Coupled Reaction/Distillation Process for Hydrolysis of Methyl Acetate*
ZHAO Suying (趙素英)**, HUANG Jingzhao (黃鏡釗) WANG Liang’en (王良恩) and HUANG Guoqiang (黃國強)
1 College of Chemistry and Chemical Engineering, Fuzhou University, Fuzhou 350002, China
2 National Engineering Research Center for Distillation Technology (NERCDT), Tianjin 300072, China
1 INTRODUCTION
Methyl acetate (MA) is a byproduct in the industry of polyvinyl alcohol (PVA) and purified terephthalic acid (PTA). In order to obtain more valuable compound, MA is usually hydrolyzed to methanol (MeOH) and acetic acid (HAc), which are recycled to the production process of PVA or PTA. The conventional hydrolysis process contains a fix-bed reactor and four separation columns [1]. The hydrolysis reaction is carried out in the fixed-bed reactor catalyzed by ion exchange resin. Limited with a equilibrium constant ~0.14 at 25 °C, the hydrolysis ratio is relatively low (~23%), resulting in a large amount of recirculation [2]. Furthermore, in order to deal with the azeotropes of methyl acetate-methanol and methyl acetate-water existing in the system, a complex separation process required 4 columns is introduced. A number of improved processes have been developed to overcome the disadvantages mentioned above [3-6]. Reactive distillation, a process that combines reaction and separation together, is an attractive alternative process and it gives clear advantages for systems with small equilibrium constant. Fuchigami [7] proposed a reactive distillation configuration with total reflux on the top and bottom product withdrawal for the hydrolysis process.The reactive zone placed in the mid-section is packed with catalyst which consists of ion exchange resin and polyethylene powder. With a molar ratio of water to methyl acetate (RW/MA) more than 8, a nearly complete conversion (~99%) can be achieved. However, owing to the large amount of excess water, the concentration of acetic acid in the hydrolysate is so low that it cannot be accepted by PVA and PTA factory. Xiao et al. [8]proposed replacing the fixed-bed reactor by a catalytic distillation column to achieve 56% MA conversion as twice that of fixed-bed reactor technology. Wang et al.[9] reported an industrial application of catalytic distillation for MA hydrolysis with 56% conversion. The mixture of MA and water was fed in the top of catalytic distillation column, and the hydrolysate was withdrawn from the bottom. MA recycle ratio was reduced with the raise of hydrolysis ratio in the two process mentioned above, yet the separation problem of the azeotrope MA-MeOH still cannot be solved.
Moreover, due to the use of heterogeneous catalyst, typically strong acidic cation ion exchanger resin in the hydrolysis process, there must be some internal equipments to place catalyst in the reactive distillation column [10-13]. Three additional distillation columns are still needed in order to separate the hydrolysate. A new process which consists of a fixed-bed reactor for hydrolysis and a conventional distillation column for separation was developed in this paper. In this process,MeOH is withdrawn from the side of the column and HAc from the bottom of the column. Feasibility of the process was evaluated and the optimal technology conditions were obtained through experiment and simulation.
2 EXPERIMENTAL
A lab-scale experiment was carried out to study the coupled process. The experimental schematic diagram is shown in Fig. 1.
Methyl acetate and SK-1A ion exchanger resin catalyst used in the experiment were supplied by the Fujian Spinning and Chemical Fiber Group Company and the Mo-Ti stainless steel θ shape packing by Tianjin University.
