Synthesis and characterization of carboxymethyl cellulose/organic montmorillonite nanocomposites and its adsorption behavior for Congo Red dye
Min-min WANG, Li WANG*
College of Material Science and Art Design, Inner Mongolia Agricultural University, Huhhot 010018, P. R. China
Synthesis and characterization of carboxymethyl cellulose/organic montmorillonite nanocomposites and its adsorption behavior for Congo Red dye
Min-min WANG, Li WANG*
College of Material Science and Art Design, Inner Mongolia Agricultural University, Huhhot 010018, P. R. China
A series of carboxymethyl cellulose/organic montmorillonite (CMC/OMMT) nanocomposites with different weight ratios of carboxymethyl cellulose (CMC) to organic montmorillonite (OMMT) were synthesized under different conditions. The nanocomposites were characterized by the Fourier transform infrared (FT-IR) spectrophotometer, X-ray diffraction (XRD) method, transmission electron microscope (TEM), scanning electron microscope (SEM), and thermal gravimetric (TG) analysis. The results showed that the introduction of CMC may have different influences on the physico-chemical properties of OMMT and intercalated-exfoliated nanostructures were formed in the nanocomposites. The effects of different reaction conditions on the adsorption capacity of samples for Congo Red (CR) dye were investigated by controlling the amount of hexadecyl trimethyl ammonium bromide (CTAB), the weight ratio of CMC to OMMT, the reaction time, and the reaction temperature. Results from the adsorption experiment showed that the adsorption capacity of the nanocomposites can reach 171.37 mg/g, with the amount of CTAB being 1.0 cation exchange capacity (CEC) of MMT, the weight ratio of CMC to OMMT being 1?1, the reaction time being 6 h, and the reaction temperature being 60℃. The CMC/OMMT nanocomposite can be used as a potential adsorbent to remove CR dye from an aqueous solution.
carboxymethyl cellulose; organic montmorillonite;nanocomposite; adsorption; Congo Red dye
1 Introduction
Dyes are widely used in the textile, rubber, paper, and plastics industries. Over 7 × 105tons of different commercial dyes and pigments are produced annually all over the world (Wang et al. 2011). Most dyes released during textile manufacturing, clothing, printing, and dyeing processes are considered hazardous and toxic to some organisms and may cause allergic dermatitis, skin irritation, and carcinogenic and mutagenic risks to humans and aquatic organisms (Zollinger 1987; Fungaro et al. 2009). Therefore, the demand for good-quality wateris continuously rising owing to an increase in the population (Srinivas et al. 2009), and it is extremely important to remove dyes from industrial wastewater before it is discharged into the environment (Luo et al. 2011). A lot of methods, such as membrane (Xu and Lebrun 1999), oxidation (Lucas and Peres 2006), coagulation/flocculation (Papic et al. 2004), and biological treatment (Akhtar et al. 2004; Kahraman et al. 2005; Gonzalez-Gutierrez and Escamilla-Silva 2009), have been used to remove dyes from wastewater. However, these methods have considerable energy requirements and thus impose both economic and environmental costs (Kittinaovarat et al. 2010). Compared with other methods, adsorption is considered superior (El Mouzdahir et al. 2010). Adsorption using activated carbon is currently of great interest for the removal of dyes and pigments. In spite of its extensive use, activated carbon remains an expensive material since the higher the quality of the activated carbon is, the greater the cost. This has led to the search for cheaper substitutes (Ahmad et al. 2007). Today, attention has been focused on the low-cost alternative adsorbent materials such as clays and biopolymers. A lot of modifications have been made with montmorillonite (MMT) and cellulose. MMT, a very rich clay mineral, consists of layers of two tetrahedral silica sheets sandwiching one octahedral alumina sheet, and it has a high adsorption capacity due to its large specific surface area and high cation exchange capacity (Fu and Qutubuddin 2001). However, natural MMT adsorbs the anionic dyes only onto the external surface with broken bonds in very small amounts, and it is difficult to make it compatible with organic polymeric material. Therefore, the modification of MMT with surfactants is a method for hydrophobizing the mineral and increasing the adsorption capacity for organic pollutants. The surfactant-modified MMT can be extensively used for a wide variety of environmental applications (Churchman et al. 2006). Cellulose is the most abundant natural polymer in nature with excellent biodegradability and biocompatibility (De Melo et al. 2009). However, the strong intermolecular and intramolecular hydrogen bonds between the hydroxyl groups along the chain backbone not only limit the water solubility but also lead to the poor reactivity of cellulose (Bao et al. 2011). By contrast, the derivative modification of cellulose, carboxymethyl cellulose (CMC), can overcome these drawbacks (Wang and Wang 2010). It is an important artificial-natural polymer derived from cellulose (Zhao et al. 2009).
