Solid Acid Used as Highly Efficient Catalyst for Esterification of Free Fatty Acids with Alcohols
Zhang Qiuyun; Li Hu; Qin Wenting; Liu Xiaofang; Zhang Yuping; Xue Wei; Yang Song
(State-Local Joint Laboratory for Comprehensive Utilization of Biomass, State Key Laboratory Breeding Base of Green Pesticides and Agricultural Bioengineering, Center for Research and Development of Fine Chemicals, Guizhou University, Guiyang 550025)
Solid Acid Used as Highly Efficient Catalyst for Esterification of Free Fatty Acids with Alcohols
Zhang Qiuyun; Li Hu; Qin Wenting; Liu Xiaofang; Zhang Yuping; Xue Wei; Yang Song
(State-Local Joint Laboratory for Comprehensive Utilization of Biomass, State Key Laboratory Breeding Base of Green Pesticides and Agricultural Bioengineering, Center for Research and Development of Fine Chemicals, Guizhou University, Guiyang 550025)
A novel solid acid catalyst, which was prepared from sodium alginate (SA) and metal chlorides and characterized with XRD and FT-IR spectrometry, was used for the preparation of biodieselviaesterification reaction. The study results showed that the aluminum-alginate complex prepared in a cheap and easy way exhibited high catalytic activity, and a 92.6% conversion of methyl oleate was obtained in the presence of 4 m% of catalyst dosage upon refluxing for 3 h of methanol and acid mixed in a molar ratio of 10:1. It should be noted that the catalyst can be applied to the esterification reaction of fatty acids with various carbon chain length on methanol or different short chain alcohols, indicating that the catalyst is suitable for the preparation of biodiesel from waste oils with a high acid value.
sodium alginate; solid acid; esterification; biodiesel
1 Introduction
In recent years, sustainable development and utilization of alternative fuels have become a hot spot all over the world, among which, biodiesel, a potential alternative fuel, has aroused wide attention of research workers. Generally, biodiesel is considered as an environmentally friendly fuel since it is biodegradable and non-toxic without producing poisonous exhaust gas such as sulfur oxides and aromatic compounds[1-2]. Biodiesel is a mixture of longchain fatty acid methyl esters (FAME) produced from free fatty acids, vegetable oil, animal oil, or waste oilviaesterification and/or trans-esteri fication with alcohols (Figure 1). The flash point, cold filter plugging point, sulphur content, and oxygen consumption of biodiesel are largely improved as compared with that of fossil-based diesel. Moreover, it was found out that biodiesel used as engine fuel can effectively reduce the emission of poisonous gases[3-4].
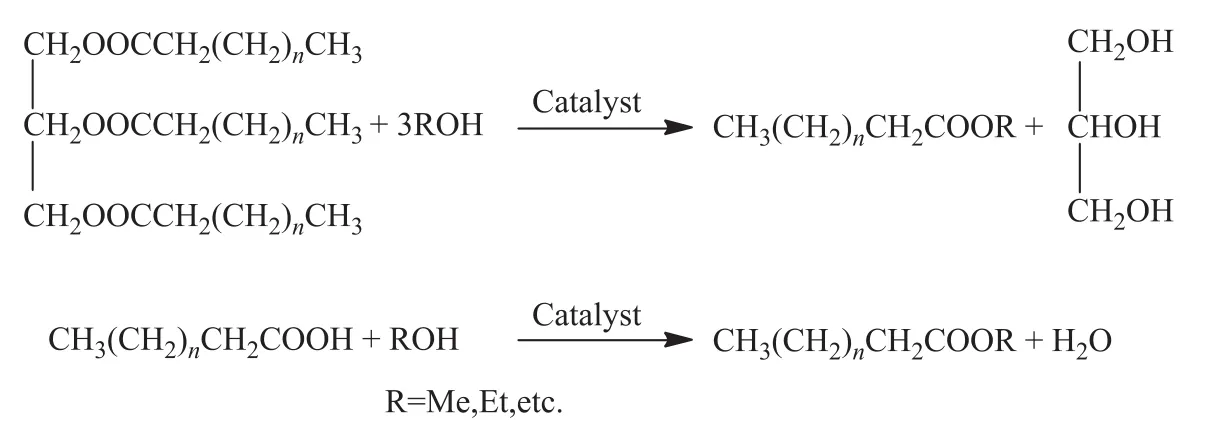
Figure 1 Reaction of esterification and transesterification
Currently, homogeneous catalysts (such as NaOH, KOH, H3PO4, H2SO4, etc.) are usually used for the preparation of biodiesel, however, this kind of catalytic system suffers from many drawbacks such as environmental pollution, difficulty in separation and purification of target product, and equipment corrosion that severely hampers the commercialization of the relevant process. The advantage of solid catalysts (such as K2CO3/silica gel[5], heteropolyacids[6-8], heterogeneous ionic liquid catalyst[9-11], and ionexchange resins[12-14]) over homogeneous catalysts is theirlow corrosiveness characteristic of esterification process, and the ease of regeneration and reuse of solid catalyst after separation. This process is quite simple with negligible environmental problems. But the solid catalysts have recently caused some problems. For example, the solid base cannot be used for catalytic esterification that involves a large amount of FFAs in oil; the heteropolyacid catalyst is prone to formation of liquid system due to its hydroscopicity, which makes the catalyst recovery more difficult; and the heterogeneous ionic liquid catalyst should be operated under high temperature, resulting in high production cost. Hereupon, the development of solid catalyst with cheap and easily reusable characteristics has become a hot spot in recent research efforts. In 2012 Boey, et al.