The concentration of acetic acid in samples was measured by acid-base titration while those of MA and methanol were measured by a VARIAN CP-3800
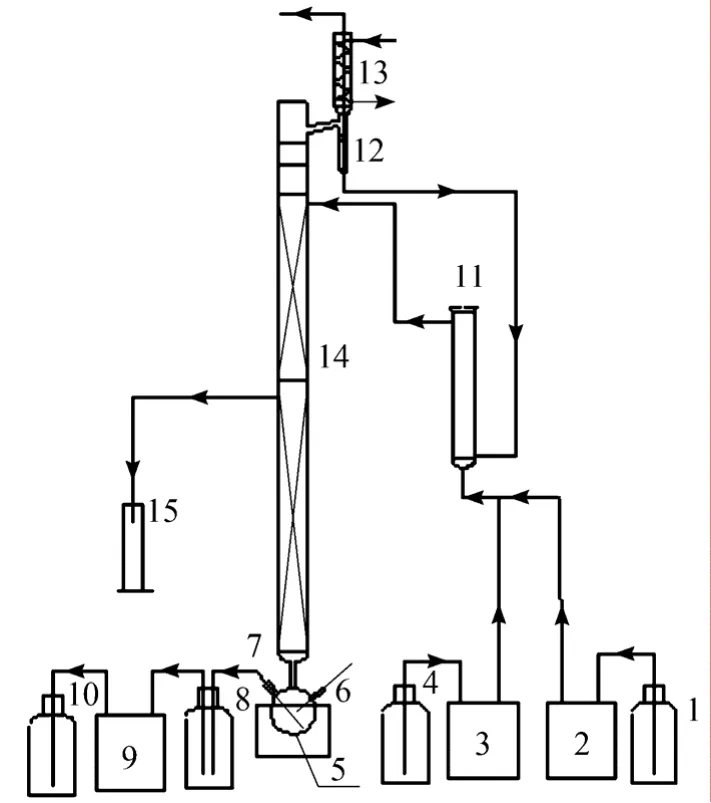
Figure 1 The schematic diagram of experimental setup1—deionized water container; 2—constant flow pump; 3—laminar current pump; 4—methyl acetate container; 5—flask;6—thermometer; 7—standby mouth; 8—bottom outlet; 9—outlet pump; 10—bottom product container; 11—fixed-bed reactor; 12—flowmeter; 13—condenser; 14—packed distillation column; 15—side product container
GC equipped with a 30 m×0.25 mm XP-1701 capillary column and hydrogen flame ionization detector.The GC conditions were: injector temperature 200 °C,detector temperature 250 °C and a stepwise column temperature. The initial column temperature was 50°C for 3 min, heated up to 120 °C at 15 °C·min-1, and then held for 1 min.
3 SIMULATION MODELING
Simulations were performed using the Aspen Plus?(Aspen Technology Inc., Aspen Plus?, Version 10.1) software package. The distillation unit was modeled using the RadFrac model in Aspen Plus?. It uses the equilibrium stage model, also called the MESH model, and consists of a set of nonlinear equations to represent material balance, vapor-liquid equilibrium, mole fraction summation, and heat balance.The RPlug model, based on plug flow model, was used to model the fixed-bed reactor. The reactions in the reactor were based on power-law kinetics.
3.1 Physical equilibrium
An important consideration in distillation simulation is the choice of physical equilibrium model and the ability to reliably predict multicomponent vaporliquid equilibrium (VLE) and liquid-liquid equilibrium (LLE). Reliable VLE and LLE are needed to establish distillation boundaries and to determine if and where azeotropes and phase separation occur.Several equations can be used to model methyl acetate-methanol-water-acetic acid system such as Van Laar, NRTL, UNIQUAC. Dirk-Faitakis [14] recommended NRTL model. Taking the non-ideality of the vapor phase caused by dimerization of acetic acid into account, Hayden-O’Connell(HOC) method was chosen. Therefore, the NRTL-HOC property method was used in this work because of the presence of high concentration of acetic acid.
Binary interaction parameters were taken from Aspen Plus?data banks. Table 1 summarizes the interactive parameters used in vapor-liquid equilibrium calculation.
It was reported that methyl acetate-methanol system exhibits an azeotrope with minimum boiling point at 53.5 °C and 0.6618 mole fraction methyl acetate in the vapor at 101.32 kPa [15]. Methyl acetate-water exhibits a homogeneous azeotrope and also displays a miscibility gap in the region at ambient pressure.Besslinget al. [16] reported a minimum boiling azeotrope at 0.92 mole fraction methyl acetate at temperature of 56.1 °C. Azeotropes predicted by NRTL-HOC model atP=101.3 kPa are shown in Table 2.