Polymer-clay nanocomposites have recently attracted significant interest in scientific research and industrial applications (Liu 2007; Pavlidou and Papaspyrides 2008). In previous studies, there has been a lot of investigation of preparing natural polymer-clay nanocomposites and using them as adsorbents. For instance, Kittinaovarat et al. (2010) successfully prepared chitosan/modified MMT beads and evaluated their adsorption capacity for Reactive Red 120. Ngah et al. (2010) synthesized crosslinked chitosan-coated bentonite for tartrazine adsorption from aqueous solutions. The main objective of this study was to report a novel CMC/OMMT (organic MMT) nanocomposite with a high adsorption capacity, acceptable biodegradability, and non-toxicity. It is a potential candidate as an environmentally friendly adsorbent. A seriesof CMC/OMMT nanocomposites were synthesized and their structures were investigated. Furthermore, the effects of different preparation conditions on the adsorption capacity of CMC/OMMT nanocomposites were investigated.
2 Experimental setup
2.1 Materials
The cation exchange capacity (CEC) of calcium montmorillonite (Ca-MMT, produced by Chifeng Xinglonghuagong Co., Ltd. of Inner Mongolia, China) is 100 mmol/100 g. The molecular weight of hexadecyl trimethyl ammonium bromide (CTAB, produced by Tianjin Finite Chemical Research Institute) is 364.45 g/mol. Chloroacetic acid is produced by Tianjin Fuchen Chemical Reagent Factory. Lignocellulose (LNC) is produced by Beijing Qinlihengtong Technology Co., Ltd. The molecular weight of Congo Red (CR, produced by Beijing Dyestuffs Plant) is 696.66 g/mol. Other agents used were all of analytical grade and all solutions were prepared with distilled water.
2.2 Preparation of CMC
LNC (10 g) was added to the NaOH solution (100 mL). After 12 h, the solution was separated by filtration. The treated LNC was dipped into anhydrous alcohol (100 mL) in a three-necked flask by stirring for 30 min, and then chloroacetic acid (10 g) was added into the reaction mixture and stirred for another 30 min. Then, the mixture was heated to 60℃ and maintained at 60℃ for 6 h. Finally, the resultant solution was filtered and the filter cake was dissolved in distilled water. The pH value of the solution obtained was adjusted to 7.0, and then the solution was precipitated by pouring it into anhydrous alcohol, dried under a vacuum at 80℃ in order to obtain products, and, finally, ground and sifted with a 200-mesh sieve.
2.3 Preparation of OMMT
OMMT was synthesized according to the following procedure. CTAB (0.25, 0.50, 1.0, 1.5, and 2.0 CEC of MMT) was dissolved in distilled water (240 mL), and then MMT (8.0 g) was added slowly. The reaction mixtures were stirred continuously at room temperature for 12 h. The mixtures were filtered and then washed several times with distilled water until no bromide ion was detected by the AgNO3solution (0.1 mol/L). The product was dried in an air oven at 105 for ℃6 h and ground and sifted with a 200-mesh sieve.
2.4 Preparation of CMC/OMMT nanocomposite
Aliquots (1.0 g) of various samples of OMMT (0.25, 0.50, 1.0, 1.5, and 2.0 CEC of MMT) were swelled by distilled water (30 mL). Then, the CMC solution was slowly added to the OMMT suspension, followed by stirring at different temperatures (30,℃ 45,℃ 60,℃ 75,℃ and 90) for℃ different time periods (2, 4, 6, 8, and 10 h), to obtain the nanocomposites, with the weight ratios of CMC to OMMT being 0.25?1, 0.5?1, 1?1, 1.5?1, and 2?1. The formedcomposites were washed with distilled water until the pH value of the supernatant reached 7.0, and were then dried at 105℃ for 12 h. All samples as adsorbents were ground and sifted with a 200-mesh sieve.
2.5 Adsorption experiments
All batch adsorption experiments were performed on a thermostated shaker (SHA-C) with a shaking rate of 120 r/min. 0.10 g of nanocomposite interacted with 25 mL of CR solution (with an initial concentration of 800 mg/L and an initial pH value of 9.6). The system was maintained under shaking conditions at 30℃ until adsorption equilibrium was established. After shaking, the suspension was separated from the adsorbent by centrifugation at a rotation speed of 6 000 r/min for 5 min. The absorbencies of the dye solutions were measured using a UV-visible spectrophotometer at a wavelength of 497 nm (at the initial pH, CR had a maximum absorbency at the wavelength of 497 nm on a UV-visible spectrophotometer).
