Single-dose and multiple-dose pharmacokinetics of zaltoprofen after oral administration in healthy Chinese volunteers
Lingjun Li, Pengcheng Ma, Yuping Cao, Lei Tao, Yue Tao
Department of Pharmacology, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College,Nanjing, Jiangsu 210042, China
INTRODUCTION
Zaltoprofen, a propionic acid derivative of nonsteroidal anti-inflammatory drug (NSAID), has strong inhibitory effects on acute and chronic inflammation[1]. Zaltoprofen may produce its analgesic effects through a peripheral mechanism and especially for the treatment of inflammatory conditions accompanied by pain[2]. However, the adverse effects of zaltoprofen on gastric and small intestinal mucosa were very weak[3].In addition, zaltoprofen has more powerful inhibitory effects on bradykinin (BK)-nociception than other NSAIDs[4].
The therapeutic dose of zaltoprofen is about 80-240 mg/d given orally. High performance liquid chromatography with ultraviolet detector (HPLC-UV)is a sensitive determination method for zaltoprofen in human plasma of the elimination phase. Although the pharmacokinetic profile of one single-dose of zaltoprofen at 160 mg in Korean healthy volunteers was studied[5], the pharmacokinetic profiles of zaltoprofen following single and multiple oral doses have not yet been fully characterized in Chinese volunteers. In addition, dose proportionality of the plasma levels over the therapeutic range has not been determined. Therefore, the purpose of this study was to evaluate the pharmacokinetics of zaltoprofen after single escalating oral doses (80-240 mg)and multiple doses (80 mg, 3 times daily)in healthy Chinese volunteers. The effects of gender on the pharmacokinetics of zaltoprofen were also evaluated.
MATERIALS AND METHODS
Materials and reagents
Zaltoprofen in human plasma was determined by HPLC. The Shimadzu HPLC system consisted of LC-10ATvp pumps and a SPD-10Avp UV-Vis absorbance detector (Shimadzu), operated at 332 nm, and an N2000 chromatographic workstation (Intelligent Information Institute, Zhejiang University, Hangzhou, China). Liquid chromatographic separation was achieved on a Waters Symmetry C18 column (250 mm, 4.6 mm,5 μm). The mobile phase consisted of acetonitrile-10 mmol/L ammonium acetate (pH 3.0; 55:45, v/v)pumped at an isocratic flow rate of 1 mL/min.
Zaltoprofen test tablets (Batch No. 080401, 80 mg zaltoprofen), zaltoprofen reference standard (100.0%purity, Batch No. ZPJ-0180707, Fig. 1)were supplied by Jiangsu Feima Pharmaceutical Co. Ltd (Nantong,China); ketoprofen reference standard (internal standard, IS, Batch No.100337-200502, 99.9% purity, Fig.1)was supplied and identified by Jiangsu Institute for Drug Control (Nanjing, China). Acetonitrile was of chromatographic grade and was purchased from Merck (Germany). Ammonium acetate was of chromatographic grade and was purchased from Tedia (Tedia Company, USA). Other chemicals were all of analytical grade. Water was purified by redistillation before use.
