Enhanced Film-Forming and Electrochromic Properties by lncorporating Bithiophene into Triphenylamine
OUYANG Mi FU Zhi-Yan Lü Xiao-Jing CHEN Huan-Le HU Bin XIAXu-Feng ZHANG Cheng
(State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology,International Science&Technology Cooperation Base of Energy Materials and Application,College of Chemical Engineering and Materials Science,Zhejiang University of Technology,Hangzhou 310014,P.R.China)
1 lntroduction
Electrochromism is a phenomenon that optical changes occur in chemical materials by a reversible electrochemical process.1-3For electrochromic(EC)conjugated polymers(CPs),the electrochromism is related to the changing of band gaps during the doping-dedoping process.Thus,it is necessary to achieve different kinds of EC propertiesviaadjusting the electronic characters of thep-orbit along the neutral polymer backbone.4Electrochromic materials have been attracting much interest for several decades,as they have several important applications,such as energy-saving“smart”windows,5rear-view mirrors,6electrochromic displays,7and electronic paper or electrochromic painting.8At present,there is significant attention in the design of new EC materials combining polyelectrochromic property,high contrast ratio(a large change in percent transmittance between the colored and bleached states),and fast switching time.9In principles,high contrast ratio originates from significant changes of molecular planarity between two redox states.Thus by designing EC materials with twisted structure in neutral state and planiform structure in charged state,high contrast ratio in EC process would be realized.
Triphenylamine(TPA)derivatives have played important roles in organic optoelectronic devices10,11and electrochromic applications during the past few decades due to their excellent charge transport properties,improved thermal and morphological stabilities.12,13TPA is a highly twisted molecule with twisted angle of 42°to 44°,while under charge state(TPA+),TPA trends to become planar and extends the electron delocalization,leading to obvious color change.In this context,the introduction of TPA and its derivatives to EC materials has become one of the important approaches to obtain high contrast ratio.Usually thepara-position of TPA needs to be protected by grafting electron-donating groups to prevent the oxidation coupling between electrogenerated radical cation of TPA to form tetraphenylbenzidine(TPB),14-17which is considered as an undesired side reaction in EC process as it might lead to irreversible defect after several redox switches.18Recently star-shaped molecule TTPA consisting of a TPA core substituted by thiophene functionalities was reported.19PTTPA as EC material exhibited reasonable electrochromic characters.However,optical contrasts and the switching time could not simultaneously achieve excellent level.Herein we would present a novel EC materialcontainingTPAcoreandperipheralbithiophene groups.
In this study,a novel EC material(TBTPA)consisting of triphenylamine core and peripheral bithiophene groups was synthesized,and the corresponding polymer(PTBTPA)was prepared by electrochemically oxidative cross-linking.The electrochromic and spectroelectrochemical properties of PTBTPA were studied in detail.The differences of the spectroelectrochemical and electrochromic properties of PTBTPA and the reported PTTPAwere also discussed.
2 Experimental
2.1 Materials
Triphenylamine(Aladdin,98%),thiophene(Aladdin,98%),Mg(Aladdin,99%),N-bromosuccinimide(NBS)(ENERGY CHEMICAL,98%),tetrabutylammonium perchlorate(TBAP)(ACROS,99%)were used without further purification.Tetrahydrofuran(THF)(Hangzhou Shuanglin Chemical Reagent Company),chromatography grade acetonitrile(ACN),dichloromethane(DCM)(Hangzhou Shuanglin Chemical Reagent Company)were distilled from CaH2and then stored over 4A molecular sieves.
2.2 Equipments
1H NMR spectra of synthesized products were recorded in CDCl3solution on a Bruker AVANCE III 500 MHz instrument(Bruker,Switzerland).The micro-Fourier-transform infrared(FTIR)spectra of monomers samples and the both polymer films samples at Neutral state peeled off from the indium tin oxide(ITO)electrode were pressed in a diamond cell and then examined between 4000 and 650 cm-1by a Nicolet 6700 FTIR(Thermo Fisher Nicolet,USA),equipped with a nitrogen cooled MCT/A detector and a continuum infrared-microscope.A CHI 660C electrochemical analyzer(CH Instruments,China)was applied to conduct the electrochemical measurements.UV-Vis spectra were recorded on a Shimadzu UV-1800 UVVis spectrophotometer(Shimadzu,Japan).The transmittance and absorbance spectra were recordedin situunder various applied potentials.All experiments were carried out at room temperature.SEM measurements were taken by using a Hitachi S-4800 scanning electron microscopy(Hitachi,Japan).Digital photographs of the polymer films were obtained through the Canon E0S 500D(Canon,Japan)digital camera.