[15]investigated the esterification of lauric acid to methyl laurate using a new heterogeneous acid catalyst prepared from inexpensive sodium alginate and ferric sulfate, and the ferric-alginate beads showed high catalytic activity for esterification. However, they just studied the catalytic activity of ferric-alginate beads without exploring the function of other metal-alginates in the esterification reaction. Based on previous literature information, we prepared aluminum-alginate catalysts from inexpensive and nontoxic sodium alginate, which were further used for the esterification reaction of oleic acid with methanol. The influence of various reaction parameters such as catalyst dosage, reaction time and molar ratio of the reactants on methyl oleate formation was evaluated to optimize the reaction conditions. Furthermore, the performance of catalytic system for the esterification of various free fatty acids with different kinds of alcohols was investigated.
2 Experimental
2.1 Materials
Oleic acid (AR), methanol (AR, >99%), aluminum chloride hexahydrate (AlCl3·6H2O, AR), and sodium alginate (CP) were purchased from the Sinopharm Chemical Regent Co., Ltd. All other chemicals were of analytically pure grade and used as received, unless otherwise noted.
2.2 Preparation of catalyst
Al (III)-alginate was synthesized according to the literature information[15-17]with slight modifications. During the experiment 2 g of sodium alginate was added to 100 mL of distilled water, followed by stirring until a clear viscous solution was obtained. Then, the viscous solution was added stepwise into 100 mL of 0.1 mol/L AlCl3solution at 28—30 ℃. The resulting solution system was stirred vigorously for 2 h to form the aluminum-alginate complex. Finally, the aluminum-alginate granules were washed and dried at about 105 ℃ under vacuum. This catalyst was denoted as Al-SA.
2.3 Characterization of the catalyst
The X-ray diffraction (XRD) patterns of the catalyst samples were obtained using a Rigaku D/Max 2000 Ultima Plus diffractometer (equipped with a monochromatic nickel filter using CuKα radiation). The FT-IR spectra were obtained on a VERTEX70 spectrophotometer using the KBr disc technique (4000—400 cm-1).
2.4 Typical procedure for fatty acid alkyl ester (biodiesel) production
A specified amount of free fatty acid (FFA), alcohol and catalyst was added to a flask equipped with a reflux condenser and a magnetic stirring apparatus. Upon completion of this reaction, the catalyst was separated from the reaction mixture by filtration; the excess methanol with water was removed by distillation under reduced pressure to purify the product. The acid value of the product was determined according to the ISO 660—1996 standard. The conversion of FFA was calculated based on the following equation[12,18]:

3 Results and Discussion
3.1 Characterization of the Al-SA catalyst
3.1.1 XRD analysis
Figure 2 shows the XRD patterns of the Al-SA catalyst. It is reported that sodium alginate does not have any distinguished peaks for the polymers as expected from polysaccharides[15], and correspondingly, the spectrogram of the Al-SA catalyst sample exhibits stronger peak intensity, which is caused by the Al (III) ions that have beenexchanged with Na ions from the sodium alginate solution. The peaks at 2θangles of approximately 31o, 35o, 45o, and 56o are similar to the pure diaspore (β-AlOOH) peaks as reported by Li, et al.[19]and Sarikaya, et al.[20], indicating to the formation of aluminum oxyhydroxide particles on the grid of alginate. In other words, this procedure is successful in the preparation of the aluminum-alginate catalyst.