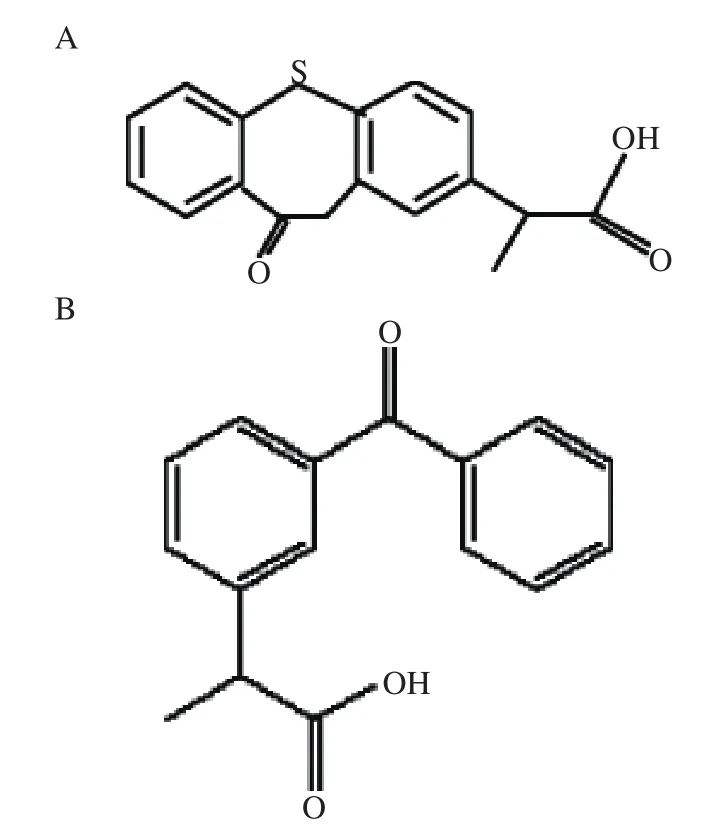
Fig.1 Chemical structure of zaltoprofen (A)and ketoprofen (B)
Subjects
Twelve healthy Chinese volunteers (6 male and 6 female volunteers, aged 22-29 years, body mass index 19.84-23.95 kg/m2)were enrolled in the study.All subjects were in good health after the assessment of medical history, physical examination, electrocardiogram and standard laboratory test results (blood cell count, biochemical profile and urinalysis)and a negative pregnancy test result. Subjects were excluded for the following reasons: a history of clinically significant cardiovascular, renal, hepatic, pulmonary,gastrointestinal, hematological or vascular disease; a history of nervous system or a psychiatric disorder;women during breastfeeding or menstruating; a history of alcohol or drug abuse; allergy or hypersensitivity to the study drugs; a positive test result for hepatitis B virus; blood donation within the previous 3 months;participation in another investigational drug study within the previous 3 months; treatment with any drug during the previous 2 w or the entry into the study.
Study designs
All subjects received in sequential order zaltoprofen orally 80, 160 and 240 mg. All doses were taken in the morning after an overnight fast (10 h). Subjects fasted 4 h after drug administration. Venous blood samples about 3 ml were collected in heparinized tubes at predose (0 h)and 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10,12 and 24 h post drug administration. The plasma samples were separated by centrifugation at 4,000 g for 3 min and stored at -20°C until analysis. After the single dose study was finished, all subjects received 80 mg of zaltoprofen 3 times a day for 6 consecutive days. On the seventh day, all subjects received 80 mg zaltoprofen only in the morning and the blood samples were also collected at the above time points. To establish that the steady-state condition had been reached,additional predose blood samples were collected prior to the morning dose on d 5, 6 and 7. In each period,the volunteers stayed in the Clinical Pharmacology Unit. Every period washout interval was 1 week.
The study was performed in accordance with good clinical practice (GCP)guidelines of the State Food and Drug Administration in China and the Declaration of Helsinki. The protocol was reviewed and approved by the Ethics Committee of the Institue of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College (Nanjing, China), and all subjects gave a written consent for their participation after having been informed by the medical supervisor about the detailed information of the study.
Biological samples
Human blank plasma samples for the development,validation and control of the method were obtained from healthy, drug-free volunteer blood donors at the Jiangsu Blood Center (Jiangsu, China). For the pharmacokinetic assessment of zaltoprofen formulations,plasma samples were collected from the volunteers enrolled in the study.
Preparation of stock solutions
Stock solutions of zaltoprofen and ketoprofen (IS)were prepared in methanol at the concentration of 1 mg/mL. These solutions were found to be stable for 10 h at room temperature and 31 d at -20°C.
Working solutions of zaltoprofen were prepared in methanol by appropriate dilution at a working concentration of 0.1, 0.5, 1, 5, 10, 50 and 80 μg/mL.