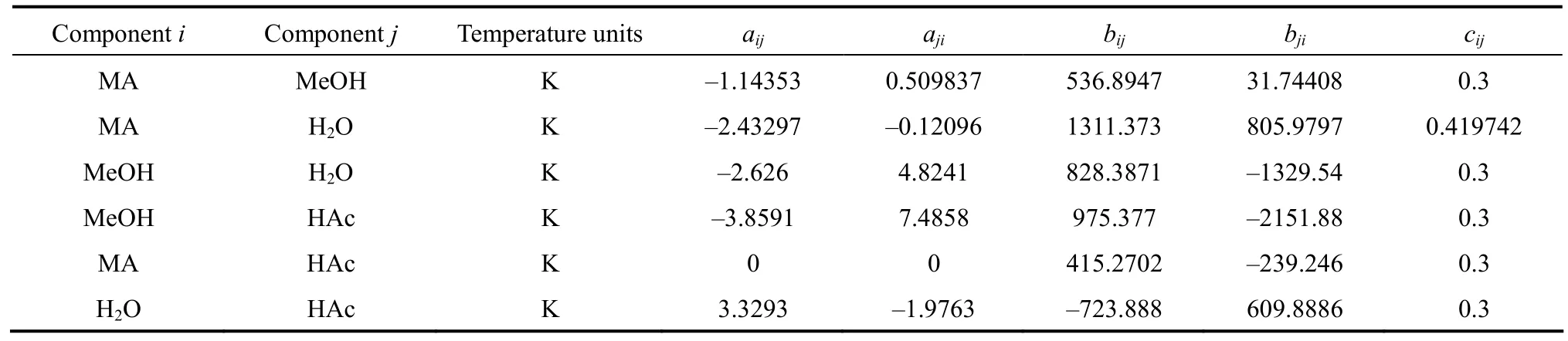
Table 1 Summary of NRTL binary interaction parameters used in the prediction of equilibrium data
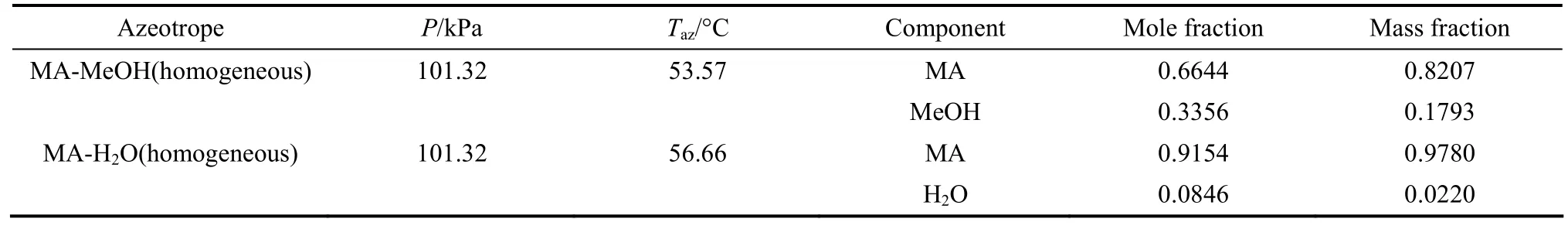
Table 2 Azeotropes predicted by Aspen Plus? using NRTL-HOC model
3.2 Reaction kinetics
The hydrolysis of methyl acetate is a reversible reaction with the following expression:
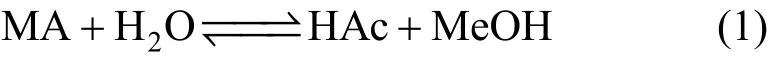
The equilibrium constant of this reaction is 0.15 at 55 °C. According to the reaction mechanism the pseudohomogeneous reaction kinetics can be expressed as:
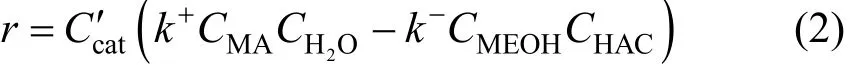
A detailed study of this reaction has been done in our previous work [17] and the forward and reverse reaction rate constant (k+andk-, respectively ) can be expressed by following equations.