The amount of adsorbed CR was calculated through the following equation:

whereqeis the amount of adsorbed dye (mg/g) at equilibrium,C0is the initial concentration of CR in the solution (mg/L),Ceis the equilibrium concentration of CR in the solution (mg/L),mis the mass of adsorbent (g), andVis the volume of the CR solution (L). In the method of calculatingqe, it was assumed that there were no losses of CR due to any other mechanism (volatilization, sorption to the glassware, degradation, etc.).
2.6 Characterization
Fourier transform infrared (FT-IR) spectra of the samples were characterized using a FT-IR spectrophotometer (Bruker Tensor 27) in KBr pellets spectra in a range from 4 000 to 400 cm?1. X-ray diffraction (XRD) analysis of the powdered samples were performed on an X-ray powder diffractometer with a Cu anode (Shimadzu XRD-6000), running at 40 kV and 30 mA and scanning from 3° to 18° at a rate of 3° per minute with a scanning electron microscope (SEM, JSM-5600LV). The morphology of the nanocomposite was characterized by transmission electron microscope (TEM, JSM-5600LV) at an acceleration voltage of 80 kV.
3 Results and discussion
3.1 FT-IR analysis of nanocomposite
The FT-IR spectra of OMMT, CMC, and the CMC/OMMT nanocomposite are shown in Fig.1. Compared with the IR spectra of CMC and OMMT, the IR spectrum of the CMC/OMMT nanocomposite showed that the symmetric and asymmetric stretching vibrations of ―CH3and ―CH2(2 922 cm?1and 2 850 cm?1, respectively) and the stretching vibration of Si―O (1 024 cm?1) of OMMT were widened and weakened after reaction. The stretchingvibrations of ―OH (3 425 cm?1and 2 924 cm?1) of CMC disappeared. In addition, the characteristic absorption bands at 1 600 cm?1(asymmetric stretching of COO―) and 1 446 cm?1(symmetric stretching of COO―) of CMC were observed on the FT-IR spectrum of the nanocomposite. The information observed from the FT-IR spectra indicates that chemical reactions have occurred on both CMC and OMMT, which may have an influence on the absorption properties of the nanocomposite.
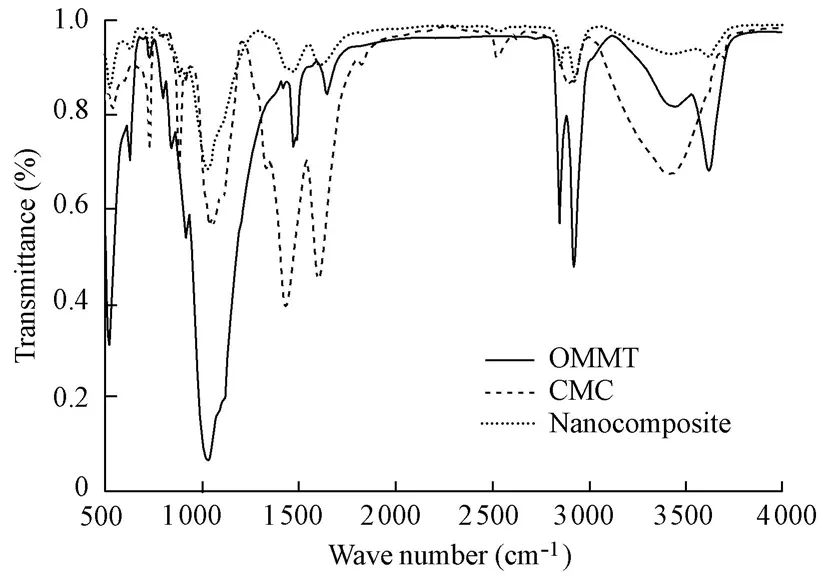
Fig. 1 FT-IR spectra of OMMT, CMC, and CMC/OMMT nanocomposite
3.2 XRD analysis
XRD is an effective method for the investigation of the intercalation existence of MMT. The XRD patterns of MMT, OMMT, and CMC/OMMT nanocomposite are shown in Fig. 2.