2.3 Synthesis
The synthetic approach of both monomers was outlined in Scheme 1.
2.3.1 tris[4-(2-thienyl)phenyl]amine
The monomer of tris[4-(2-thienyl)phenyl]amine(TTPA)was synthesized according to the reported method by palladiumcatalyzed cross-coupling reactions oftris(4-bromophenyl)amine with Grignard thiophene in THF for 12 h.20Yield:78%.MS:m/z=491(M+).FTIR(KBr):1598,1533,1498,1431,1290,1274,1184,848,818,696 cm-1.1H NMR(CDCl3,500 MHz)δ:7.08(t,3H),7.14(d,6H),7.27(d,6H),7.54(d,6H).
2.3.2 4,4′,4″-tris[4-(2-bithienyl)pheny]amine
The monomerof4,4′,4″-tris[4-(2-bithienyl)pheny]amine(TBTPA)was synthesized from Grignard thiophene and tris(4-(2-bromothienyl)phenyl)amine which was obtained from bromination of PTBTPA.20Yield:70%.MS:m/z=737(M+).FTIR(KBr):1596,1518,1457,1322,1290,1182,833,796,639 cm-1.1H NMR(CDCl3,500 MHz)δ:7.04(dd,3H),7.17(m,9H),7.23(m,9H),7.52(d,6H).
2.4 Electrochemistry
The electropolymerization and measurements were performed in a conventional three-electrode cell with an ITO-coated glass(CSG holding Co.LTD,Rs≤10 Ω-1.the active area:1.0 cm×2.0 cm)as working electrode which was sequencely washed with ethanol,acetone and deionized water under ultrasonic before use,a platinum sheet and a double-junction Ag/AgCl electrode(silver wire coated with AgCl in saturated KCl solution,0.1 mol·L-1TBAP in DCM/ACN(7:3,by volume)solution as the second junction)were applied as the counter electrode and the reference electrode,respectively.All the electrochemistry experiments were carried out at room temperature under N2atmosphere.
3 Results and discussion
3.1 Electrochemical characterization and electropolymerization
The redox behaviors of TTPA and TBTPA were investigatedviacyclic voltammetry(CV)in DCM/ACN(7:3,by volume)using 0.1 mol·L-1tetrabutylammonium perchlorate(TBAP)as supporting electrolyte with scan intervals 0 V/1.4 V and 0 V/1.2 V(vsAg/AgCl),respectively(Fig.1a).In the first CV cycle,TTPA showed a couple of quasi-reversible redox peaks with the oxidative onset potential at 0.92 V,oxidative peak at 1.12 V,and reductive peak at 0.91 V,respectively.By comparison,TBTPA exhibited a couple of reversible redox peaks with the oxidative onset potential at 0.87 V,oxidative peak at 0.99 V,and reductive peak at 0.88 V,respectively.TBTPA showed lower oxidative onset potential than TTPA due to the enhanced conjugated length by adding another thiophene unit.21TBTPA presented better redox reversibility compared with TTPA,which was benefit to the stability in EC process.On subsequent scans in the potential range from 0 to 1.4 V for TTPA and 0 to 1.2 V for TBTPA as shown in Fig.1b,the redox peak current increased in the successive cycles,indicating cross-linking between the thiophene units and the growth of the film on the electrode.After washed in ACN solution,the PTTPA and PTBTPA film exhibited yellow and orange color,respectively.It was worth mentioning that PTTPA film was automatically peeled off from electrode even in polymerization process,which revealed poor adhesion to electrode.This phenomenon was commonly observed in other TPA based EC materials.The reason could be concluded as the low cross-linking efficiency because the oxidative potential of TPA was lower than that of thiophene,resulting in coulometric wastage.By contrast,the adhesion of PTBTPA film to electrode was very better,and no films peeled off from electrode even under sonication.We pointed out that the contact of film and electrode was a basic requirement to achieve high EC performance,and PTBTPA film exhibiting stronger adhesion to electrode clearly satisfy it.

