尿素對土壤中碳鋼微生物腐蝕的影響
孫 成 李喜明 許 進 閆茂成 王福會 王振堯
(中國科學(xué)院金屬研究所,金屬腐蝕與防護國家重點實驗室,沈陽110016)
1 Introduction
Corrosion of carbon steel in soil is affected by the metal characteristics,but is more attributed to the ambient soil environment such as soil humidity,acidity,soil salinity,and composition of microbes and so on.1-4Microbes are especially abundant in soil and have been widely recognized as one of the major factors that influence the corrosion behavior of buried metal structures,known as microbiological induced corrosion (MIC).5-11One of the key elements of MIC is sulphate-reducing bacteria(SRB),12-16the role of which in the corrosion of metals is due to the chemical activities associated with their metabolism,growth,and reproduction.Numerous studies on SRB corrosion mechanism have been reported:7,12,17,18the classical cathodic depolarization theory,the metabolite product theory,the effect of iron sulfide and hydrogen sulfide(H2S)and so on.Although conflicts exit among these mechanisms,agreement has been reached on the aggressive activity of SRB and their corrosive role.SRB also has influences on the environment parameter.Javaherdashti19mentioned that SRB had deteriorating impact on engineering materials performance,as well as inducing harmful effect on health and agriculture.Environment features,such as the moisture content,the type of soil,re-sistivity,pH value,and other factors,should be used as a basis for assessing the probable causes of the bio-corrosion.
Previous researches have almost been in simulated soil solution or in uncontaminated soil.It is unavoidable that a large amount of carbon steel is buried in farmland.With agricultural development,more fertilizers are used on farmland in order to raise crop outputs,of which nitrogenous fertilizer,urea,is the most common.It is known that nitrogen is one of the essential elements to constitute protein and nuclelic acid of bacterial. Hence urea can be served as energy source for microbe growth.Whether urea can promote the growth of SRB in soils has not been studied.Besides,Li et al.20reported that urea can inhibitor corrosion of the magnesium alloy AZ91D.Mozheiko et al.21showed that urea application decreased the corrosion of the carbon steel in the presence of the chorine ions.The effect of urea on corrosion of carbon steel in soil is totally unknown.
In the present paper,the effect of urea on the microbe corrosion of carbon steel Q235 has been investigated at constant soil moisture(10%)by using X-ray photoelectric spectroscopy (XPS),scanning electron microscopy(SEM),as well as energy dispersive X-ray(EDX)analysis,and microbiological test methods.
2 Experimental
2.1 Materials
Q235 carbon steel,with a composition(w,%)of 0.30C, 0.019P,0.029S,0.01Si,0.42Mn,and balance Fe,was cut into two different shapes of sheet:10 mm×10 mm×3 mm and 20 mm×20 mm×3 mm.The coupons were abraded according to the national standards GB5776-86 with a series of grit papers (200#,400#,600#,800#,and 1000#)and cleaned in acetone and alcohol,and then dried.The working and counter electrodes were embedded in epoxy resin to give working areas of 10 mm×10 mm for electrochemical measurements in a threeelectrode cell.The working surface was abraded as described above and cleaned with acetone and distilled water.All the prepared coupons were sterilized under ultraviolet rays prior to an experiment.
The soil used in this work was taken from Shenyang,Liaoning province of China.The chemical compositions of the soil are given in Table 1.The soils were sterilized by heating at 121.8°C at high pressure.The moisture content of the soil was kept at 10%during the experiment.
The SRB used in this study,desulfovibrio desulfuricans,was isolated from the soil.SRB cultures were incubated in an anaerobic environment in theAPI RP-38 medium,containing MgSO4· 7H2O 0.2 g·L-1;KH2PO40.5 g·L-1;NaCl 10.0 g·L-1;ascorbic acid 1.0 g·L-1;sodium lactate 4.0 g·L-1;yeast extract 1.0 g· L-1;Fe(NH4)2(SO4)20.02 g·L-1.The pH value of the culture solution was between 7.0 and 7.1.Prior to an experiment,SRB species were activated in an incubator for 24 h,and then were added to the inoculated soil in the testing devices.
Urea was manufactured in Sinopharm,the nitrogen content was 46.4%(w)according to the national standard GB2440-2001.Before an experiment,different amounts of urea were measured by electronic analytical balance up to 0.0001 g,and then were sterilized under ultraviolet rays.
2.2 Electrochemical measurements
All the experiments were performed in a sterile plastic container with a diameter of 20 cm and a height of 25 cm.The container was sealed to prevent soil moisture evaporation.Specimens were buried at a depth of 10 cm below the surface of the soil.The prepared urea-containing soils were divided into two groups,one with SRB and the other without SRB.All the experiments were performed for 65 days at room temperature.
When experiments were carried through for 1,15,40,65 days,the amount of SRB,obtained from the soil around the specimens,was tested according to the most probable number (MPN)methods.
The soil redox potential is mainly affected by the amount of soil organic matter,salt compositions,and aeration conditions. It has been considered indicative of the risk to bacterial corrosion.In this study,the soil redox potential was measured by a multimeter,5 Pt electrodes and a saturated calomel electrode (SCE).The redox potential was calculated from the following relationship:
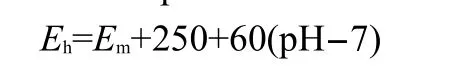
where,Ehis the redox potential(mV)at pH 7(standard hydrogen scale);Emis the mean of the potential measured from the five platinum electrodes(mV).22
During an experiment,electrochemical impedance spectroscopy(EIS)and the polarization curves of the carbon steel were investigated at scheduled intervals.All experiments were performed in a three-electrode electrochemical cell,with a graphite electrode used as the counter electrode,and a saturated Cu/ CuSO4electrode(CSE)as the reference electrode.The tests were conducted using the PARSTAT 2273 electrochemical measurement system manufactured by the U.S.EGG Company.In the EIS,an alternating current signal with a frequency range from 100 kHz to 1 mHz and an amplitude of 10 mV was applied to the working electrode at the corrosion potential.The EIS data obtained were modeled and simulated using the ZSimpWin software supplied by the PARSTAT2273.When measuring the polarization curves,potentiodynamic polarization was used and the potential scanning velocity was 0.5 mV· s-1,the scope being±0.25 V.The results were modeled and simulated using the Origin software.
2.3 Weight loss tests