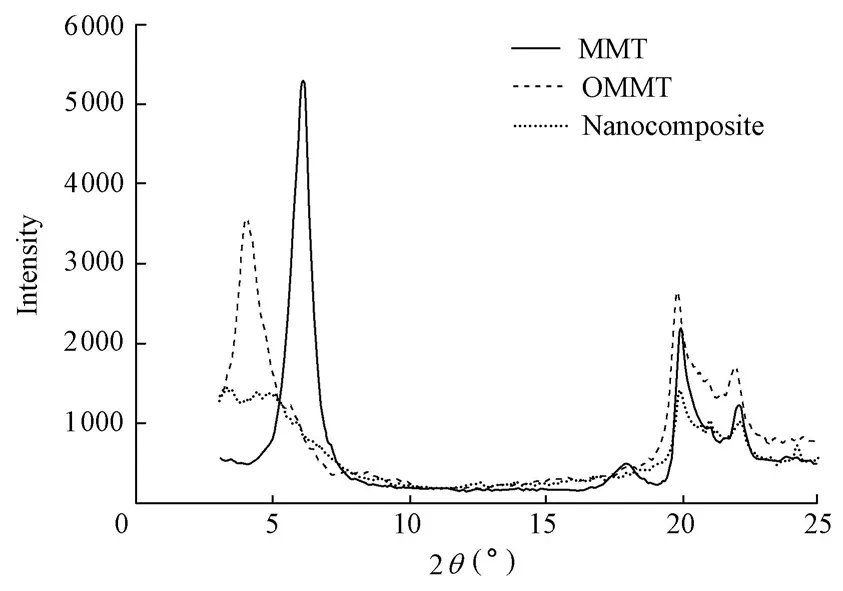
Fig. 2 XRD patterns of MMT, OMMT, and CMC/OMMT nanocomposite Bragg’s law can be expressed as

wheredis the distance between two consecutive clay layers,λis the wavelength of the intercept X-rays at the incident angleθ, andnis the diffraction order.
According to Bragg’s law, it can be noted that OMMT shows a typical diffraction peak at 2θ= 4.43°, corresponding to a basal spacing of 19.92 nm (the interlayer distance of naturalMMT is 1.49 nm). The presence of CMC chains destroyed the layer structure of OMMT. It can be seen from Fig. 2 that the diffraction peak of the nanocomposite nearly disappears. This result indicated that the CMC molecules were successfully intercalated into the spacing of layers of OMMT and destroyed the crystal structure of OMMT.
3.3 TEM image analysis
Fig. 3 shows the TEM microphotograph of the CMC/OMMT nanocomposite. The dark lines and agglomerates in the photograph are the intersections of the OMMT sheets, and the spaces between the dark lines are CMC molecules. Almost all the CMC molecules are well dispersed in the OMMT interlayers. Although some of the clays were agglomerates, the TEM results also suggest that the average particle size was below 50 nm in thickness, and CMC intercalated into OMMT interlayers, destroying the crystalline structure. These phenomena indicated that the disordered intercalated and exfoliated structures of OMMT were formed in the nanocomposite. These results accord with the XRD analysis.
3.4 SEM image analysis
SEM has been a primary tool for characterizing the surface morphology and fundamental physical properties of an adsorbent surface. It can be used to determine the particle shape, porosity, and appropriate size distribution of an adsorbent. The SEM images of MMT (Fig. 4(a)) and nanocomposite (Fig. 4(b)) show distinct differences. It is clear that the surface of MMT is compact and flat stretch, showing crystal features. The nanocomposite has considerable numbers of pores and a large surface area, and there is a good possibility of dyes being absorbed into these pores.
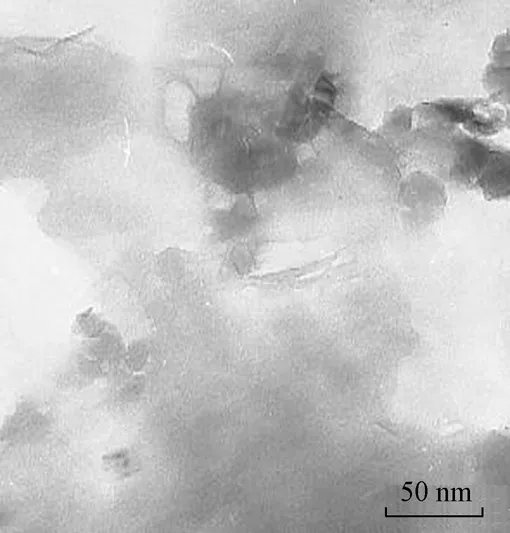
Fig. 3 TEM image of CMC/OMMT nanocomposite
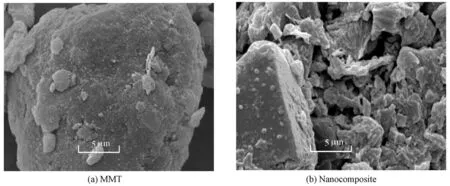
Fig. 4 SEM images of MMT and CMC/OMMT nanocomposite
3.5 TG analyses of CMC and nanocomposite
Generally, the incorporation of clay into the polymer matrix was found to enhance the thermal stability by acting as a superior insulator and mass transport barrier to the volatile products generated during decomposition (Sinha Ray and Okamoto 2003). The characteristic TG curves of CMC and the CMC/OMMT nanocomposite are shown in Fig. 5. The TG results indicated the improvement of the thermal stability of the CMC/OMMT nanocomposite. At 500℃, the weight percents of the residues of CMC and CMC/OMMT nanocomposite were 47.4% and 74.6%, respectively. Thus, 17.2% remained, indicating that the thermal stability as well as the combustion behavior of the polymer-clay nanocomposite was affected by the degradation mechanism of alkylammonium ion clay modifier. The clay acted as a heat barrier, which enhanced the overall thermal stability of the polymer layered silicate nanocomposites and assisted in the formation of char after thermal decomposition. OMMT occurred on the surface of the nanocomposite, creating a protective barrier on the surface of the material. These factors may lead to improved thermal properties of the nanocomposite.