Scheme 1 Synthetic route and chemical structures for monomers TTPAand TBTPA
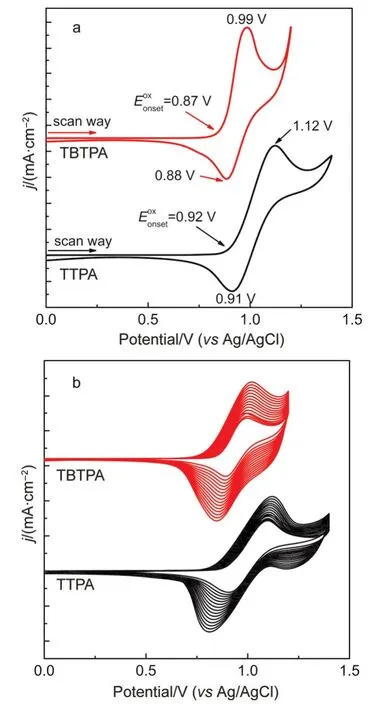
Fig.1 (a)The first CV cycles and(b)repeated cyclic voltammograms of 1.5 mmol·L-1TTPAand TBTPAon ITO electrode in 0.1 mol·L-1TBAPin DCM/ACN(7:3,by volume)at a scan rate of 100 mV·s-1
The polymer films could be preparedviaconstant potentiostatic orviacyclic voltammetry,22And the films of PTTPA and PTBTPA were synthesizedviaconstant potential electrolysis on ITO electrode in DCM/ACN(7:3,by volume)containing 0.1 mol·L-1TBAP as supporting electrolyte and 1.5 mmol·L-1monomer.The redox behaviors of the films were carried out in DCM containing 0.1 mol·L-1TBAP,which were presented in Fig.2a.PTTPA film showed better reversibility than the precursor in solution with redox peaks at 0.91 and 1.07 V,respectively.By comparison,the PTBTPA film showed more polarized redox peaks at 0.87 and 1.07 V.This revealed that there were stronger interactions between electroactive groups(TPA,thiophene)and supporting electrolytes in PTBTPA film.The redox currents of PTTPA and PTBTPA films were correspondingly enhanced by the increase of scan rate(Fig.2b).Linear relationships between scan rates and current intensities indicated that all polymers were well adhered on ITO surface and the electrochemical processes were not limited by diffusion control.23,24
3.2 Morphology
The polymer films of PTTPA and PTBTPA were prepared through constant potential electrolysis in 0.1 mol·L-1TBAP/DCM containing relevant monomers on ITO electrodes.And the morphologies of the neutral PTTPA and PTBTPA films were investigated comparatively by SEM after dedoping at-0.2 V for 30 min in a precursor-free solution.
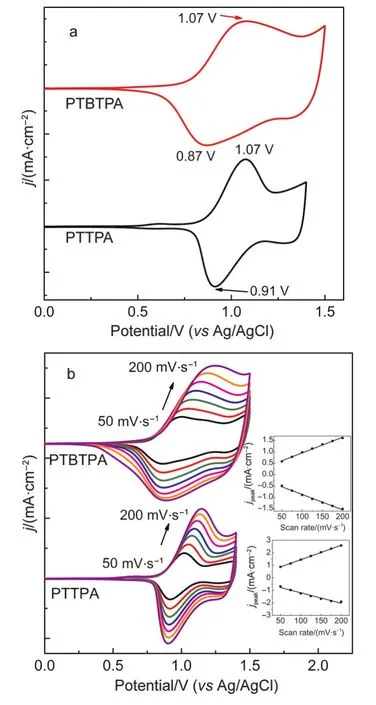
Fig.2 (a)CV curves of PTTPAand PTBTPAfilms in 0.1 mol·L-1TBAPin DCM/ACN(7:3,by volume)at a scan rate of 100 mV·s-1,(b)scan rate dependence of PTTPAand PTBTPAfilms on ITO electrode in 0.1 mol·L-1TBAPin DCM/ACN(7:3,by volume)solution
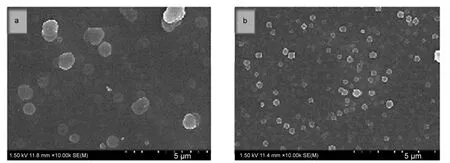
Fig.3 SEM images of(a)PTTPAfilm(1.40 V)and(b)PTBTPAfilm(1.20 V)
As depicted in Fig.3,the surface of two polymer films showed an accumulation state of clusters of globules,and the approximate diameters of these globules were in the range of 500-1000 nm.But the diameter of PTBTPA(500 nm)was smaller than that of the PTTPA(1000 nm).The smaller size of globules of PTBTPA film could provide larger surface area and more contact interface for the insert/extract of charge carrier,which could offer the proof for the following enhanced electrochromic performance.