Table 1 Compositions of the soil(mg·g-1)
The samples of 20 mm×20 mm×3 mm were used for weight loss tests.Prior to the experiment,the samples were weighed to a precision of 0.1 mg.After the experiment,the extracted specimen was pickled in a mixture containing HCl 500 mL, urotropine 20 g,and H2O 500 mL for 10 min at room temperature,then cleaned with water,dried at 105.8°C for 30 min in a furnace,cooled,and weighed.The same process was repeated until the difference between the last two results was less than 0.0001 g.Weight-losses were calculated and converted into uniform corrosion rates.Each experiment used triplicate specimens to guarantee the reliability of the results.
2.4 Analysis of corrosion products
After the experiment,the surface appearance of the buried samples was observed and analyzed using scanning electron microscopy in combination with energy-dispersive X-ray analysis.Additionally,X-ray photoelectron spectroscopy was used to analyze qualitatively the elements in the corrosion product.
3 Results
3.1 Characteristics of SRB growth
The number of SRB in inoculated soils is shown in Fig.1. The amount of SRB in soil(CFU·g-1,Colony-Forming Units per gramme soil)with urea was larger than that in soils without urea.It has indicated that urea can promote the growth of SRB as energy resource.Moreover,at the early stage,the amount of SRB in soils with a lower urea concentration(0.05%and 0.1%)was considerably more than that found in the other experimental groups.It shows that a lower urea concentration is more helpful for the survival of SRB.As the experiments progressed,the amount of SRB greatly decreased due to the exhaustion of the nutrition.Because some urea in the soils had been consumed,a previously high urea concentration(0.2% and 0.3%)changed to be more suitable for the survival of SRB.So the amount of SRB in soils containing a higher urea concentration(0.2%and 0.3%)was larger than that for other groups.
3.2 Soil redox potential and corrosion potential
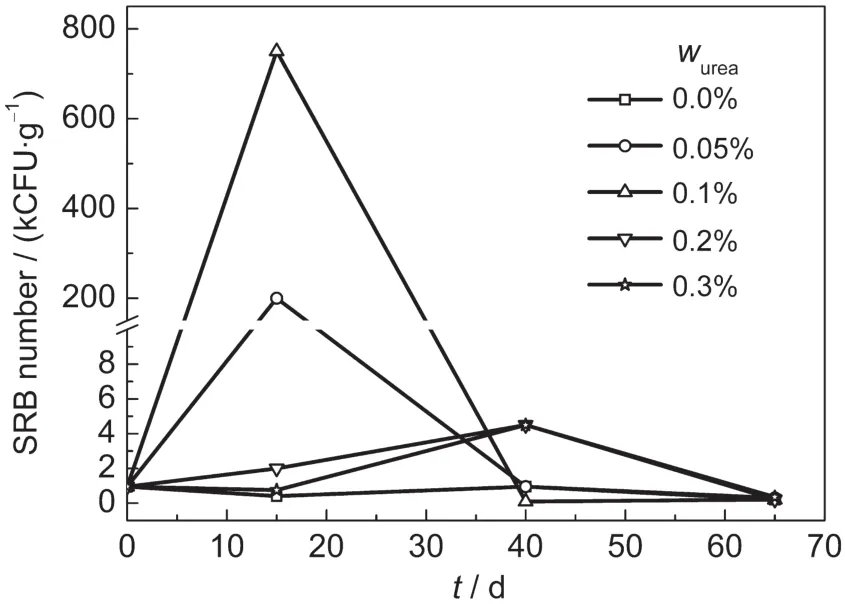
Fig.1 Growth curves of SRB in soils containing urea