Fig. 5 TG curves of CMC and CMC/OMMT nanocomposite
3.6 Effect of amount of CTAB on adsorption
Fig. 6 shows the effect of the amount of CTAB on the adsorption capacity of CR onto the nanocomposite under the preparation conditions with the weight ratio of CMC to OMMT being 1?1, the reaction time being 6 h, and the reaction temperature being 60℃. As can be seen from this figure, the adsorption capacity of the nanocomposite increased with the amount of CTAB. The reason is that the amount of surfactant, which intercalated into the MMT galleries, increased with the amount of CTAB, resulting in increasing layer spacing and more space for CMC molecules to intercalate. However, when the addition amount of CTAB exceeds 1.0 CEC of MMT, the adsorption capacity hardly increases, which may be due to the fact that the amount of intercalated CTAB is saturated, and more CTAB molecules cannot intercalate into the MMT galleries and affect the formation of the nanocomposite. Therefore, under these experimental conditions, the amount of CTAB of 1.0 CEC of MMT is optimal.
3.7 Effect of weight ratio of CMC to OMMT on adsorption
The effect of the weight ratio of CMC to OMMT on the adsorption capacity of the CMC/OMMT nanocomposite for CR is shown in Fig. 7. The preparation conditions were that the amount of CTAB was 1.0 CEC of MMT, the reaction time was 6 h, and the reaction temperature was 60℃. It is obvious that the amount of CMC is an important factor affecting the adsorption capacity of the CMC/OMMT nanocomposite. It can be seen that, compared with the nanocomposite, OMMT exhibited better adsorption capacity for CR (201.3 mg/g). This is because its chemical properties and internal structure make it easier for it to adsorb anionic dyes. When introducing CMC, the adsorption capacity of the nanocomposite decreased slowly with the increase of the weight ratio of CMC to OMMT from 0.25?1 to 1?1, and decreased sharply with a further increase of the weight ratio of CMC to OMMT. This may be explained by the reason that the amount of CMC improved the flocculation capacity of OMMT and facilitated the separation of the adsorbents from the solution (Wang and Wang 2008). This made it hard for CR molecules to diffuse into the pores in the nanocomposite and approach the adsorptive sites. However, through the comparison we can see that when the weight ratio of CMC to OMMT is 1?1, the adsorption capacity of the nanocomposite (171.37 mg/g) is higher than that of their physical mixture (130.4 mg/g). Considering the adsorption ability and economic cost, the weight ratio of CMC to OMMT of the nanocomposite was selected as 1?1.
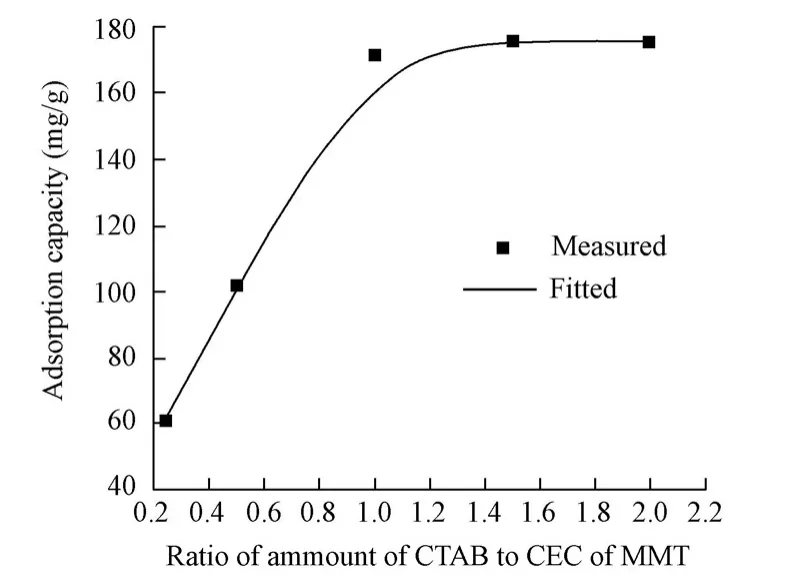
Fig. 6 Effect of amount of CTAB on adsorption capacity of nanocomposite for CR

Fig. 7 Effect of weight ratio of CMC to OMMT on adsorption capacity of nanocomposite for CR
3.8 Effect of reaction time on adsorption
The effect of the reaction time on the adsorption capacity of the CMC/OMMT nanocomposite was investigated and the results are shown in Fig. 8. The preparation conditions were that the amount of CTAB was 1.0 CEC of MMT, the weight ratio of CMC to OMMT was 1?1, and the reaction temperature was 60℃. It can be seen from Fig. 8 that the adsorption capacity of the CMC/OMMT nanocomposite increased from 141.05 mg/g to 171.37 mg/g from 2 to 6 h, and decreased with longer reaction time. This fact may be explained by the reason that the reaction between CMC and OMMT molecules had not achieved equilibrium before 6 h.After 6 h, the molecular repulsion between CMC molecular chains weakened the dispersion of OMMT and affected the formation of nanocomposite. Under the experimental conditions, the optimal time for preparing CMC/OMMT nanocomposite is 6 h.