Sample preparation and extraction procedure
A 1 mL aliquot of the collected plasma sample from a human volunteer was pipetted into a 10 mL centrifuge tube. The 50 μL working IS solution (1 mg/mL),and 50 μL hydrochloric acid solution (1 mol/L)were added and then vortexed for 10 s. Then, 5 mL diethyl ether were added again and vortexed for 1 min. After centrifugation of the samples at 4, 000 g for 10 min at room temperature, the organic layer was transferred to another 10 mL centrifuge tube and evaporated to dryness in a water bath at 40°C. The residue was redissolved in 100 μL methanol. After vortex and centrifugation, an aliquot of 20 μL was injected into the HPLC system.
Standard curves and quality-control samples
Calibration curves were prepared by spiking blank plasma with proper volume of one of the abovementioned working solutions to produce the standard curve points equivalent to 0.1, 0.5, 1, 5, 10, 50 and 80 μg/mL of zaltoprofen. Each sample also contained 50 μg/mL of the IS. The following assay procedures were the same as described above. In each run, a blank plasma sample (no IS)was also analyzed. Calibration curves were prepared by concentration vs the best fit of peak-area ratios (peak area of analyte/peak area of IS), and fitted to the equation (y = bx+a)by unweighted least-square regression.
Quality-control (QC)samples were prepared daily by spiking different samples of 1 mL blank plasma each with proper volume of the corresponding standard solution to produce a final concentration equivalent to low level (0.2 μg/mL), middle level (6.0 μg/mL)and high level (60 μg/mL)of zaltoprofen with 50 μg/mL of IS each. The following procedures were the same as described above.
Method validation
The method validation assays were carried out following the US Food and Drug Administration (FDA)bioanalytical method validation guidance[6].
The method’s specificity was tested by analyzing blank plasma samples of the healthy human subjects obtained from six different sources.
Standard curves of seven concentrations of zaltoprofen ranging 0.1-80 μg/mL were extracted and assayed. Blank plasma samples were analyzed to confirm absence of interferences, but were not used to construct the calibration function. The lower limit of quantification (LLOQ)was the lowest concentration of the standard curves. Each concentration standard should meet the following acceptable criteria: no more than 20% deviation at LLOQ and no more than 15%deviation for the standards above LLOQ.
The precision and accuracy of the assay were determined from the QC plasma samples by replicate analyses of three concentration levels of zaltoprofen(0.2, 6.0 and 60 μg/mL).
Within-batch precision and accuracy were determined by repeated analyses of five samples of zaltoprofen at each QC level (0.2, 6.0 and 60 μg/mL)on the same day (n = 5). Between batch precision and accuracy were determined by repeated analyses on three consecutive days (n = 5 per day). The concentration of each sample was determined using the standard curves prepared and analyzed on the same day.
The absolute extraction recovery of zaltoprofen was determined by comparson of the zaltoprofen /IS peakarea ratios obtained from extracted plasma samples with those from the standard solutions at the same concentration. This procedure was repeated for the three different concentration levels of 0.2, 6.0 and 60 μg/mL.
Freeze and thaw stability: Three concentration levels of QC plasma samples were tested after each of three freeze (-20°C)-thaw (room temperature)cycles.
Short-term temperature stability: Three concentration levels of QC plasma samples were kept at room temperature for a period that exceeded the routine preparation time of samples (around 8 h).
Long-term stability: Three concentration levels of QC plasma samples kept at low temperature (-20°C)were studied for a period of 15 and 29 d.
Postpreparative stability: The postpreparative stability was conducted by analyzing extracted QC samples kept at the room temperature for 24 h.
Stock solution stability: The stability of zaltoprofen and IS working solutions was evaluated at room temperature for 10 h and at low temperature (-20°C)for 31 d.
A standard curve in each analytical run was used to calculate the concentration of zaltoprofen in the run. It was prepared along with the unknown samples in the same batch.