3.3 Spectroelectrochemistry
Spectroelectrochemical analyses of the resulted polymers were performed in order to investigate their optical and structural responses upon doping process,which proved the evolution of the charge carries in the structure.25,26The electrochromic absorption spectra were monitored by an UV-Vis spectrometer in DCM/ACN(7:3,by volume)containing 0.1 mol·L-1TBAP as supporting electrolyte at different applied potentials.
As shown in Fig.4,TPA-based polymers displayed two transitions due to the TPA-thiophene hyperbranched structure.The largest energy transitions affected the colors of neutral films because the other transitions were not in the visible region dominantly.27PTTPA and PTBTPA films showed the relative maximum absorption peak located at about 440 and 470 nm due to theπ→π*electron transition.Correspondingly,they exhibited yellow and orange color in their neutral state,respectively.The absorption peak of films was red-shifted with the increasing number of peripheral thiophene groups.The reason was mainly attributed to the appearance of 2,2′-bithienyl which located at thepara-position of triphenylamine as donor group and possessed higher electron density and better conjugated characteristic.With the increasing of oxidative potential,formation of charge carriers such as polarons and bipolarons in the PTTPA and PTBTPA backbones caused new absorption bands in near infrared(NIR)while absorptions for the neutral states were decreasing.The new broad absorption tails for PTTPA and PTBTPA were located at around 680 and 720 nm,respectively.During the process,the PTTPA film showed the color changed from olivedrab to dimgry.On the other hand,the films of PTBTPA presented the color from olivegreen to dimgry(see Fig.4b).Moreover,PTBTPA exhibited better ratio of color change(44.7%,calculated by the change of maximum absorption intensity)than PTTPA(40.7%),which revealed the enhanced peripheral thiophene group benefited for the doping process and the ratio of color change.
3.4 Kinetic studies
Optical contrasts were monitored at the maximum absorption of PTTPA and PTBTPA in order to characterize their response times and switching abilities.The films of PTTPA and PTBTPA(coated on ITO electrode with the polymerization charge of 0.02 C)were carried out with a residence time of 3 s at visible region(680 and 720 nm).At NIR wavelength(1100 nm),both the polymer films were carried out with a residence time of 4 s.The switching time was defined as the time required for reach 95%of the full change in absorbance after the switching of the potential.

Fig.4 Spectroelectrochemical spectra of PTTPAand PTBTPAfilms as applied potentials
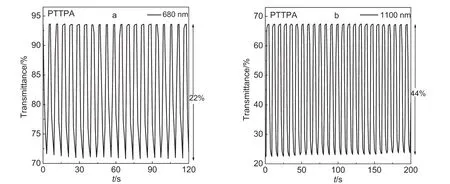
Fig.5 Electrochromic switching response for PTTPAfilm monitored at 680 and 1100 nm in 0.1 mol·L-1TBAPin DCM/ACN(7:3,by volume)solution between-0.2 and 1.3 V
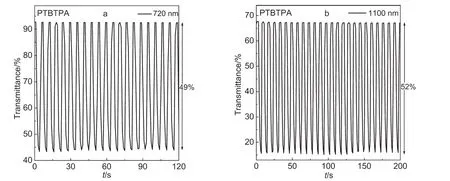
Fig.6 Electrochromic switching response for PTBTPAfilm monitored at 720 and 1100 nm in 0.1 mol·L-1TBAPin DCM/ACN(7:3,by volume)solution between-0.2 and 1.4 V
PTTPA film was switched between-0.2 and 1.3 V.As depicted in Fig.5,the percentage transmittance changes(ΔT)between the neutral and oxidized states were 22%for 680 nm and 44%for 1100 nm.And the switching times were 0.96 s at 680 nm and 0.93 s at 1100 nm,respectively.While PTBTPA film was switched between-0.2 and 1.4 V(Fig.6).The percentage transmittance changes between the neutral and oxidized states were found to be 49%for 720 nm and 52%for 1100 nm.And the switching times were 0.93 s at 720 nm and 0.91 s at 1100 nm.These results showed that TBTPA could simultaneously achieve excellent level in optical contrasts and switching time.
