Donor liver natural killer cells alleviate liver allograft acute rejection in rats
Jian-Dong Yu, Tian-Zhu Long, Guo-Lin Li, Li-Hong Lv, Hao-Ming Lin, Yong-Heng Huang, Ya-Jin Chen and Yun-Le Wan
Guangzhou, China
Donor liver natural killer cells alleviate liver allograft acute rejection in rats
Jian-Dong Yu, Tian-Zhu Long, Guo-Lin Li, Li-Hong Lv, Hao-Ming Lin, Yong-Heng Huang, Ya-Jin Chen and Yun-Le Wan
Guangzhou, China
BACKGROUND:Liver enriched natural killer (NK) cells are of high immune activity. However, the function of donor liver NK cells in allogeneic liver transplantation (LTx) remains unclear.
METHODS:Ten Gy of whole body gamma-irradiation (WBI) from a60Co source at 0.6 Gy/min was used for depleting donorderived leukocytes, and transfusion of purified liver NK cells isolated from the same type rat as donor (donor type liver NK cells,dtlNKs) through portal vein was performed immediately after grafting the irradiated liver. Post-transplant survival observation on recipients and histopathological detection of liver grafts were adoptive to evaluate the biological impact of donor liver NK cells on recipients' survival in rat LTx.
RESULTS:Transfusion ofdtlNKs did not shorten the survival time among the recipients of spontaneous tolerance model (BN to LEW rat) after rat LTx, but prolonged the liver graft survival among the recipients depleted of donor-derived leukocytes in the acute rejection model (LEW to BN rat). Compared to the recipients in the groups which received the graft depleted of donor-derived leukocytes, better survival and less damage in the allografts were also found among the recipients in the two different strain combinations of liver allograft due to transfusion ofdtlNKs.
CONCLUSIONS:Donor liver NK cells alone do not exacerbate liver allograft acute rejection. Conversely, they can alleviate it, and improve the recipients' survival.
(Hepatobiliary Pancreat Dis Int 2011; 10: 386-392)
liver transplantation; natural killer cell; donor; transfusion; graft rejection
Introduction
Liver allograft tolerance is a special case in transplantation,[1]not only could liver graft be spontaneously accepted across complete major histocompatibility (MHC) barrier, but also protect other homologous organs (such as heart, kidney and skin grafts), which have originally been rejected by the recipient, from the recipient's immune attack without immunosuppression. The mechanism underlying this special transplant tolerance is unclear at present. Sun and his colleagues[2,3]attributed the passenger leukocytes deriver-from donor liver to this spontaneous tolerance, since they have demonstrated that depletion of passenger leukocytes via whole body gamma-irradiation (WBI) of donor rat could delete the original liver allograft tolerance, and intravenous injection of donor liver leukocytes could reconstitute the spontaneous acceptance. However, it is still controversial on which leukocytes derived from liver graft would play an important role in promoting transplant tolerance, since the leukocytes in the liver graft belong to many lineages with diverse activities, and the lymphocyte constitution of which is complicated, including T and B lymphocytes, antigen presenting cells (APCs), monocytes/macrophages, natural killer (NK) cells, etc.
NK cells, first defined by their ability to kill certain tumor cells and virus-infected cells without prior immunization, are one of the major cell types in the innate immune system and constitute the third largest population of lymphocytes besides T cells and B cells in peripheral blood.[4]Owing to the hypothesis of "missingself" and "stress-induced" recognition pattern,[5,6]NKcells are increasingly favored by transplant researchers. Compared with peripheral blood, a striking feature of the liver in the lymphocytes constitution and properties is that liver NK cells predominate in the lymphocyte proportion and immunological activity. Liver NK cells comprise 30%-50% of all lymphocytes present in a normal adult liver while NK cells in peripheral blood account for 10%-15% of lymphocytes.[7,8]The liver NK cells detached from human liver graft belong to the CD56brightsubset and highly express some molecules such as CD69, Nkp44, and so on, which are associated with NK cell activation.[9]
Nevertheless, except a pronounced role in allogeneic hematopoietic stem cell and corneal transplant rejection, it is hard to ascribe the precise role of NK cells in reactivity to solid-organ transplantation at present.[10-12]Some of animal experimental studies have demonstrated that recipient NK cells were associated with allograft rejection since alloreactive NK cells have been shown to be activated in some models of allogeneic cardiac transplantation and skin transplantation, and inhibition of recipient NK cells could result in acceptance of cardiac allografts in CD28-/- mice.[13,14]Recently, NK cells acting as effector cells have been found to be involving in graft acceptance. Beilke and his colleagues[15]firstly reported that NK cells promoted islet allograft tolerance via a perforin-dependent mechanism. Subsequently, skin allograft tolerance was also found to be associated with the killing of APC by recipient NK cells.[16]However, it is a pity that much of the work, which has been performed on alloreactive NK cells in transplantation, was dedicated to the study of recipient NK cells rather than the donor-derived populations. The properties of donor liver NK cells in LTx remain unclear and need further investigation.