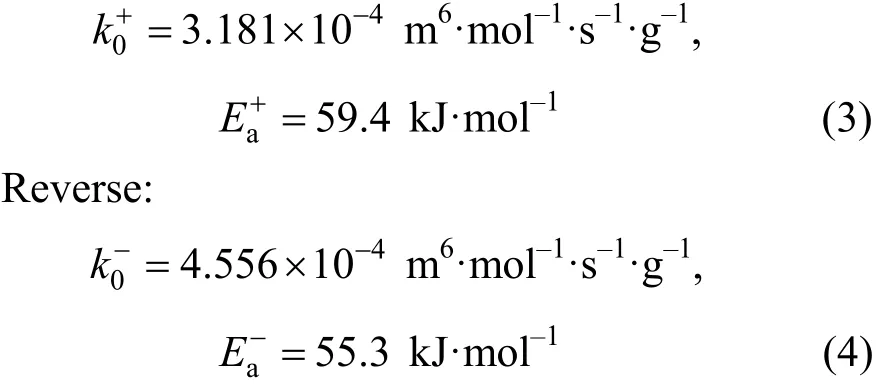
Forward:ThecatC′ in Eq. (2) was 7.8×105g·m-3(catalyst mass/reaction liquid) in this experiment. And this kinetics equation was verified by experiments.
4 RESULTS AND DISCUSSION
4.1 Simulation of coupled reactor/column process
After confirmed the physical equilibrium and reaction kinetics, the continuous reaction and distillation process shown in Fig. 1 can be simulated. The hydrolysate distillation column consists of a 20 stages column with none condenser and a partial reboiler. The height of packed section of the experimental column is 1.6 m. According to the manufacturer, one meter θ shape packing equals 12 theoretical stages, so the theoretical stages of packed section is 20 including reboiler.Compared the results of simulation with experimental data in the next part, 20 theoretical stages is suitable.The side product location is 0.9 m from the bottom of the column, thus the stage is 11. In order to meet demands of MeOH mass concentration of less than 0.5%in bottom product in the PTA and PVA plants, we set a design value of 0.4%.RL/Fof 4.5 andRW/MAof 5.0 are feasible parameters obtained by several simulations.Other parameters are the same as the experimental data. All the parameters for the base case simulation are given in Table 3. All feed streams enter at 25 °C.
The mass basis distribution in the hydrolysate distillation column is shown in Figs. 2 and 3. Fig. 2 indicates the methanol concentrations in vapor phase at 6th-14th stages are more than 0.8 with less than 0.15 water, 0.03HAc and rare MA. That means 6th-14th stages are the feasible locations for side withdrawal and the side product is readily to be refined by simple distillation to attain high purity methanol. In this way the separation problem of the azeotrope MA-MeOH has been solved. The bottom product only composes of acetate acid and water. The mass concentration of acetic acid is higher than 40% and meets the requirement of PTA plant.
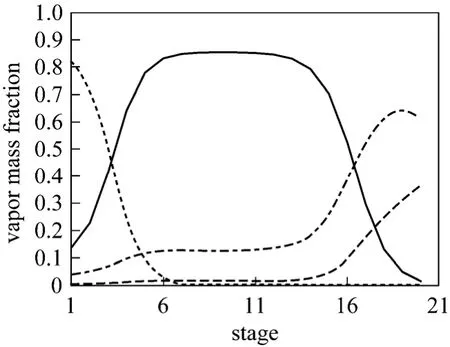
Figure 2 Vapor profiles in the hydrolysate separation columnMEOH; HAc; MA; Water
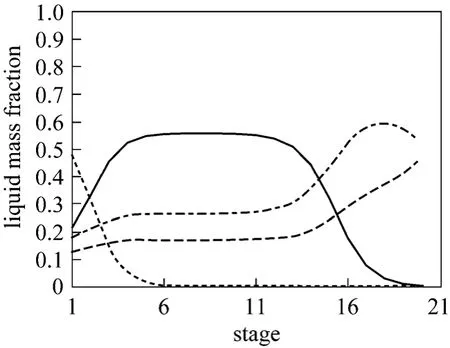
Figure 3 Liquid profiles in the hydrolysate separation columnMEOH; HAc; MA; Water

Table 3 Parameters used in the simulations

Table 4 The results of experiment and simulation (mass fraction)
4.2 Experimental verification
The results of the experiment and simulation are compared in Table 4. Here, the experimental value ofRSP/Fwere adopted by simulation. As shown in the table,most of the side product is composed of more than 80%(by mass) methanol(xSP-MeOH) and less than 2% (by mass)MA(xSP-MA), which is readily to get >96% (by mass)methanol in the following distillation. At the same time, the mass concentration of the HAc in the bottom(xW-HAc) is more than 46%, which satisfied the requirement in the PVA manufacture. The experimental results agree with those predicted by simulation. Thus,the simulation model can be used to discuss the influence of different parameters on the process.