The coloration efficiency(CE)was also an important characteristic for the electrochromic materials.It was defined as the change in the optical density(ΔOD)for the charge consumed per unit electrode area(ΔQ).28The corresponding equations are given below:

whereTbandTcwere the transmittances and colored states,respectively,andηrepresented the coloration efficiency.CE values of PTBTPA and PTTPA were measured at the corresponding wavelength under full doped state(PTTPA:108 cm2·C-1(680 nm),212 cm2·C-1(1100 nm);PTBTPA:198 cm2·C-1(720 nm),285 cm2·C-1(1100 nm)).Both of PTTPA and PTBTPA showed reasonable coloration efficiency.From the result of CE of the two polymers,PTBTPA showed higher coloration efficiency compared with PTTPA.
The above results demonstrated PTBTPA film possessed higher optical contrast than PTTPA film which may be attributed to stronger adhesion to electrode,better doping/dedoping process and smaller globule morphology.The EC performance is acceptable in EC devices,indicating that PTBTPA had its potentials to an efficient EC material.
4 Conclusions
The EC material TBTPA was synthesized and characterized.TBTPA presented better redox reversibility,excellent filmforming property and strong adhesion to electrode relative to the reported TTPA,which satisfied the basic requirement to achieve high EC performance.Furthermore,compared to reported PTTPA,PTBTPA displayed the enhanced EC performance with better contrast ratio of 44.7%,percentage transmittance changes(ΔT)of 49%for 720 nm and 52%for 1100 nm and faster switching response of 0.93 s(720 nm)and 0.91 s(1100 nm).Meanwhile,PTBTPAhad higher coloration efficiency(198 cm2·C-1(720 nm),285 cm2·C-1(1100 nm)).Besides,SEM images illustrated that the surface of PTBTPA film showed an accumulation state of clusters of globules,which was smaller than that of PTTPA.The excellent performance indicated that PTBTPA had its potentials to be an efficient EC material.
(1)Argun,A.A.;Aubert,P.H.;Thompson,B.C.;Schwendeman,I.;Gaupp,C.L.;Hwang,J.;Pinto,N.J.;Tanner,D.B.;MacDiarmid,A.G.;Reynolds,J.R.Chem.Mater.2004,16,4401.doi:10.1021/cm049669l
(2)Verghese,M.M.;Ram,M.K.;Vardhan,H.;Malhotra,B.D.;Ashraf,S.M.Polymer1997,38,1625.doi:10.1016/S0032-3861(96)00655-6
(3) Beaupré,S.;Dumas,J.;Leclerc,M.Chem.Mater.2006,18,4011.doi:10.1021/cm060407o
(4) Quinto,M.;Bard,A.J.J.Electroanal.Chem.2001,498,67.doi:10.1016/S0022-0728(00)00266-7
(5) Azens,A.;Granqvist,C.G.J.Solid State Electrochem.2003,7,64.