Fig.2 Soil redox potential(Eh)distributions with time
The soil redox potential is generally considered indicative of the risk to bacterial corrosion.3Fig.2 shows the changes of soil redox potential(vs standard hydrogen electrode(SHE))in inoculated soils and in sterile soils.The redox potential decreased with increasing urea concentration both in inoculated soils and in sterile soils.In inoculated soils,it is true that the more negative the soil redox potential,the more aggressiveness the steel suffered.23However,in sterile soils,the soil redox potential is not related to the tendency of corrosion.That was because urea changed the content of organic matter of the soil,the higher urea concentration the higher organics content.Thus it is arbitrary to address soil redox potential as indication of soil erosion.

The corrosion potential of the carbon steel(Fig.3)increases over time with some fluctuations,and then remains stable.This is due to dynamic changes of corrosion products on the surface of samples.The corrosion potential in soils with SRB was more negative than that without SRB,which indicates that the presence of SRB increases the corrosive tendency of the steel. In sterile soils,the corrosion potential of the carbon steel tended to be more positive with urea concentration.However,in inoculated soils,the corrosion potential fluctuated with increasing urea concentration.
3.3 Polarization behaviors
The polarization curves of Q235 carbon steel in soils at different urea concentrations with or without SRB after 20 and 65 days are shown in Fig.4 and the fitting results are listed in Table 2.
In soils with SRB,the corrosion rate increases with urea concentration(Fig.4 and Table 2).What is more,the differences between the anode and cathode Tafel slopes(βaand βc)are not great except in soils containing urea of 0.3%after 20 days, which shows that the corrosion process is controlled by both anode and cathode processes.In soil containing 0.3%urea after 20 days,βais dramatically larger than βc,which indicates that the corrosion rate is controlled by the anode process.24This is probably because the corrosion products prevent the dissolution of the iron.
In soils without SRB,after 20 days,compared with the corrosion rate of the carbon steel in soil without urea,the corrosion rate increased in soil with 0.05%and 0.3%urea and yet decreased in soil with 0.1%and 0.2%urea.After 65 days,the anode process was prevented with the increase of urea concentration.The same phenomena that βawas larger than βcespecially in the group with 0.2%and 0.3%urea,indicates that the corrosion rate is also controlled by the anode process.In addition,the corrosion rate of the carbon steel in soil with SRB is dramatically larger than that in sterile soil,which further indicates that the presence of SRB increases the corrosion tendency of the steel.