4.3 Feasible technology conditions
RW/MAandRL/Fare the most important parameters in this process. Their value decide whether MA can be hydrolyzed completely or not. Simulation work has been done to study the effect ofRL/Fon the side product, whileRW/MAis kept constant. As shown in Figs. 4 and 5, the increase ofRL/Fwill raise MeOH concentration in the side product and reduce MA impurity whenRW/MAis 4 and 5. Fig. 5 shows that there is a critical value ofRL/F. Below it, MA in the side product is quite high, which means that MA is not hydrolyzed completely. Above it, MA is near zero, indicating about 100% conversion of MA. The critical value is 3 atRW/MA=5.0 and about 4.5 atRW/MA=4.0. Attentions must be paid that atRW/MA=3.0 there always contains a large amount of MA in the side product no matter how muchRL/Fis. It can be predicted that the critical value will further decrease if a largerRW/MAis provided. But the acid concentration in the bottom will decrease. Proper operation conditions can be chosen with the specification of the manufacturer. Under conditions thatRW/MA=4.0-5.0 andRL/Fabove critical value, more than 80% (by mass) MeOH in the side product and more than 46% (by mass) HAc in the bottom can be obtained (Fig. 6).
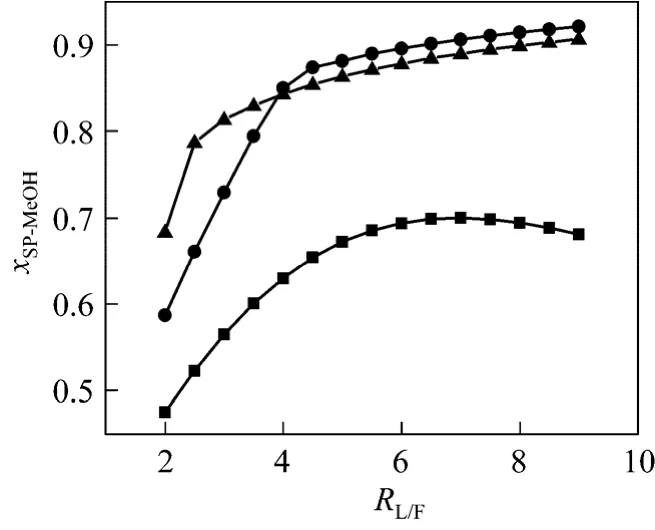
Figure 4 Effect of RL/F on MeOH concentration inside productRW/MA: ■ 3; ● 4; ▲ 5
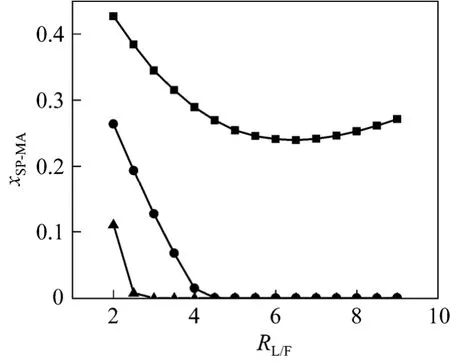
Figure 5 Effect of RL/F on MA concentration in side product RW/MA: ■ 3; ● 4; ▲ 5
In the simulation mentioned above, a Design specs-Vary was set, which varied the amount of side product to keep MeOH mass concentration <0.4% in the bottom. At the sameRW/MA, the volume of side product decreases with the increase ofRL/F. TakingRW/MA=4.0 for example, theRSP/Fchanges from 0.68 to 0.57, whileRL/Fchanges from 2 to 9. Because of the decrease ofRSP/F, the quantity of water in the side product reduces. On consequence, the concentration of HAc in the bottom product decreases with the increase ofRL/Fwhen the MA is totally hydrolyzed.