3.9 Effect of reaction temperature on adsorption
Fig. 9 shows the effect of the reaction temperature on the adsorption capacity of the CMC/OMMT nanocomposite. The preparation conditions were that the amount of CTAB was 1.0 CEC of MMT, the weight ratio of CMC to OMMT was 1?1, and the reaction time was 6 h. As can be seen, the adsorption capacity of nanocomposite increased from 149.97 mg/g to 171.37 mg/g with the increase of temperature from 30℃ to 60℃, and then decreased about 25 mg/g with the further increase of temperature from 60℃ to 90℃. This can be explained as follows: at low temperatures, high viscosity limited the mobility of CMC and OMMT molecules, so it was difficult to form nanocomposite; at high temperatures, CMC began to degenerate, and its viscosity and performance decreased significantly. As a result, 60℃ is the most appropriate temperature in this study.
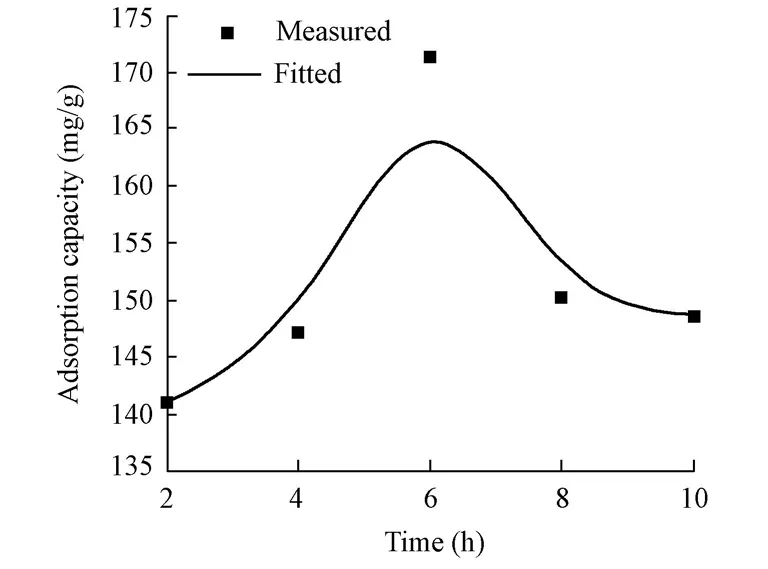
Fig. 8 Effect of reaction time on adsorption capacity of nanocomposite for CR
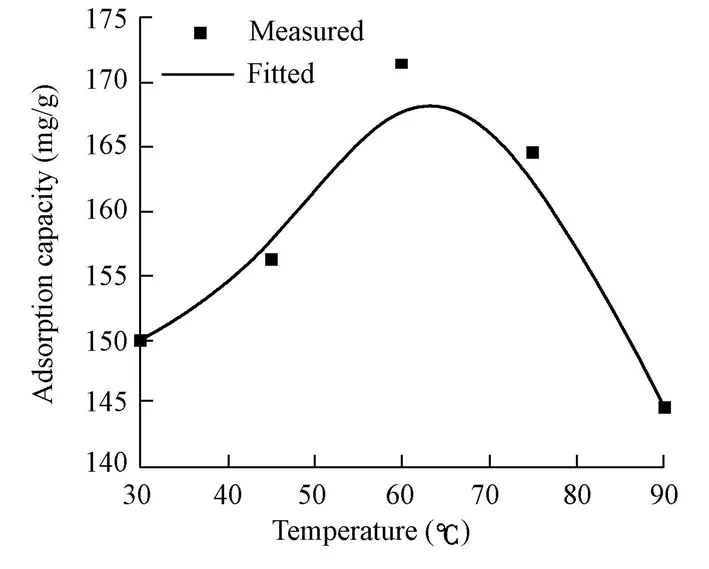
Fig. 9 Effect of reaction temperature on adsorption capacity of nanocomposite for CR
4 Conclusions
The novel CMC/OMMT nanocomposite was synthesized with the intercalation method. FT-IR, XRD, TEM, SEM, and TG analyses indicated that it was not physical but chemical reactions that had occurred on both CMC and OMMT, CMC molecules had intercalated into the interlayer of OMMT, and intercalated-exfoliated structures were formed in the CMC/OMMT nanocomposite. Furthermore, the nanocomposite exhibited an improved thermal performance. Experiments on the adsorption of CR dye with the CMC/OMMT nanocomposite were carried out and the results showed that when the amount of CTAB was 1.0 CEC of MMT, the weight ratio of CMC to OMMT was 1?1, the reaction time was 6 h, and the reaction temperature was 60℃, the nanocomposite showed a high adsorption capacity. The maximum adsorption capacity was 171.37 mg/g. As a novel nanocomposite material, the CMC/OMMT nanocomposite is a promising biosorbent for the removal of dyes from wastewater.