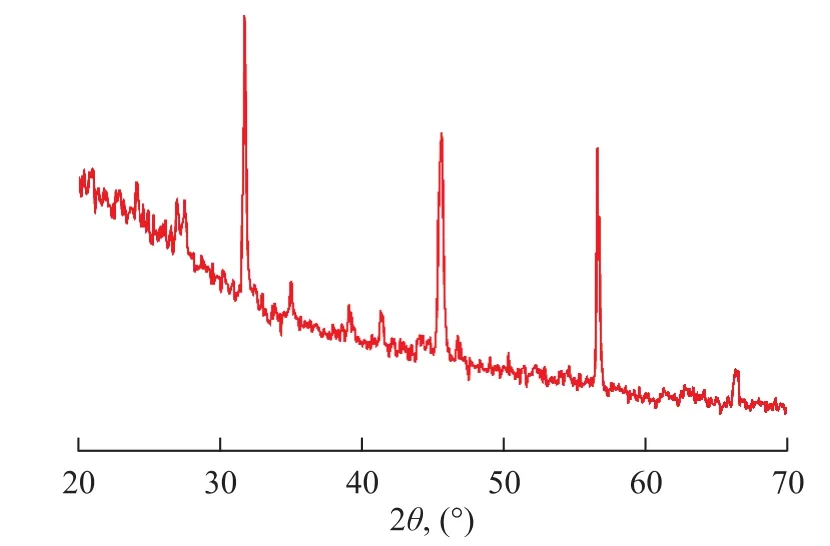
Figure 2 XRD patterns of Al-SA
3.1.2 FT-IR analysis
Figure 3 illustrates the FT-IR spectroscopic analysis of sodium alginate (SA) and Al-SA catalyst samples. It can be seen from Figure 3 that two weak peaks at the wavenumber of 1 651 cm-1and 1 417 cm-1,which were attributed to the asymmetrical stretching and symmetrical stretching band of —OCO— group in sodium alginate, were similar to the wavenumber of 1 649 cm-1and 1 417 cm-1vibrations found in the Al-SA catalyst. Similar results were also reported in the literature[16]. However, the weak peak at the wavenumber of 1633 cm-1could be assigned to the stretching vibration of free carboxylate anions, and the broad absorption band between the wavenumber of 500 cm-1and 700 cm-1was probably assigned to the stretching of Al—O bond in the diaspore. Thus, it is assumed that the aluminum ion and alginate have formed the aluminumalginate complex, as reported in previous studies[15-17].