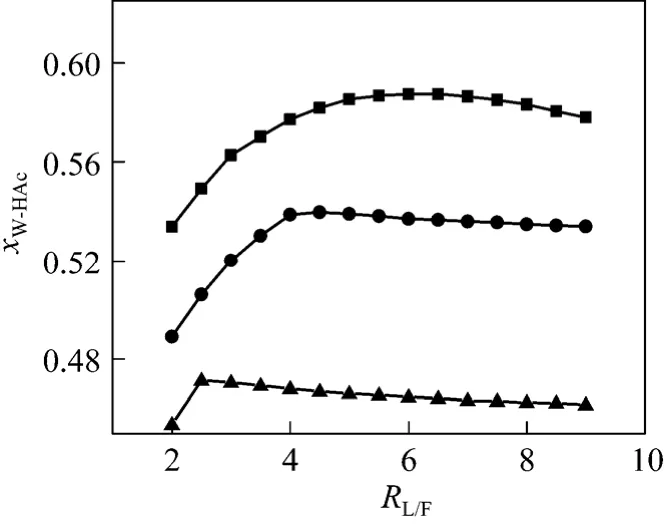
Figure 6 Effect of RL/F on HAc concentration inbottom productRW/MA: ■ 3; ● 4; ▲ 5
5 SUPERIORITIES OF THIS PROCESS
A further simulation was carried out to examine the energy consumption of the proposed process. A simple distillation column was added to purify methanol. As shown in Fig. 7, the side product is distillated to get methanol with high purity and the bottom product is sent back to fixed-bed reactor. Process flow diagram (PFD) of the catalytic distillation process is shown in Fig. 8. This technology [18, 19] has industrialized in several PTA and PVA plants. Ref. [19] describes the process in details.
Based on data given by a certain petrochemical corporation, energy consumption was compared between this process and the catalytic distillation one(the old process). The mass compositions of the feed MA stream are as follows: MA 94.625%, MeOH 0.625%,and water 3.75%. The purity specifications that the HAc in the bottom equals to 46% (by mass) and product MeOH amounts to 96% (by mass) are required.
The two processes were both simulated by Aspen plus based on the thermodynamics and kinetics which have been discussed earlier. The operation conditions for catalytic column are: the volume ratio of reflux to MA feed, 3.0, and the molar ratio of H2O to MA feed,4.0. The catalytic column can be simulated using these data by the model introduced by Wuet al. [20]. The energy consumption of other distillation columns can be calculated easily according to separation requirements. The energy consumptions of the main equipments of the two processes are summarized in Tables 5 and 6,respectively.
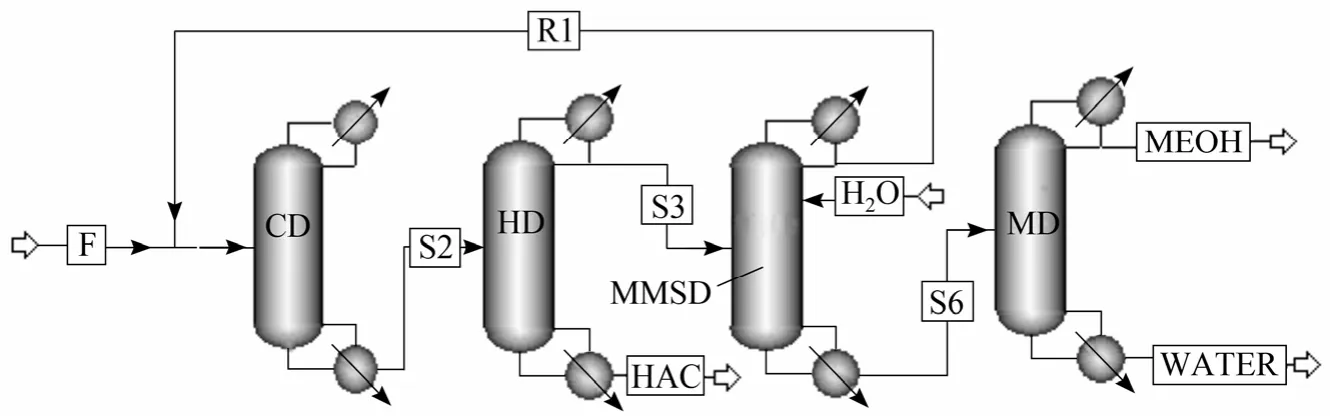
Figure 8 PFD of the catalytic distillation process

Table 5 The energy consumptions of the main equipments of the old process (kW)

Table 6 The energy consumptions of the main equipments of the new process (kW)
Obviously the new process have saved a main equipment by comparing Figs. 7 and 8. The MA-MeOH separation column is not needed in which the side-withdraw of the hydrolysate distillation column can solve the separation problem of the azeotrope MA-MeOH. Table 5 shows that the sum of energy consumptions of fixed-bed and hydrolysate distillation column in the new process is 747.81 kW. It is much smaller than that of CD column and hydrolysate distillation column of 1222.31 kW in the old process. As known, MA hydrolyzes with water in liquid phase [21].But MA is vaporized in the reactive distillation zone of the CD column. Thus, the reflux quantity on the top of the CD column needs to be increased to promote reaction. More reflux quantity, more energy consumption. The energy consumption of MeOH distillation column are also reduced greatly as shown in Table 6.In the old process, extra water enters on the top of the MA-MeOH distillation column to extract MeOH from MA, so methanol concentration is much lower than that of the side product in the new process. The vapor feeding used in the new process also saves a lot of energy. The energy consumption gap between the new and the old is up to 47.6%. Therefore, no matter in the capital investment or in the operation cost, the new process takes every superiority.