Ahmad, A. A., Hameed, B. H., and Aziz, N. 2007. Adsorption of direct dye on palm ash: Kinetic and equilibrium modeling.Journal of Hazardous Materials, 141(1), 70-76. [doi:10.1016/j.jhazmat. 2006.06.094]
Akhtar, N., Iqbal, J., and Iqbal, M. 2004. Enhancement of Lead(II) biosorption by microalgal biomass immobilized onto Loofa (Luffa cylindrica) sponge.Engineering in Life Sciences, 4(2), 171-178. [doi:10.1002/elsc.200420019]
Bao, Y., Ma, J. Z., and Li, N. 2011. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly (AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel.Carbohydrate Polymers, 84(1), 76-82. [doi:10. 1016/j.carbpol.2010.10.061]
Churchman, G. J., Gates, W. P., Theng, B. K. G., and Lagaly, G. 2006. Clays and clay minerals for pollution control. Bergaya, F., Theng, B. K. G., and Lagaly, G., eds.,Handbook of Clay Science, Vol. 1, 625-675. Amsterdam: Elsevier.
De Melo, J. C. P., Da Silva Filho, E. C., Santana, S. A. A., and Airoldi, C. 2009. Maleic anhydride incorporated onto cellulose and thermodynamics of cation-exchange process at the solid/liquid interface.Colloids and Surfaces A:Physicochemical and Engineering Aspects, 346(1-3), 138-145. [doi:10.1016/j.colsurfa.2009. 06.006]
El Mouzdahir, Y., Elmchaouri, A., Mahboub, R., Gil, A., and Korili, S. A. 2010. Equilibrium modeling for the adsorption of methylene blue from aqueous solutions on activated clay minerals.Desalination, 250(1), 335-338. [doi:10.1016/j.desal.2009.09.052]
Fu, X., and Qutubuddin, S. 2001. Polymer-clay nanocomposites: Exfoliation of organophilic montmorillonite nanolayers in polystyrene.Polymer, 42(2), 807-813. [doi:10.1016/S0032-3861(00)00385-2]
Fungaro, D. A., Bruno, M., and Grosche, L. C. 2009. Adsorption and kinetic studies of methylene blue on zeolite synthesized from fly ash.Desalination and Water Treatment, 2(1-3), 231-239.