Fig.4 Polarization curves of carbon steel buried in soils with and without SRB for 20 and 65 days

Table 2 Fitting results of polarization curves
3.4 EIS
Electrochemical impedance spectroscopy(EIS)was used to investigate the electrochemical properties of the corroded surface on the carbon steel over time.10,25,26Fig.5 shows EIS plots of the carbon steel Q235 in soils containing different urea concentrations with SRB after 1 day,20 days and 65 days,respectively.In the Bold plot,the time constant at high frequencies describes the electrical properties of corrosion products,while the constant at low frequencies is associated with the charge transfer resistance related to the iron dissolution reaction and inversely proportional to the active area.27The corresponding Nyquist plot and equivalent circuits are given in Fig.6 and Fig.7,respectively,and the fitting data are listed in Table 3.Rsrepresents the soil electrolyte resistance,and Rfrepresents all the resistance of products on the surface of sample.Rctand Qdlrepresent the charge transfer resistance and the double layer capacitance respectively,and W represents the Warburg impedance.

Fig.5 EIS plots of the carbon steel Q235 in soils containing different urea concentrations with SRB after 1,20,and 65 days

Fig.6 Nyquist plot of the EIS of the carbon steel Q235 containing different urea concentrations with and without SRB after 1,20,and 65 days in soils

Fig.7 Equivalent circuits of the different time constants(a)one time constant;(b)two time constants;(c)two time constants and one Zw
On the first day,there was little corrosion products on the steel surface,so the resistance at high frequency was generally low in all experiment groups.One time constant was found (Fig.7(a)).In inoculated soils,the resistance at low frequency (Rt,shown in Table 3)decreased dramatically in soils with urea which indicated that urea promoted the steel corrosion.However,with increasing urea concentration,Rtincreased because a lower urea concentration was more beneficial for the growth of SRB,in accordance with the results discussed before.In sterile soils,the soil electrolyte resistance was much higher than that in inoculated soils,and Rtdramatically increased with increasing urea content.It was because urea was adsorbed on the steel surface to prevent the iron dissolving.
After 20 days,in inoculated soils,there were corrosion products on the steel surface,so two time constants were observed and the equivalent circuit is shown in Fig.7b.Rtin soils with 0.05%and 0.1%urea was larger than that in other experiment groups,because at the early experiment there were more corrosion products on the steel surface in soils containing a lower urea concentration.In soils with urea concentrations of 0.2% and 0.3%,Rtdecreased considerably which further indicated the former conclusion that a higher urea concentration became helpful for the growth of SRB.In sterile soils,there were several pits,so two time constants were also observed.Rtalso increased greatly with increasing urea concentration which showed that urea prevented corrosion of the carbon steel in soil,and this influence strengthened with increasing the amount of urea.
Over experiment time,there were more and more corrosion products on the surface in soils with SRB,and then the diffu-sion of corrosion products became more difficult.Hence after 65 days,there are two time constants and a Warburg impedance in inoculated soils,which indicates that the process is controlled by concentration polarization.28The resistance in high frequency decreased with urea addition,especially in soils with urea concentrations of 0.2%and 0.3%,that was due to the loose corrosion products and some of the corrosion products had shed off.In soils without SRB,Rtstill increased with increasing the content of urea,except in soils with 0.2%urea which appeared a Warburg impedence.That was probably because of the ion diffusion through the pits.

Table 3 Fitting results of the EIS in soils after 1 day,20 days,and 65 days
3.5 Weight loss analysis
Average corrosion rates(Fig.8)were calculated from the mass losses of the samples.In soils without SRB,the average corrosion rate decreased with the increase of urea concentration,which proved that urea may decrease the corrosion tendency of the carbon steel in soils.In soils with SRB,the average corrosion rate of the carbon steel went up with an increase in urea concentration,with the highest corrosion rate at a urea concentration of 0.2%.A proper amount of urea can benefit the growth of SRB,which promotes the corrosion of the carbon steel.The excessive urea may be served as protective organic matter on the steel surface.That is why the corrosion rate of steel in soils with 0.3%decreased compared to that in soils with 0.2%.The results also show that the corrosion rate of the carbon steel in inoculated soil is dramatically larger than that in sterile soil,which further proves that the presence of SRB does increase the corrosive tendency of the steel.
The macroscopic photograph of the Q235 steel(Fig.9) shows that the surface of the carbon steel in soil with SRB shows a great deal of corrosion pits,while in soil without SRB, there are only several small pits.Pitting corrosion is characteristic of the action of the sulfate reducers on steels.12
3.6 Analysis of corrosion products