6 CONCLUSIONS
Both simulation and experiment have proven that MA can be hydrolyzed completely in the coupled system of fix-bed reactor and distillation column with vapor side product. However, RW/MAshould be greater than 3.0 and RL/Fabove the critical value at every RW/MA. Under the conditions that RW/MA=4.0-5.0 and RL/Fabove the critical value the side product will contain more than 80% (by mass) MeOH and less than 2% (by mass) MA, while the bottom will contain more than 46% (by mass) HAc. Only a simple column is needed to attain MeOH with high purity. Compared with the catalytic distillation process we proposed before, this process can save 47.6% of energy consumption and a distillation column.
NOMENCLATURE
Ciconcentration of component i, mol·m-3
Eaapparent activation energy, kJ·mol-1
k rate constant, m6·mol-1·s-1·g-1
k0pre-exponential factor, m6·mol-1·s-1·g-1
R universal gas constant, J·mol-1·K-1
RL/Fdistillate to feed MA volume ratio, m3·m-3
RSP/Fvolume ratio of side product to feed MA, m3·m-3
RW/MAmolar feed ratio of water to methyl acetate, mol·mol-1
r reaction rate, mol·s-1·m-3
xSP-HAcmass concentration of acetate acid in the side product
xSP-MAmass concentration of methyl acetate in the side product xSP-MeOHmass concentration of methanol in the side product
xW-HAcmass concentration of acetate acid in the bottom product
xW-MeOHmass concentration of methanol in the bottom product
Superscripts
+ forward reaction
- reverse reaction
Subscripts
az azeotrope cat catalyst
1 Ma, Y.G., Mou, C.G., Wu, S.H., Technology of PVA Production, Textile Industry Press, Beijing (1986). (in Chinese)
2 Wang, C.X., “Study on hydrolysis of methyl acetate in a catalytic distillation column”, Chin. J. Chem. Eng., 9, 382-387 (2001).
3 Pan, Y.B., Li, W.X., Shen, P.D., Wan, H., Han, M.J., Guan, G.F.,“Study on hydrolysis of methyl acetate from production of purified terephthalic acid by catalytic distillation”, Chem. Reac. Eng. & Tech.,25, 132-136 (2009). (in Chinese)
4 Wang, J.F., Ge, X.D., Wang, Z.W, Jin, Y., “Experimental studies on the catalytic distillation for hydrolysis of methyl acetate”, Chem.Eng. Technol., 24, 155-159 (2001).
5 Sander, S., Flisch, C., “Methyl acetate hydrolysis in a reactive divided wall column”, Chem. Eng. Res. Des., 85,149-154 (2007).
6 Yuan, P.Q., Chen, Z.M., Liu, T., Yuan, W.K., “Hydrolysis of methyl acetate under near or supercritical condition”, Chem. J. Chinese U.,24, 1241-1245 (2003). (in Chinese)
7 Fuchigami, Y., “Hydrolysis of methyl acetate in distillation column packed with reactive packing of ion exchange resin”, J. Chem. Eng.Jpn., 23, 354-359 (1990).