Pharmacokinetic analysis
Single- and multiple-dose pharmacokinetic parameters were calculated from plasma concentration-time data by noncompartmental methods. The maximal plasma concentration (Cmax)and the peak time (Tmax)were obtained from observed data. The area under the plasma concentration versus time curve (AUC)from 0 to the last measurable concentration (Ct), AUC0-24, was calculated by the linear trapezoidal rule. The area under the plasma concentration versus time curve from 0 to infinity (AUC0-∞)was calculated as AUC0-24+ Ct/λz, where λzis the slope of the log-linear regression of the terminal concentration data points. The terminal elimination half-life (t1/2)was calculated as (ln2)/λz.The mean steady-state concentration (Css)was calculated using AUCss(0-τ)/τ, where τ is the dosing interval.
Statistical analysis
Pharmacokinetic parameters were compared among dose levels using analysis of variance (ANOVA). The linearity of zaltoprofen pharmacokinetics was assessed by examining Cmax, AUC, and t1/2, as a function of the single-dose administration. Prior to comparisons,dose-dependent parameters (Cmaxand AUC)were normalized to the lowest dose. Data were presented as mean±standard deviation (SD). Paired Student t tests and a significance level of 5% were applied. The Drug and Statistics Software (DAS, Version 2.1.1; Mathematical Pharmacology Professional Committee of China, China)was used.
RESULTS
Method validation
Fig. 2A shows an HPLC chromatogram of a blank plasma sample indicating no endogenous peaks at the retention positions of zaltoprofen or IS. Fig. 2B and 2C shows the typical chromatograms of a blank, spiked plasma sample with zaltoprofen (5.07 μg/mL), and a plasma sample obtained at 2 h from a subject who received a single oral dose (160 mg). The retention time of zaltoprofen and the IS was about 10.8 and 6.8 min,respectively.
The method was validated and found to be linear over the concentration range of 0.1-80 μg/mL (r =0.9998)using (1/C2)weighted least squares regression. The relative standard deviation (RSD)values for within-batch precision and accuracy were in the range 3.07%-7.12% and 101.61%-103.75%, respectively,whereas the corresponding between-batch values were 3.75%-7.97% and 100.84%-102.48% (Table 1).The mean recoveries of the three concentration levels(0.2, 6.0 and 60 mg/mL)were 71.67%, 72.85% and 80.81%, respectively. The stability study showed that zaltoprofen and IS were stable in plasma for at least 8 h at room temperature and 29 d at –20°C. They were also stable at room temperature for at least 24 h after postpreparation as well as at –20°C after 3 freeze-thaw cycles (Table 2).

Pharmacokinetics
The method was applied to analyze plasma samples obtained from 12 healthy volunteers who received a single dose of 80, 160 and 240 mg zaltoprofen each and administered 80 mg zaltoprofen subsequently to achieve steady states in the pharmacokinetic study.The procedure developed was sensitive enough to assure the quantitative analysis of zaltoprofen in plasma with acceptable accuracy over a period of 24 h after a single oral administration. The mean plasma concentration–time curve of 12 volunteers after oral administration of zaltoprofen is shown in Fig. 3. The mean plasma concentration-time profile following 3 times a day oral dosing of zaltoprofen is shown in Fig. 4. Kinetic parameters are listed in Table 3 and Table 4. The results show that the t1/2and the Tmaxdid not change noticeably with the dose. The t1/2and the Tmaxof consecutive administrations of 80 mg zaltoprofen were similar to those of single-dose of 80 mg zaltoprofen.
The correlation analysis of dose and Cmaxshowed that there was good linear response of different doses and Cmax. The linear regression equation was C =0.069×d + 0.33, r = 0.99, where d corresponded to the dose and C refered to the Cmaxof three dose groups of zaltoprofen.