Fig.8 Average corrosion rates of the Q235 steel
A micrograph of the carbon steel Q235 in inoculated soils containing 0.2%urea has been selected to analyze the changing of surface appearances.
In the cross-section of the corrosion products(Fig.10),there were four films of different morphologies and chemical compositions.The outmost layer included two different films with different atomic ratios of O/Fe:the upper larger than the lower. As the amount of corrosion products gradually increased with experimental time,the upper part contacted the soil directly which meant more access to oxygen,and this tended to produce ferric oxide.The lower part,on the other hand,tended to produce ferrous oxide due to the lower availability of oxygen. Over time,the outer layer could not withstand the tension of the increased corrosion products,and cracks appeared which allowed some kinds of ions in the soil to infiltrate.This was the cause of the loose second layer.The infiltration of oxygen led to the third layer which was very thin and not stable.Over time,the third layer cracked,and soil grain went through.The ratio of O/Fe in the third layer was also larger than that in the fourth layer for the same reasons as given before.The interface between different layers was not clear which was not recognized by the EIS,so only two time constants were observed.

Fig.9 Macroscopic photographs of the samples after 65 days
Small amounts of Ca2+and Cl-were present in the corrosion products.There were impurities introduced from the soils.The element sulfur was not observed,which may be due to the amount of iron sulfide being insufficient to be detected by EDX,but it was detected by X-ray photoelectric spectroscopy (Fig.11).After brushing the upper corrosion products,the sample was investigated after varying the etching time by XPS. The outer products were found to be mainly made up of Fe2O3, while the inner products included FeO and FeS2.The ratio of FeO/FeS2increased with etching time,which indicates that the nearer to the steel surface,the greater the amount of FeS2. When approaching closer to the steel surface the amount of oxygen is declining,and this promotes the metabolism of SRB, one of facultative anaerobes,so the amount of SRB is increased.The content of SRB directly influences the metabolism product H2S that results in FeS2.
In other experiment groups in soils with SRB,there were similar layered corrosion products.Some interesting phenomena were found in the micrographs of corrosion products.There were different shapes of corrosion products(Fig.12),such as lamellar,global,‘needle’,and blowball structures,as the results of the different components(as shown in Table 4).
4 Discussion
In sterile soils,urea inhibits corrosion of carbon steel.Some of urea was adsorbed on the steel surface to protect it from ambient environment,21and corrosion potential increases with urea(Fig.3).Some urea could be decomposed into ammonia and carbon dioxide.At first,urea was adsorbed on the steel surface to form a film.One time constant appears in EIS(Fig.5). And Rtincreases in soil with urea compared to that in soil without urea,which proves the existence of the film on the steel surface.However it should be noticed that Rtis smaller in soil with 0.3%urea due to the characteristics of the film.If the film is complete and compacted,the iron dissolution is inhibited and corrosion rate decreases.However,when this formed film is not complete,which makes the situation of“small anode and big cathode”,the corrosion rate would increase,and the pitting corrosion would happen.And two time constants are observed (Fig.5).The value of Rtincreased with urea concentration, which indicated that higher content of urea inhibit the iron dissolving,in accordance with the results of polarization curves. After 65 days,in soil with low urea concentration(0.05%and 0.1%),not enough urea could form a compete protective film, and corrosion rate increased,while in soil with high urea content,the protective film was still complete,and corrosion rate decreased(Table 2).
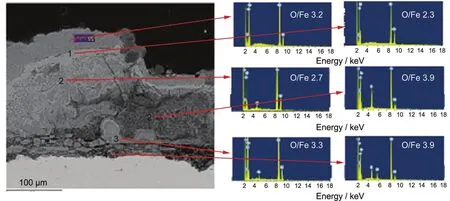
Fig.10 Morphology and chemical composition(atomic ratio,%)in the cross-section of the sample in soils containing 0.2%urea