In this study, two different rat liver allograft combinations of BN→LEW and LEW→BN were established, 10 Gy of WBI from a60Co (a radioactive isotope of cobalt) source at 0.6 Gy/min was used for depleting donor-derived leukocytes, and transfusion of purified donor type liver NK cells (dtlNKs) through the portal vein was performed immediately after grafting the irradiated liver, for exploration the function of donor liver NK cells in allogeneic liver transplantation (LTx).
Methods
Animals
Inbred male Lewis (LEW, RT1l) rats and Brown Norway (BN, RT1n) rats, weighing 180-200 g, were purchased from Beijing Vital-river Laboratory Animal Technology Company. The rats were maintained under standard conditions and fed clean rodent food and water, and were also treated in accordance with the guidelines of the European Community Standards on the Care and Use of Laboratory Animals (No. 28871-22A9).
Experimental design
Except those for isolation of liver NK cells, the rest rats were used as donors and recipients. The liver allograft combinations were randomly divided into 6 groups as follows: group A (n=8) BN→LEW (without special treatment), group B (n=10) BN (WBI of donor)→LEW, group C (n=12) BN (WBI of donor)→LEW (transfusion ofdtlNKs), group D (n=8) LEW→BN (without special treatment), group E (n=8) LEW (WBI of donor)→BN, and group F (n=11) LEW (WBI of donor)→BN (transfusion ofdtlNKs).
Donor WBI
After being confined to a translucent-ventilation plastic box, donor rats were treated with 10 Gy of WBI from a60Co source at 0.6 Gy/min. Three days later, the irradiated livers of donors were transplanted into the appropriate recipients.
Purification of liver NK cells
With reference to the method described by Bouwens et al,[17]liver NK cells were isolated by liver perfusion and digestion. After resection under sterile conditions, the rat's liver was perfused with liver digested liquid (contained 0.05% collagenase IV, 500 μg/mL DNaseiand 2% fetal bovine serum) through via the portal vein and was cut into small pieces. The perfusate as well as the liver tissue was collected and continued to digest in a 37 ℃ constant temperature shaker for 15 minutes. Liver tissue suspension was filtered with a steel mesh (100 μm pore size) to collect the digest liquid which contained a large number of liver parenchymal cells and nonparenchymal cells (NPCs). The mesh was washed with phosphate buffer solution (PBS) to collect the filtrate and removed the liver tissue that was not completely digested. The digested liquid was then depleted via centrifugation and got the cell suspension rich in hepatic parenchymal cells and NPCs. Hepatic parenchymal cells were depleted by lowspeed centrifugation (300 rpm), while erythrocytes, granulocytes and cell debris were depleted by Ficoll density gradient centrifugation (1500 rpm) for 25 minutes at 25 ℃. The mononuclear cells recovered from the interface of Ficoll-Paque gradient contained a large number of liver NK cells (with the purity of NK cells53.54±8.23%). Further purification of the mononuclear cells mentioned above was performed with Dynabeads FlowCompTMFlexi system. After DSB-X-conjugated anti-CD3 negative sorting and DSB-X-conjugated anti-NKR-P1 positive sorting, CD3-NKR-P1+NK cells of higher purity isolated from rat liver (with the purity of NK cells 88.64± 4.36%) were resuspended and counted. The viability of the cells without contamination by bacteria, fungi and endotoxins was assessed by the Trypan blue dye-exclusion test (the viability of liver NK cells >95%).