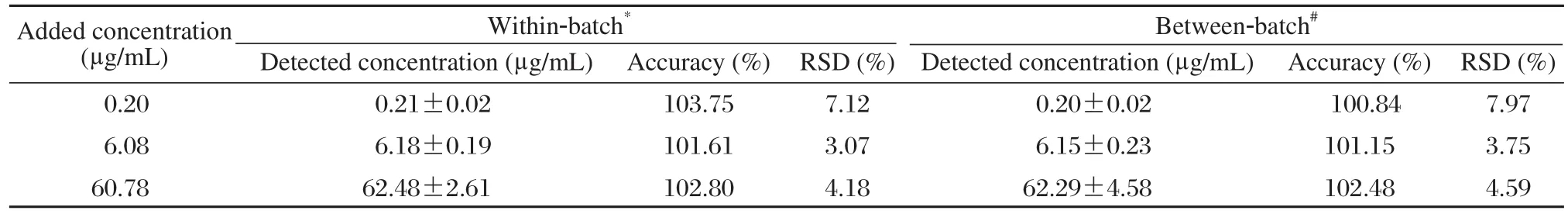
Table 1 The within- and between-batch precision and accuracy of the method
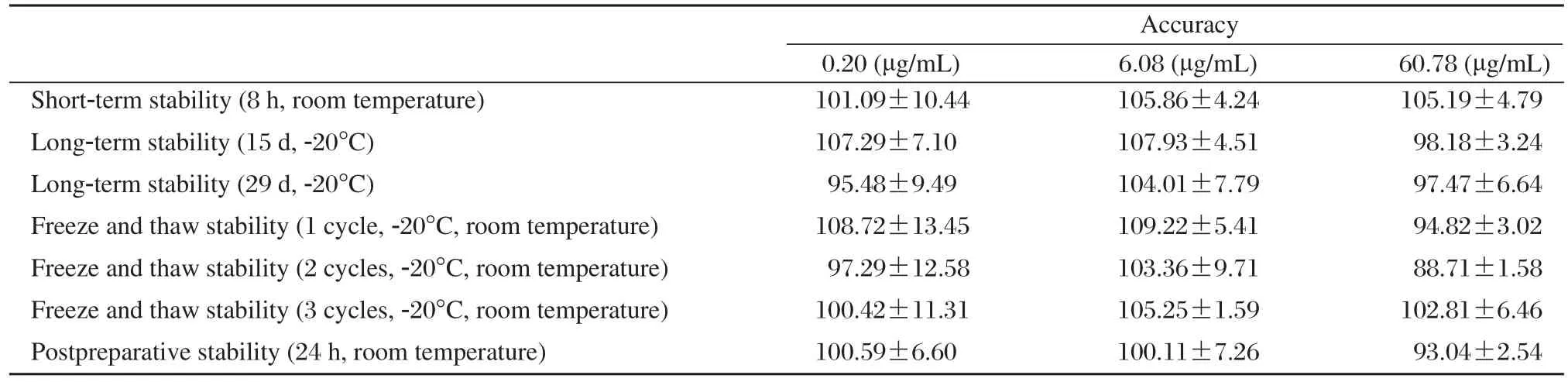
Table 2 The stability of zaltoprofen in human plasma at different levels(n = 5)
The correlation analysis of dose and AUC0-24showed that there was a linear response of different doses and AUC. The linear regression equation was A = 0.233×d–1.33, r = 0.98, where d corresponded to the dose and A refered to the AUC0-24of three dose groups of zaltoprofen.
Through one-way ANOVA of the main pharmacokinetic parameters between male and female volunteers after oral administration of zaltoprofen, Cmaxand AUC0-24of three dose groups of male and female volunteers showed no significant difference (P > 0.05).
Single and multiple doses of zaltoprofen were well tolerated. There were no withdrawals and no adverse events in this study.