Fig.11 XPS results of carbon steel in soil containing 0.2%urea

Fig.12 Different shapes of corrosion products of Q235 steel in inoculated soils(a)lamellar,(b)global,(c)needle,(d)blowball
In inoculated soils with SRB,urea plays a complicated role in corrosion behavior of Q235 steel.On the one hand,urea could also be adsorbed on the steel surface to protect it from corrosion;on the other hand,urea may serves as energy resource to promote the growth of SRB.Therefore,the influence of urea on corrosion of Q235 steel depends on which factor takes the main part.Corrosion potential fluctuates with increasing urea concentration(Fig.3),which may be due to the competition of SRB that promoted corrosion and urea that prevented corrosion.The population of SRB(Fig.1)over time shows that proper amount of urea promotes the growth of SRB.At the early experiment stage,low urea concentration stimulates the growth of SRB.Urea was adsorbed on the steel surface togeth-er with SRB which promoted the electron transfer process,and Rtdecreased compared to that in soil without urea.However, low content of urea benefits the growth of SRB more than high urea content at first,so Rtincreases with the increases of urea content.After 20 days,there are corrosion products on the steel surface,and two time constants are observed in EIS(Fig.5).At the later stage,some urea has been consumed or decomposed, consequently high urea concentration promotes the growth of SRB better than low urea content,however the population is still very low(Fig.1),which indicates that most of SRB are in death.Along with the death of SRB,corrosion rate increases due to the complicated metabolic products,in accordance with the present work.7,26
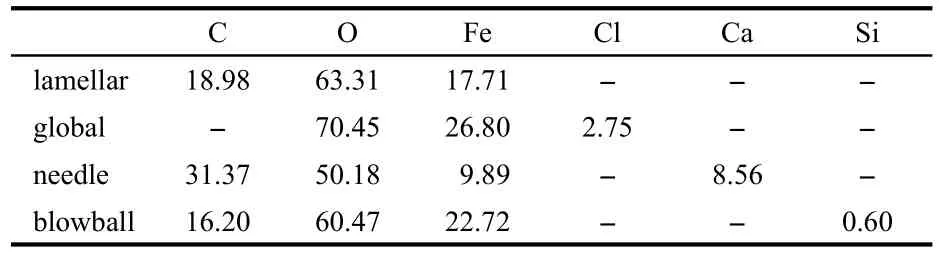
Table 4 EDX results(atomic fraction,%)of the corrosion products of different shapes after 65 days
5 Conclusions
In sterile soils,urea is adsorbed on the steel surface to form a protective film,and prevents corrosion of the carbon steel.In inoculated soils,a proper amount of urea promotes the growth of SRB.Urea accelerates carbon steel corrosion due to its promotion in the growth of SRB.The results of EIS show that in inoculated soils containing urea,the process was controlled by concentration polarization at the later experiment stage.The element sulfur can be detected by XPS.The nearer to the steel surface,the larger the ratio of FeS2/FeO was detected due to the increasing content of the SRB.
(1) Chaker,V.;Palmer,J.D.Effects of Soil Characteristics on Corrosion,ASTM STP 1013,American Society of Testing and Materials(ASTM)International,West Conshohocken, Pennsylvania,1989;p 37.
(2) Benmoussa,A.;Hadjel,M.;Traisnel,M.Materials and Corrosion-Werkstoffe Und Korrosion 2006,57(10),771.doi: 10.1002/(ISSN)1521-4176