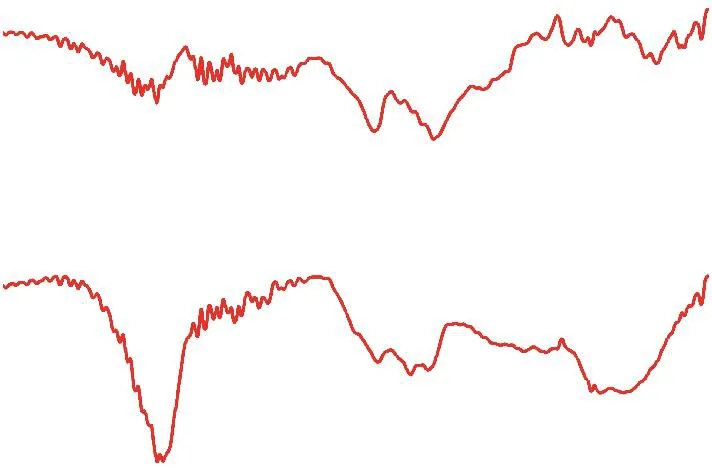
Figure 3 FT-IR spectra of sodium alginate (SA), Al-SA
3.2 Esterification of oleic acid with Al-SA
3.2.1 Influence of methanol/oleic acid molar ratio on oleic acid conversion
The molar ratio of oleic acid to methanol significantly affects the FFA conversion. The esterification reaction is a reversible reaction, so the amount of methanol must be in excess to force the reaction proceeding towards the formation of methyl oleate. The influence of oleic acid to methanol molar ratio in the range of between 1:6 to1:12 on the conversion rate was investigated in the presence of 4 m% of the Al-SA catalyst while the reactants were refluxed for 3 h. The test results are shown in Figure 4. The more the methanol was added, the higher the conversion would be obtained within the same reaction time. The FFA conversion increased from 74.0% to 92.6% with the oleic acid/ methanol molar ratio increasing from 1:6 to 1:10, and when the molar ratio of oleic acid to methanol was 1:10, a highest conversion of oleic acid was achieved, which could reach 92.6%. However, when the molar ratio further increased, the oleic acid conversion decreased slightly, probably because the oleic acid and catalyst were diluted by excess methanol. Similar finding has been reported in the literature[21].
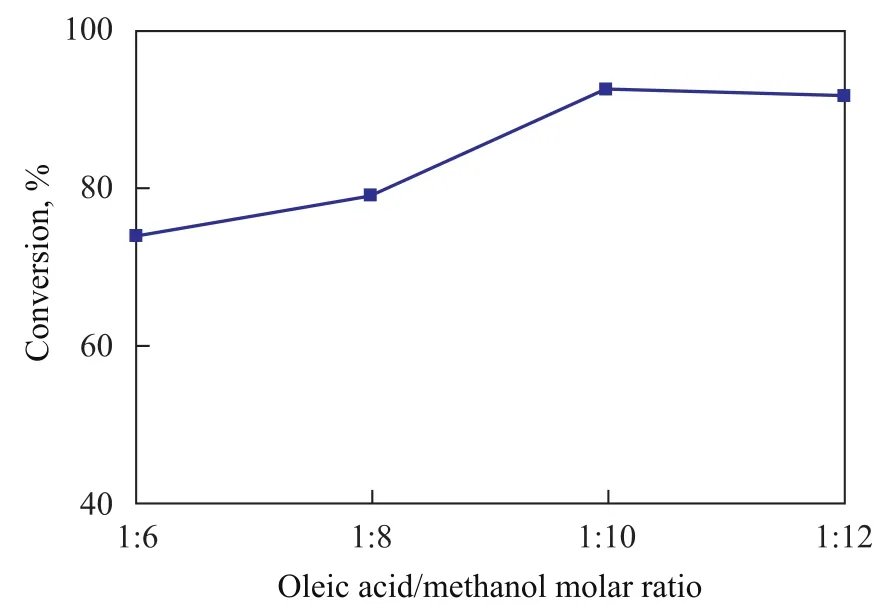
Figure 4 Effect of molar ratio of oleic acid to methanol on oleic acid conversion
3.2.2 Influence of Al-SA catalyst dosage on oleic acid conversion
The influence of catalyst amount was tested during the esterification of oleic acid with methanol. Figure 5 showsthe effect of the catalyst amount on FFA conversion. In the absence of catalyst, oleic acid conversion rate was low (less than 5%). However, as the amount of catalyst increased to 3 m% and 4 m%, the FFA conversion reached 72.2% and 92.6%, respectively. Furthermore, when the amount of catalyst was above 4 m%, the oleic acid conversion rate remained almost the same. So the optimum amount of Al-SA catalyst was 4 m%.