DISCUSSION
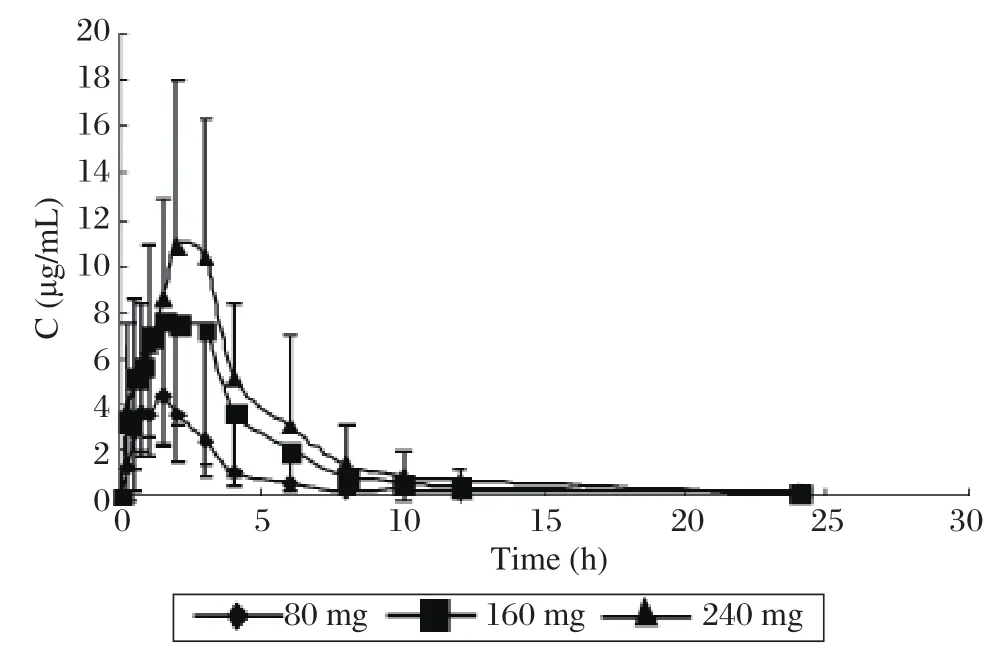
Fig.3 Mean concentration-time curves of zaltoprofen in 12 volunteers after oral administration of zaltoprofen in single-dose study (80, 160, and 240 mg). The plasma concentration increased in a proportional manner with the increasing single-dose of 80,160 and 240 mg.

Fig.4 Mean concentration-time curves of zaltoprofen after oral administration of zaltoprofen in multiple doses (80 mg).
Zaltoprofen is a NSAID with anti-inflammatory and analgesic effects on inflammatory pain[2], and it possesses novel anti-nociceptive mechanism by inhibiting B2-type bradykinin (BK)receptor function in nerve endings and selectively inhibiting PGE2 production at inflammatory sites and exhibits a powerful antiinflammatory effect with a good safety margin. and a more strong inhibitory effect on BK-nociception than other NSAIDs[4].