(6) Heuer,H.W.;Wehrmann,R.;Kirchmeyer,S.Adv.Funct.Mater.2002,12,89.doi:10.1002/(ISSN)1616-3028
(7) Mortimer,R.J.;Dyer,A.L.;Reynolds,J.R.Displays2006,27,2.doi:10.1016/j.displa.2005.03.003
(8) Shah,J.;Brown,R.M.,Jr.Appl.Microbiol.Biotechnol.2005,66,352.doi:10.1007/s00253-004-1756-6
(9) Sapp,A.S.;Sotzing,A.G.;Reynolds,J.R.Chem.Mater.1998,10,2101.doi:10.1021/cm9801237
(10)Chu,Z.Z.;Wang,D.;Zhang,C.;Zou.D.C.Acta Phys.-Chim.Sin.2012,28(8),2000.[初增澤,王 丹,張 超,鄒德春.物理化學學報,2012,28(8),2000.]doi:10.3866/PKU.WHXB201206071
(11) Pei,J.;Liang,M.;Chen,J.;Tao,Z.L.;Xu,W.Acta Phys.-Chem.Sin.2008,24(11),1950. [裴 娟,梁 茂,陳 軍,陶占良,許 煒.物理化學學報,2008,24(11),1950.]doi:10.1016/S1872-1508(08)60077-7
(12) Hagberg,D.P.;Edvinsson,T.;Marinado,T.;Boschloo,G.;Hagfeldt,A.;Sun,L.C.Chem.Commun.2006,No.21,2245.
(13) Karthikeyan,C.S.;Peter,K.;Wietasch,H.;Thelakkat,M.Sol.Energy Mater.Sol.Cells 2007,91,432.doi:10.1016/j.solmat.2006.10.006
(14)Nelson,R.F.;Adams,R.N.J.Am.Chem.Soc.1968,90,3925.doi:10.1021/ja01017a004
(15) Lapkowskia,M.;Golba,S.;Soloduchoc,J.;Idzikc,K.Synthetic Met.2009,159,2202.doi:10.1016/j.synthmet.2009.09.005
(16)Zhang,C.;Wang,G.H.;Ouyang,M.J.Electroanal.Chem.2011,660,45.doi:10.1016/j.jelechem.2011.06.002
(17) Idzika,K.;Soloducho,J.;Lapkowski,M.;Golba,S.;Electrochim.Acta2008,53,5665.doi:10.1016/j.electacta.2008.03.019
(18) Chiu,K.Y.;Su,T.X.;Li,J.H.;Lin,T.H.;Liou,G.S.;Cheng,S.H.J.Electroanal.Chem.2005,575,95.doi:10.1016/j.jelechem.2004.09.005
(19) Cheng,X.F.;Zhao,J.S.;Cui,C.S.;Fu,Y.Z.;Zhang,X.X.J.Electroanal.Chem.2012,677-680,24.
(20) Cho,J.S.;Uchida,K.;Yoshioka,N.;Yamamoto,K.Sci.Technol.Adv.Mat.2004,5,697.doi:10.1016/j.stam.2004.03.006
(21)Ouyang,M.;Wang,G.H.;Zhang,C.Electrochim.Acta2011,56,4645.doi:10.1016/j.electacta.2011.02.103
(22) Gu,C.;Liu,H.;Hu,D.H.;Zhang,W.S.;Lv,Y.;Lu,P.;Lu,D.;Ma,Y.G.Macromol.Rapid Commun.2011,32,1014.doi:10.1002/marc.v32.13
(23)Sonmez,G.;Schwendeman,I.;Schottland,P.;Zong,K.;Reynolds,J.R.Macromolecules2003,36,639.doi:10.1021/ma021108x
(24)Gu,C.;Dong,W.Y.;Yao,L.;Lv,Y.;Zhang,Z.B.;Lu,D.;Ma,Y.G.Adv.Mater.2012,24,2413.doi:10.1002/adma.201200559
(25)Sonmez,G.Chem.Commun.2005,No.42,5251.
(26)Beyazyildirim,S.;Camurlu,P.;Yilmaz,D.;Gullu,M.;Toppare,L.J.Electroanal.Chem.2006,587,235.doi:10.1016/j.jelechem.2005.11.018
(27)Akpinar,H.;Balan,A.;Baran,D.;ünver,E.K.;Toppare,L.Polymer2010,51,6123.doi:10.1016/j.polymer.2010.10.045
(28)Deepa,M.;Awadhia,A.;Bhandari,S.Phys.Chem.Chem.Phys.2009,11,5674.