Gonzalez-Gutierrez, L. V., and Escamilla-Silva, E. M. 2009. Reactive red azo dye degradation in a UASB bioreactor: Mechanism and kinetics.Engineering in Life Sciences, 9(4), 311-316. [doi:10.1002/elsc. 200900036]
Kahraman, S., Asma, D., Erdemoglu, S., and Yesilada, O. 2005. Biosorption of Copper(II) by live and dried biomass of the white rot fungiPhanerochaete chrysosporiumandFunalia trogii.Engineering in Life Sciences, 5(1), 72-77. [doi:10.1002/elsc.200420057]
Kittinaovarat, S., Kansomwan, P., and Jiratumnukul, N. 2010. Chitosan/modified montmorillonite beads and adsorption Reactive Red 120.Applied Clay Science, 48(1-2), 87-91. [doi:10.1016/j.clay.2009.12.017]
Liu, P. 2007. Polymer modified clay minerals: A review.Applied Clay Science, 38(1-2), 64-76. [doi:10.1016/ j.clay.2007.01.004]
Lucas, M. S., and Peres, J. A. 2006. Decolorization of the azo dye Reactive Black 5 by Fenton and photo-Fenton oxidation.Dyes Pigments, 71(3), 236-244. [doi:10.1016/j.dyepig.2005.07.007]
Luo, X. B., Zhan, Y. C., Huang, Y. N., Yang, L. X., Tu, X. M., and Luo, S. L. 2011. Removal of water-soluble acid dyes from water environment using a novel magnetic molecularly imprinted polymer.Journal of Hazardous Materials, 187(1-3), 274-282. [doi:10.1016/j.jhazmat.2011.01.009]
Ngah, W. S. W., Ariff, N. F. M., and Hanafiah, M. A. K. M. 2010. Preparation, characterization, and environmental application of crosslinked chitosan-coated bentonite for tartrazine adsorption from aqueous solutions.Water, Air and Soil Pollution, 206(1-4), 225-236. [doi:10.1007/s11270-009-0098-5]
Papic, S., Koprivanac, N., Bozic, A. L., and Metes, A. 2004. Removal of some reactive dyes from synthetic wastewater by combined Al(III) coagulation/carbon adsorption process.Dyes and Pigments, 62(3), 291-298. [doi:10.1016/S0143-7208(03)00148-7]
Pavlidou, S., and Papaspyrides, C. D. 2008. A review on polymer-layered silicate nanocomposites.Progress in Polymer Science, 33(12), 1119-1198. [doi:10.1016/j.progpolymsci.2008.07.008]
Sinha Ray, S., and Okamoto, M. 2003. Polymer/layered silicate nanocomposites: A review from preparation to processing.Progress in Polymer Science, 28(11), 1539-1641. [doi:10.1016/j.progpolymsci.2003.08.002]
Srinivas, G., Sekar, S., Saravanan, R., and Renganarayanan, S. 2009. Studies on a water-based absorption heat transformer for desalination using MED.Desalination and Water Treatment, 1(1-3), 75-81.
Wang, L., and Wang, A. Q. 2008. Adsorption behaviors of Congo red on the N,O-carboxymethylchitosan/montmorillonite nanocomposite.Chemical Engineering Journal, 143(1-3), 43-50. [doi:10.1016/j. cej.2007.12.007]
Wang, L., Zhang, J. P., and Wang, A. Q. 2011. Fast removal of methylene blue from aqueous solution by adsorption onto chitosan-g-poly (acrylic acid)/attapulgite composite.Desalination, 266(1-3), 33-39. [doi:10.1016/j.desal.2010.07.065]
Wang, W. B., and Wang, A. Q. 2010. Nanocomposite of carboxymethyl cellulose and attapulgite as a novel pH-sensitive superabsorbent: Synthesis, characterization and properties.Carbohydrate Polymers, 82(1-2), 83-91. [doi:10.1016/j.carbpol.2010.04.026]
Xu, Y., and Lebrun, R. E. 1999. Treatment of textile dye plant effluent by nanofiltration membrane. Separation Science and Technology, 34(13), 2501-2519.[doi:10.1081/SS-100100787]
Zhao, Q., Qian, J. W., An, Q. F., Gao, C. J., Gui, Z. L., and Jin, H. T. 2009. Synthesis and characterization of soluble chitosan/sodium carboxymethyl cellulose polyelectrolyte complexes and the pervaporation dehydration of their homogeneous membranes.Journal of Membrane Science, 333(1-2), 68-78. [doi: 10.1016/j.memsci.2009.02.001]
Zollinger, H. 1987.Colour Chemistry: Synthesis, Properties of Organic Dyes and Pigments. New York: VCH Publishers.
(Edited by Yun-li YU)
This work was supported by the Special Fund for National Forestry Industry Scientific Research in the Public Interest of China (Grant No. 201104004), the Natural Science Foundation of China (Grant No. 20867004), and the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region.
*Corresponding author (e-mail:wl2083663@126.com)
Apr. 13, 2012; accepted Aug. 23, 2012
 Water Science and Engineering2013年3期
Water Science and Engineering2013年3期
- Water Science and Engineering的其它文章
- Impact of consolidation pressure on contaminant migration in clay liner
- Application of modified k-ω model to predicting cavitating flow in centrifugal pump
- Variability in salt flux and water circulation in Ota River Estuary, Japan
- Effectiveness of inhibitors in increasing chloride threshold value for steel corrosion
- Towards full predictions of temperature dynamics in McNary Dam forebay using OpenFOAM
- Optimized operation of cascade reservoirs on Wujiang River during 2009-2010 drought in southwest China