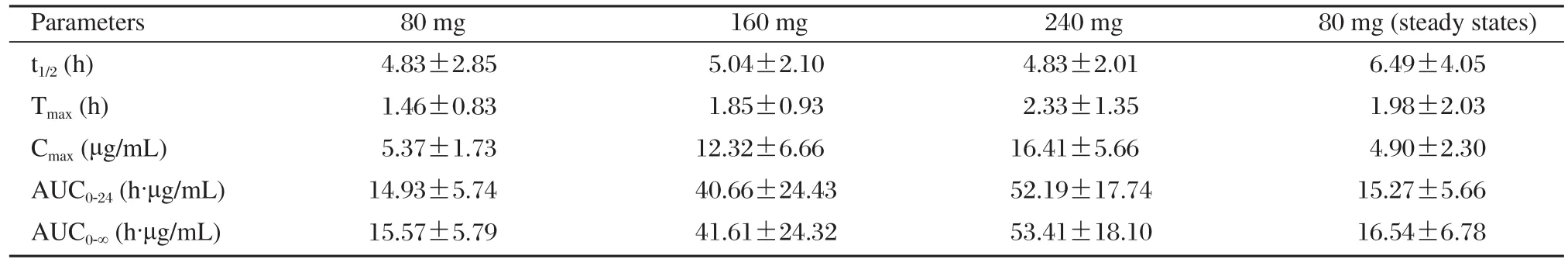
Table 3 Pharmacokinetic parameters of 12 healthy volunteers after oral administration of zaltoprofen tablets in single-dose study (80, 160 and 240 mg)and in multidose study (80 mg)
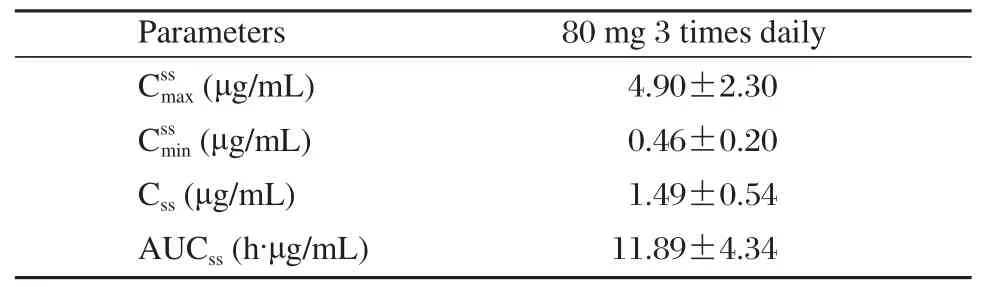
Table 4 Main pharmacokinetic parameters of zaltoprofen following multiple oral doses of zaltoprofen 80 mg 3 times daily (n = 12)
However, little information has been reported on the pharmacokinetics of zaltoprofen in humans. So the study was performed to investigate the pharmacokinetic characteristics and dose proportionality of zaltoprofen in healthy Chinese volunteers following single oral doses of 80, 160, 240 mg and multiple oral doses of zaltoprofen 80 mg 3 times daily for 6 consecutive days.
Sample preparation is usually required for the determination of pharmaceuticals in biological samples owing to complex matrices in order to remove possibly interfering matrix components and increase the selectivity and sensitivity. Liquid–liquid extraction(LLE)is a widely adopted method and often achieves satisfactory extraction recoveries of analytes from biological samples. Dichloromethane, ethyl acetate,and diethyl ether were all tested. Diethyl ether finally chosen as the extraction solvent can not only eliminate the interference of endogenous substances, but also meet the requirement of sensitivity for the assay. For zaltoprofen is a weak acid, the extraction recovery of zaltoprofen can be improved by decreasing the pH of the plasma samples with hydrochloric acid solutions.Therefore, a 50 μL aliquot of 1 mol/L hydrochloric acid solution was added to 1 mL plasma sample before extraction. IS was applied to get high accuracy when zaltoprofen was determined in human plasma.Enalapril maleate, ibuprofen, fenbufen and ketoprofen were all investigated to find the suitable one. Ketoprofen was finally chosen as the IS because of its similarity of structure, retention time, and extraction efficiency as well as less endogenous interference in plasma. This suitable IS was useful to correct volumetric errors and to improve linearity and selectivity of the detection system. In the mobile phase, 10 mmol/L ammonium acetate buffer was added to improve the chromatographic peak shapes of zaltoprofen and IS, and obtain a good separation of target compounds.