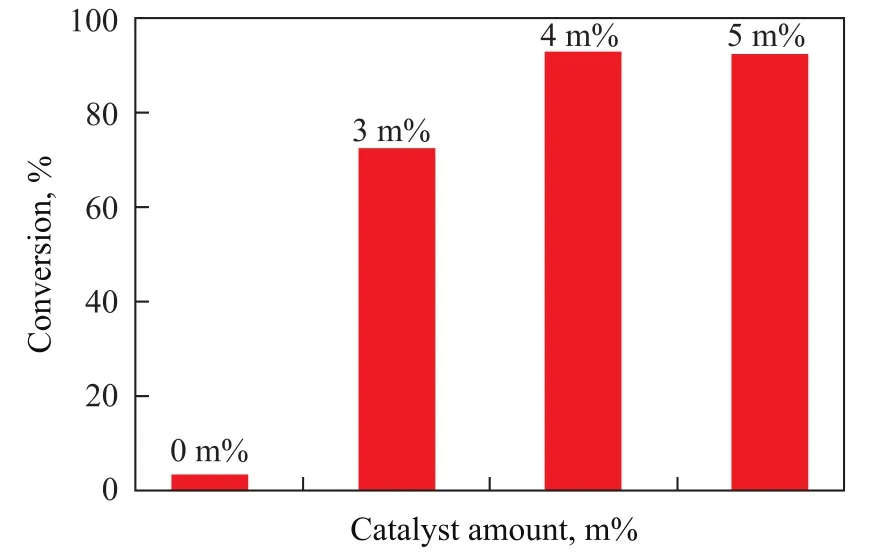
Figure 5 Effect of the amount of Al-SA on oleic acid conversion
3.2.3 Effect of reaction time on oleic acid conversion
The relationship between FFA conversion and reaction time was also investigated, with the results shown in Figure 6. It can be seen from Figure 6 that the oleic acid conversion rapidly reached up to 80% within 1 h. After 3 h of reaction, the conversion rate leveled off as the products attained a near-equilibrium composition. This fact shows that aluminum-alginate is an appropriate solid catalyst that has a very high reactivity to catalyze esterification reaction. So the optimum reaction time for Al-SA catalyst was 3 h.
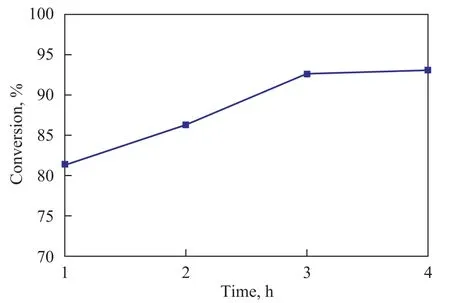
Figure 6 Effect of reaction time on oleic acid conversion
3.3 Catalytic activity of Al-SA catalyst for esterification of other short-chain alcohols
In order to further investigate the catalytic activity of Al-SA catalyst, the esterification reaction of oleic acid with different alcohols (methanol, ethanol,n-propanol, andn-butanol) was also studied (Table 1). It was discovered that excellent conversion rates (as high as 92.6%) were obtained in all cases. It also shows that the chain length of alcohols had no obvious effect on the conversion of oleic acid. Therefore, the Al-SA catalyst shows a good catalytic activity for esterification of fatty acids with various alcohols, which could produce biodiesel with different specific features[22].
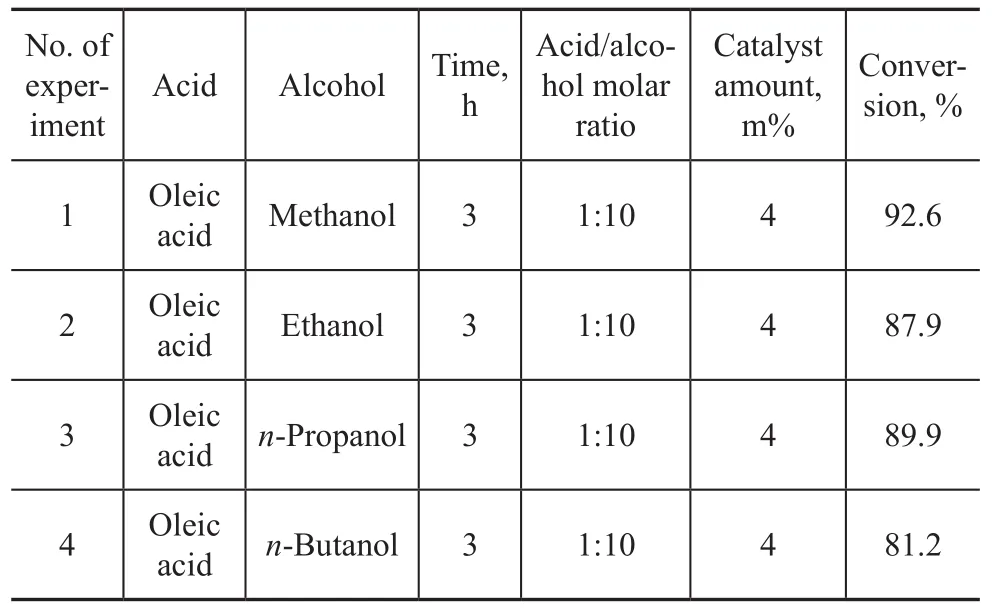
Table 1 Esterification of oleic acid with different alcohols in the presence of Al-SA cat
3.4 Catalytic activity of Al-SA catalyst for esterification of alcohols with other FFAs
