solid state reaction for methane dehydrogenation and aromatization [J]. Microporous Mesoporous Mater, 2006, 88(1/2/3): 244-253
[19] Wang H N, Li X H, Wang Y M, et al. Pt nanoparticles supported on highly dispersed alumina coated inside SBA-15 for enantioselective hydrogenation [J]. Chem Cat Chem, 2010, 2(10): 1303-1311
[20] Zhang Y W, Zhou Y M, Qiu A D, et al. Effect of alumina binder on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation [J]. Ind Eng Chem Res, 2006, 45(7): 2213-2219
[21] Zhang Y W, Zhou Y M, Huang L, et al. Sn-modified ZSM-5 as support for platinum catalyst in propane dehydrogenation [J]. Ind Eng Chem Res, 2011, 50(13): 7896-7902
[22] Zhang Y W, Zhou Y M, Qiu A D, et al. Effect of hydrothermal treatment on catalytic properties of PtSnNa/ZSM-5 catalyst for propane dehydrogenation [J]. Microporous Mesoporous Mater, 2006, 96(1/2/3): 245-254
[23] Jin H L, Ansari M B, Jeong E Y, et al. Effect of mesoporosity on selective benzylation of aromatics with benzyl alcohol over mesoporous ZSM-5 [J]. J Catal, 2012, 291: 55-62
[24] Bai L Y, Zhou Y M, Zhang Y W, et al. Effect of magnesium addition to PtSnNa/ZSM-5 on the catalytic properties in the dehydrogenation of propane [J]. Ind Eng Chem Res, 2009, 48(22): 9885-9891
[25] Hartmann M. Hierarchical zeolites: A proven strategy to combine shape selectivity with efficient mass transport [J]. Angew Chem Int Ed, 2004, 43(44): 5880-5882
[26] Bocanegra S A, Ballarini A D, Scelza O A, et al. The influence of the synthesis routes of MgAl2O4on its properties and behavior as support of dehydrogenation catalysts [J]. Mater Chem Phys, 2008 ,111(2-3): 534-541
[27] Vogelson C T, Barron A R. Particle size control and dependence on solution pH of carboxylate–alumoxane nanoparticles [J]. J Non-Cryst Solids, 2001, 290(2/3): 216-223
[28] Barthos R, Lónyi F, Onyestyák Gy, et al. An NH3-TPD and -FR study on the acidity of sulfated zirconia [J]. Solid State Ionics, 2001, 141-142: 253-258
[29] Lónyi F, Valyon J. On the interpretation of the NH3-TPD patterns of H-ZSM-5 and H-mordenite [J]. Microporous Mesoporous Mater, 2001, 47(2/3): 293-301
[30] Guisnet M, Gnep NS. Aromatization of propane over GaHMFI catalysts. Reaction scheme, nature of the dehydrogenation species and mode of coke formation [J]. Catal Today, 1996, 31(3/4): 275-292
[31] Katranas T K, Vlessidis A G, Tsiatouras V A, et al. Dehydrogenation of propane over natural clinoptilolite zeolites [J]. Microporous Mesoporous Mater, 2003, 61(1/2/3): 189-198
Recieved date: 2013-04-15; Accepted date: 2013-05-04.
Professor Zhou Yuming, Telephone: +86-25-52090617; E-mail: ymzhou@seu.edu.cn.
- 中國煉油與石油化工的其它文章
- Experimental and Molecular Dynamics Simulations for Investigating the Effect of Fatty Acid and Its Derivatives on Low Sulfur Diesel Lubricity
- Synthesis of TS-1 Films on Porous Supports for Epoxidation of Allyl Chloride by Hydrogen Peroxide
- Effects of Moisture on Rheological Properties of PAV Aged Asphalt Binders
- Spectroscopic Analysis of Structural Transformation in Biodiesel Oxidation
- Microwave-Assisted Decarboxylation of Sodium Oleate and Renewable Hydrocarbon Fuel Production
- Research and Commercial Application of CPP Technology for Producing Light Olefins from Heavy Oil