(3) Ismail,A.I.M.;El-Shamy,A.M.Applied Clay Science 2009, 42(3-4),356.doi:10.1016/j.clay.2008.03.003
(4)Liu,T.M.;Wu,Y.H.;Luo,S.X.;Sun,C. Materialwissenschaft und Werkstofftechnik 2010,41(4),228. doi:10.1002/(ISSN)1521-4052
(5) Pope,D.H.;Duquette,D.J.;Johannes,A.H.;Wayner,P.C. Materials Performance 1984,23(4),14.
(6) Little.,B.;Wagner,P.;Mansfeld,F.Electrochimica Acta 1992, 37(12),9.
(7) Wagner,P.;Little,B.Materials Performance 1993,32(9),65. (8) Hernandez,G.;Kucera,V.;Thierry,D.;Pedersen,A.; Hermansson,M.Corrosion 1994,50,603.doi:10.5006/ 1.3293532
(9) Pikas,J.L.Corrosion 1996,96,7.
(10) Li,S.Y.;Kim,Y.G.;Jeon,K.S.;Kho,Y.T.;Kang,T.Corrosion 2001,57,815.doi:10.5006/1.3280616
(11) Rao,T.S.Corrosion Reviews 2009,333.
(12) Hamilton,W.A.Annual Review of Microbiology 1985,39,195. doi:10.1146/annurev.mi.39.100185.001211
(13)Lee,W.;Lewandowski,Z.;Nielsen,P.H.;Hamilton,W.A. Biofouling 1995,8(3),165.doi:10.1080/08927019509378271
(14) Rao,T.S.;Sairam,T.N.;Viswanathan,B.;Nair,K.V.K. Corrosion Science 2000,42(8),1417.doi:10.1016/S0010-938X(99)00141-9
(15) Videla,H.A.Biofouling 2000,15(1-3),37.doi:10.1080/ 08927010009386296
(16) Liu,T.;Liu,H.;Hu,Y.;Zhou,L.;Zheng,I.Materials and Corrosion-Werkstoffe Und Korrosion 2009,60(3),218.doi: 10.1002/maco.v60:3
(17) Booth,G.H.;Tiller,A.K.Transactions of the Faraday Society 1960,56,1689.doi:10.1039/tf9605601689
(18) Booth,G.H.;Tiller,A.K.Corrosion Science 1968,8(8),583. doi:10.1016/S0010-938X(68)80094-0
(19) Javaherdashti,R.Applied Microbiology and Biotechnology 2011,91(6),1507.doi:10.1007/s00253-011-3455-4
(20) Li,Y.;Zhang,T.;Wang,F.H.Journal of Chinese Society for Corrosion and Protection 2004,24,334 [李 瑛,張 濤,王福會.中國腐蝕與防護學(xué)報,2004,24,334.]
(21) Mozheiko,F.F.;Potkina,T.N.;Goncharik,I.I.Russian Journal of Applied Chemistry 2008,81(9),1705.doi:10.1134/ S1070427208090437
(22)Booth,G.H.;Cooper,A.W.;Cooper,P.M.;Wakerley,D.S. British Corrosion Journal 1967,2,104.doi:10.1179/ 000705967798326957
(23) Stations National Soil Corrosion Test Networks.Test Methods of Soil Corrosion of Materials;Science Press:Beijing,1990. [全國土壤腐蝕試驗網(wǎng)站編.材料土壤腐蝕試驗方法.北京:科學(xué)出版社,1990.]
(24) Wei,B.M.The Corrosion Theory and Application of Metals; Chemical Industry Press:Beijing,2004. [魏寶明.金屬腐蝕理論及應(yīng)用.北京:化學(xué)工業(yè)出版社,2004.]
(25) Li,M.C.;Lin,H.C.;Cao,C.N.Journal of Chinese Society for Corrosion and Protection 2000,20,111.[李謀成,林海潮,曹楚南.中國腐蝕與防護學(xué)報,2000,20,111.]
(26)Xu,J.;Wang,K.;Sun,C.;Wang,F.;Li,X.;Yang,J.;Yu,C. Corrosion Science 2011,53(4),1554.doi:10.1016/ j.corsci.2011.01.037
(27) Castaneda,H.;Benetton,X.D.Corrosion Science 2008,50(4), 1169.doi:10.1016/j.corsci.2007.11.032
(28) Cao,C.N.Principles of Electrochemistry of Corrosion,3rd ed.; Chemical Industry Press:Beijing,2008;pp 93-94.[曹楚南.腐蝕電化學(xué)原理.第三版.北京:化學(xué)工業(yè)出版社,2008: 93-94.]