The esterification of other FFAs (or mixed acid) with methanol was also investigated (Figure 7). When lauric acid, myristic acid, palmitic acid and stearic acid were successively subjected to esterification reaction with methanol, the FFA conversion reached 97.3%, 96.6%, 95.8% and 91.6%, respectively. The catalyst which was also used to catalyze the reaction of mixed acid (with the lauric acid/stearic acid molar ratio equating to 1:1) with methanol, a highest FFA conversion rate (95.3%) was achieved. Our investigation showed that the Al-SA catalyst could be a good candidate for catalytic conversion of cheap feedstocks containing high amount of FFAs (such as inedible oils[23], waste vegetable oils[24], waste cooking oils[25], etc.) into biodiesel.
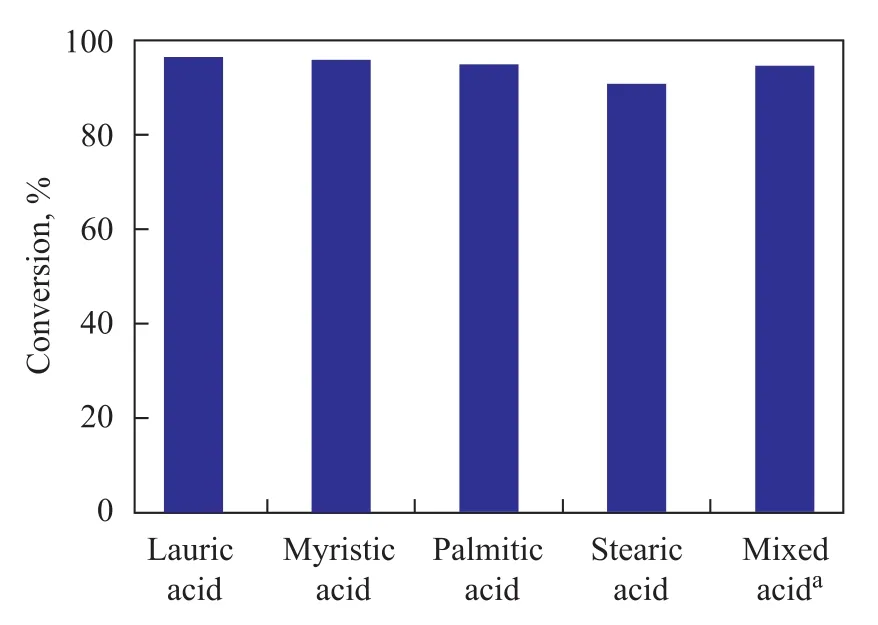
Figure 7 Esterification of different free fatty acids and mixed acid
3.5 Reusability of catalyst
The catalysts were separated from the reaction mixture by filtration and washed with cyclohexane, and then the catalyst was dried at 60 ℃ for 12 h prior to being reused for the next run. Reusability studies were carried out at a methanol /acid molar ratio of 10:1 in the presence of 4 m% of catalyst with the reactants subjected to refluxing for 3 h. The performance of the recycled Al-SA catalyst is shown in Figure 8. The catalyst could be reused three times and a 92.6% conversion was achieved in the first cycle and a 47.1% conversion could be obtained after 3 cycles. This fact indicates that the Al-SA catalyst is not thermally stable for repeatedly conducting esterification reaction. The possible reason is that the water formed during esterification of oleic acid with methanol tends to hydrolyze Al3+ions in the mixing process, leading to the loss of active component[26].