The results of the study showed that zaltoprofen was rapidly absorbed following single oral doses,with maximum plasma concentrations occurring 1.46 to 2.33 h after administration. Mean t1/2ranged from 4.83 to 5.04 h in the subjects. The Cmaxof zaltoprofen increased in a linear and proportional manner with increasing oral doses. The AUC and dose are also positively correlated. The t1/2and the Tmaxdid not change largely with the doses. In comparing the single-dose with the multiple-dose profile, the pharmacokinetic analysis of zaltoprofen showed similar properties.The t1/2and the Tmaxof consecutive administrations of 80 mg zaltoprofen were similar to those of singledose 80 mg zaltoprofen,which means that there is no significant accumulation of zaltoprofen with repeated doses. The linear pharmacokinetics and the low pharmacokinetic variability should facilitate the definition of dose regimens and the prediction of plasma levels after multiple oral administrations. As another main objective of the study, we used these data to examine the effects of sex on the pharmacokinetics of zaltoprofen. In both study parts, there were no significant differences in AUC and Cmaxbetween the female and male subjects. Hence, no adjustment of dosage on the basis of sex appears needed. Though the body weight of male and female volunteers have significant difference, we need not adjust dose according to body weight.
In conclusion, the results of the present study indicate that zaltoprofen has predictable pharmacokinetics. Zaltoprofen displays linear pharmacokinetics in the dose range of 80 to 240 mg after single oral doses.The compound is rapidly absorbed and cleared from plasma, which supports the use of zaltoprofen for a 3 times daily dose regimen. Zaltoprofen was not accumulated in the human body. No marked effect of sex on the disposition of zaltoprofen was observed. All these conclusions can provide reference for clinical medication.
Lee et al[5]. described the pharmacokinetic profiles of Korean healthy volunteers after oral administration of 160 mg zaltoprofen in single-dose study. The pharmacokinetic parameters of zaltoprofen were generally similar between Korean and Chinese volunteers.
[1]Tsurumi K, Kyuki K, Niwa M, Kokuba S, Fujimura H.Pharmacological investigations of the new antiinflammatory agent 2-(10, 11-dihydro-10-oxodibenzo[b, f]thiepin-2-yl)propionic acid. 1st communication: inhibitory effects of rat paw edema. Arzneimittelforschung 1986;36:1796-800.
[2]Kameyama T,Nabeshima T, Yamada S, Sato M. Analgesic and antiinflammatory effects of 2-(10, 11-dihydro-10-oxo-dibenzo[b,f]thiepin-2-yl)propionic acid in rat and mouse. Arzneimittelforschung 1987;37:19-26.
[3]Tsurumi K, Kyuki K, Niwa M, Mibu H,Fujimura H. Pharmacological investigations of the new antiinflammatory agent 2-(10,11-dihydro-10-oxodibenzo[b,f]thiepin-2-yl)propionic acid. 2nd communication: inhibitory effects on acute inflammation and prostaglandin-related reactions.Arzneimittelforschung 1986;36:1801-5.
[4]Misaki Matsumoto, Makoto Inoue,Hiroshi Ueda. NSAID zaltoprofen possesses novel anti-nociceptive mechanism through blockage of B2-type bradykinin receptor in nerve endings. Neurosci Lett 2006;397:249-53.
[5]Heon-Woo Lee, Ji-Hyung Seo, Yong-Won Kim, Seo-Young Jeong, Kyung-Tae Lee. Determination of zaltoprofen in human plasma by liquid chromatography with electrospray tandem mass spectrometry: Application to a pharmacokinetic study. Rapid Commun Mass Spectrom 2006;20:2675-80.
[6]US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry, Bioanalytical Method Validation, May 2001.
 THE JOURNAL OF BIOMEDICAL RESEARCH2011年1期
THE JOURNAL OF BIOMEDICAL RESEARCH2011年1期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- The role of microRNAs during the genesis of medulloblastomas induced by the hedgehog pathway
- Development and optimization of an antibody array method for potential cancer biomarker detection☆
- A mutation in the type II hair keratin KRT86 gene in a Han family with monilethrix☆
- Instructions for Authors
- 《生物醫(yī)學研究雜志(Journal of Biomedical Research)》宣布與湯森路透合作——采用國際一流的在線投審稿系統(tǒng)ScholarOne Manuscripts
- Successful conservative management with methotrexate and mifepristone of cervical pregnancy