8 Xiao, J., Liu, J.Q., Li, J.T., Jiang, X.H., Zhang, Z.B., “Increase MeOAc conversion in PVA production by replacing the fixed bed reactor with a catalytic distillation column”, Chem. Eng. Sci., 56, 6553-6562 (2001).
9 Wang, L.E., Su, W.R., Zhao, Z.S., Liu, J.Q., Wu, Y.X., Shen, J.N., Qiu, T.,“Industrial application of the catalytic distillation technique in hydrolysis of methyl acetate”, Viny. Commu., 21, 10-12 (2001). (in Chinese)
10 Wu, Y.X., Wang, L.E., Zhao, Z.S., Tan, T.E., “Mass transfer model for catalyst capsule in catalyst distillation (I) mathematical model”,CIESC J., 53, 503-507 (2002). (in Chinese)
11 Wu, Y.X., Wang, L.E., Zhao, Z.S., Tan, T.E., “Mass transfer model for catalyst capsule in catalyst distillation (II) measurement and calculation of effectiveness factor”, CIESC J., 53, 508-512 (2002). (in Chinese)
12 Kim, K., Roh, H.D., “Reactive distillation process and equipment for the production of acetic acid and methanol from methyl acetate hydrolysis”, U.S. Pat., .5970770 (1998).
13 Moritz, P., Hasse, H., “Fluid dynamics in reactive distillation packing Katapak?-S”, Chem. Eng. Sci., 54, 1367-13741 (1999).
14 Dirk-Faitakis, C.B., An, W.Z., Lin, T.B., Chuang, K.T., “Catalytic distillation for simultaneous hydrolysis of methyl acetate and etherification of methanol”, Chem. Eng. Process., 48, 1080-1087 (2009).
15 Gmehling, J., B?lts, R., “Azeotropic data for binary and ternary systems at moderate pressures”, J. Chem. Eng. Data., 41, 202-209 (1996).
16 Bessling, B., L?ning, J.M., Ohligschl?ger, A., Schembecker, G.,Sundmacher, K., “Investigations on the synthesis of methyl acetate in a heterogeneous reactive distillation process”, Chem. Eng. Technol.,21, 393-400 (1998).
17 Wu, Y.X., Zhao, Z.S., Wang, L.E., Zhao, S.Y., “Kinetics of hydrolysis of methyl acetate and the effectiveness factor of catalyst capsule”,Eng. Chem. Meta., 20, 241-246 (1999). (in Chinese)
18 Wang, L.E., Liu, J.Q., Su, W.R., Zhao, Z.S., Zheng, W.H., Zhang,J.L., “Catalytic distillation process for methyl acetate hydrolysis and its equipments”, China Pat., 97101306.3 (1997).
19 Wang, L.E., Zhao, Z.S., Qiu, T., Zhao, S.Y., Zheng, H.D., Qiu, T.R.,Su, W.R., Xie, Y.H., Luo, D.H., “Byproduct methyl acetate hydrolysis process and its equipments in the industry of PTA”, China Pat.,200610124556.7 (2006).
20 Wu, Y.X., Tan, T.E., Wang, L.E., Zhao. Z.S., “Simulation of the catalytic distillation process for hydrolysis of methyl acetate”, Eng.Chem. Meta., 21, 24-29 (2000). (in Chinese)
21 P?pken, T., G?tze, L., Gmehling, J., “Reaction kinetics and chemical equilibrium of homogeneously and heterogeneously catalyzed acetic acid esterification with methanol and methyl acetate hydrolysis”, Ind.Eng. Chem. Res., 39, 2601-2611 (2000).
 Chinese Journal of Chemical Engineering2010年5期
Chinese Journal of Chemical Engineering2010年5期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis of Petroleum Sulfonate Surfactant by Different Sulfonating Agent with Application of HIGEE Technology*
- Caffeine Crystallization Induction Time Measurements Using Laser Scattering Technique and Correlation to Surface Tension in Water and Ethanol
- On-line Measurement for Ohmic Resistance in Direct Methanol Fuel Cell by Current Interruption Method*