Figure 8 Reaction activity versus recycle times of Al-SA catalyst
4 Conclusions
This work described the preparation of the solid acid catalyst consisting of aluminum-alginate complex by using inexpensive raw materials; and the obtained catalyst was further applied to the esterification of FFAs with methanol for the production of biodiesel. Through investigating various parameters such as the amount of catalyst, the methanol/oleic acid molar ratio and the reaction time, an optimum reaction condition was established. Under the optimized condition, namely, upon conducting esterification in the presence of 4 m% of Al-SA catalyst at an oleic acid/methanol molar ratio of 1:10 in a reaction time of 3 h at a methanol refluxing temperature, as high as a 92.6% conversion of FFA could be achieved. The Al-SA catalyst also showed good catalytic performance for the esterification of various FFAs with alcohols. This kind of solid acid can have promising application prospects in production of biodiesel from high acidity feedstocks available in the industry.
Acknowledgements:This work was financially supported by the International Science & Technology Cooperation Program of China (No.2010DFB60840), the Key Science and Technology Project of Guizhou Province (No.20076004), the Social Development S&T Program (No. SZ-[2009]3011), and the National Key Technology R&D Program (No.2006BAD07A12).
[1] Wan Omar W N N, Amin N A S. Biodiesel production from waste cooking oil over alkaline modi fied zirconia catalyst [J]. Fuel Processing Technology, 2011, 92: 2397-2405
[2] Zillillah, Tan G W, Li Z. Highly active, stable, and recyclable magnetic nano-size solid acid catalysts: ef ficient esteri fication of free fatty acid in grease to produce biodiesel [J]. Green Chemistry, 2012, 14: 3077-3086
[3] Ng J H, Ng H K, Gan S. Characterisation of engine-out responses from a light-duty diesel engine fuelled with palm methyl ester (PME) [J]. Applied Energy, 2012, 90(1): 58-67
[4] Ng H K, Gan S. Combustion performance and exhaustemissions from the non-pressurized combustion of palm oil biodiesel blends [J]. Applied Thermal Engineering, 2010, 30(16): 2476-2484
[5] Zhang Q Y, Xu C, Zhou K Z, et al. Synthesis of biodiesel with silica gel column chromatography catalyzed with immobilized K2CO3[J]. Journal of Fuel Chemistry and Technology, 2011, 39(10): 754-758 (in Chinese)
[6] Zhang S, Zu Y G, Fu Y J, et al. Rapid microwave-assisted transesterification of yellow horn oil to biodiesel using a heteropolyacid solid catalyst [J]. Bioresource Technology, 2010, 101(3): 931-936
[7] Fermande S A, Cardoso A L, Silva M J. A novel kinetic study of H3PW12O40-catalyzed oleic acid esterification with methanol via1H NMR spectroscopy [J]. Fuel Processing Technology, 2012, 96: 98-103
[8] Leng Y, Jiang P P, Wang J. A novel Br?nsted acidic heteropolyanion-based polymeric hybrid catalyst for esterification [J]. Catalysis Communications, 2012, 25: 41-44
[9] Liu F J, Wang L, Sun Q, et al. Transesterification catalyzed by ionic liquids on superhydrophobic mesoporous polymers: heterogeneous catalysts that are faster than homogeneous catalysts [J]. Journal of the American Chemical Society, 2012, 134(41): 16948-16950
[10] Miao J M, Wan H, Guan G F. Synthesis of immobilized Br?nsted acidic ionic liquid on silica gel as heterogeneous catalyst for esterification [J]. Catalysis Communications, 2011, 12 (5): 353-356
[11] Zhang L, Xian M, He Y C, et al. A Br?nsted acidic ionic liquid as an efficient and environmentally benign catalyst for biodiesel synthesis from free fatty acids and alcohols [J]. Bioresource Technology, 2009, 100(19): 4368-4373
[12] Feng Y H, He B Q, Cao Y H, et al. Biodiesel production using cation-exchange resin as heterogeneous catalyst [J]. Bioresource Technology, 2010, 101(5): 1518-1521
[13] Kouzu M, Nakagaito A, Hidaka J S. Pre-esterification of FFA in plant oil transesterified into biodiesel with the help of solid acid catalysis of sulfonated cation-exchange resin [J]. Applied Catalysis A: General, 2011, 405(1/2): 36-44
[14] Weerachanchai P, Tangsathitkulchai C, Tangsathitkulchai M. Effect of reaction conditions on the catalytic esterification of bio-oil [J]. Korean Journal of Chemical Engineering, 2012, 29(2): 182-189
[15] Boey P L, Ganesana S, Maniamb G P, et al. A new heterogeneous acid catalyst system for esterification of free fatty acids into methyl esters [J]. Applied Catalysis A: General, 2012, 433-434: 12-17
[16] Zaafarany I A. Non-isothermal decomposition of Al, Cr and Fe cross-linked trivalent metal-alginate complexes [J]. Journal of King Abdulaziz University: Science, 2010, 22(1): 193-202
[17] Zaafarany I A. Khairou K S, Hassan R M. et al. Physicochemical studies on cross-linked thorium (IV)-alginate complex especially the electrical conductivity and chemical equilibrium related to the coordination geometry [J]. Arabian Journal of Chemistry, 2009, 2: 1-6
[18] Melo Junior C A R, Albuguergue C E R, Carneiro J S A, et al. Solid-acid-catalyzed esterification of oleic acid assisted by microwave heating[J]. Industrial & Engineering Chemistry Research, 2010, 49(23): 12135-12139
[19] Li B, Shao L L. Appraisal of alumina and aluminum hydroxide by XRD[J]. Inorganic Chemicals Industry, 2008, 40(2): 54-57(In Chinese)
[20] Sarikaya Y, Sevinc I? Akinc M. The effect of calcination temperature on some of the adsorptive properties of fine alumina powders obtained by emulsion evaporation technique [J]. Powder Technology, 2001, 116, 109-114
[21] Huang B H, Wang Y F, Zhang K, et al. Synthesis of pyrrolidonium acidic ionic liquids and their catalytic activity for esterification of acetic acid and butanol [J]. Chinese Journal of Catalysis, 2007, 28(8): 743-748 (In Chinese)
[22] Salis A, Pinna M, Monduzzi M, et al. Biodiesel production from triolein and short chain alcohols through biocatalysis[J]. J Biotechnol, 2005, 119: 291-299
[23] Wang R, Hanna M A, Zhou W W, et al. Production and selected fuel properties of biodiesel from promising nonedible oils: Euphorbia lathyris L., Sapium sebiferum L. and Jatropha curcas L.[J]. Bioresource Technology, 2011, 102(2): 1194-1199
[24] Ceclan R E, Pop A, Ceclan M. Biodiesel from waste vegetable oils [J]. Chemical Engineering Transactions, 2012, 29: 1177-1182
[25] Gan S Y, Ng H K, Chan P H, et al. Heterogeneous free fatty acids esterification in waste cooking oil using ion-exchange resins [J]. Fuel Processing Technology, 2012, 102, 67-72
[26] Guo F, Fang Z, Tian X F, et al. One-step production of biodiesel from Jatropha oil with high-acid value in ionic liquids [J]. Bioresource Technology, 2011, 102(11): 6469-6472
Recieved date: 2012-11-30; Accepted date: 2013-01-21.
Yang Song, Telephone: +86-851-829-2171; Fax: +86-851-829-2170; E-mail: jhzx.msm@gmail.com.
- 中國煉油與石油化工的其它文章
- Degradation of Nitrobenzene-Containing Wastewater with O3and H2O2by High Gravity Technology
- A Novel Modeling, Simulation and Optimization Approach of Crude Oil Cold Stripping Process
- Feasibility of Intermediate Fluid Vaporizer with Spiral Wound Tubes
- Selecting Suitable Heat Source in Refinery for Multi-effect Distillation Based on Grey System Theory
- Oxidative Desulfurization of Model Sulfur Compound by Potassium Ferrate in the Presence of Phosphomolybdic Acid Catalyst
- Preparation of Alumina Binder-Added PtSnNa/AlSBA-15 Catalyst for Propane Dehydrogenation