Liver transplantation and transfusion ofdtlNKs
Orthotopic liver transplantation (OLTx) was performed as described by Kamada and Calne without hepatic artery reconstruction.[18]Cold ischemic time and anhepatic phase were restricted to 70 minutes and 16 minutues, respectively. The recipient survived for more than 24 hours after OLTx was considered to be surgically successful. Immediately after grafting the irradiated liver, the suspension ofdtlNKs (contained 3.0×106) was transfused into the recipient through the portal vein.
Post-transplant survival observation on recipients
The post-transplant changes of general status and body weight in recipients were observed daily to evaluate the quality of life. Here we defined the adverse events associated with the poor survival condition of the recipients, as the post-transplant general status such as depilation, jaundice, ascites, and post-transplant complication including liver abscess or liver necrosis. The percentage of post-transplant weight change among the recipients of each group was calculated in accordance with the following formula: the percentage of post-transplant weight change=(the values of posttransplant weight change in the recipients/the weight of recipients before liver transplantation)×100%.
Histopathological detection
Liver graft specimens of BN recipients were immediately cut once the recipients died from acute rejection, while the liver graft specimens of the recipients of long-term survival were harvested on 100 days after liver transplantation. The liver graft specimens were dehydrated, and fixed with 10% formalin solution once removed from recipients. Then the paraffin-embedded liver tissue was sectioned and stained with hematoxylin and eosin.
Statistical analysis
All data were analyzed with SPSS13.0 software. The survival analysis was performed by the Kaplan-Meier method and log-rank test. The chi-square test and analysis of variance (ANOVA) were performed in data analysis when appropriate. The differences in the groups were compared by Bonferroni's test. A P<0.05 was considered statistically significant.
Results
Survival time of recipients not shortened by transfusion ofdtlNKs
BN liver grafts were spontaneously accepted by LEW recipients in group A, and all rats survived more than 100 days (Table 1). Depletion of passenger leukocytes via WBI of donor before transplantation did not shorten the median survival time (MdST) of Lewis recipients in group B, even though 4 of 10 recipients survived for less than 100 days (Table 1). Transfusion ofdtlNKs through the portal vein also did not shorten the MdST of the recipients in group C, since 8 of 12 recipients survived for more than 100 days (Table 1).
Of note, LEW→BN has been proved the existence of liver allograft acute rejection, but it is not a high responder rejection strain combination due to the relatively long mean survival time (MaST, with MaST=44.0±10.2 d) as described by Dresske et al.[19]Our data also supported that the BN recipients in group D could not survive for more than 60 days after LTx (Table 1), with a MdST of 42 days and a MaST of 45.0±3.4 days. Depletion of donorderived leukocytes via WBI resulted in further reductionof MdST and the MaST in the BN recipients in group E (Table 1, P<0.05) as the recipients in this group died within 22 days after transplantation, with a MdST of 11 days and a MaST of 13.7±1.5 days. In contrast to the recipients of group E, transfusion ofdtlNKs through the portal vein would prolong the MdST and MaST in the recipients of group F (Table 1, P=0.03), with a MdST of 17 days and a MaST of 26.0±5.0 days. All these findings suggest that transfusion ofdtlNKs does not shorten the survival time, but in some ways increases the survival rate after LTx.

Table 1. Effect of donor liver NK cells on survival after rat LTx
Transfusion ofdtlNKs improving the general status of recipients after transplant
Depilation was not found in the recipients of group A in the period of observation (Table 2), but there was a higher incidence of depilation in the recipients depleted of passenger leukocytes whetherdtlNKs was transfused or not [Table 2, with the incidence of depilation of 7/10 (group B) and 7/12 (group C)]. In contrast to the recipients of group A, the higher incidences of ascites, jaundice and liver abscess or liver necrosis were not found in the LEW recipients of group B, but the accumulated incidence of adverse events would significantly increase in these recipients due to depletion of passenger leukocytes [accumulated incidence of adverse events: 21/10 (group B) vs 7/8 (group A), P=0.039]. However, the accumulated incidence of adverse events in the recipients of group C was significantly reduced after transfusion ofdtlNKs, compared with that in the recipients of group B (Table 2, group C vs group B, P=0.045). As for the recipients of LEW→BN liver allograft combination, the accumulated incidence of adverse events was higher in the recipients of group E, than in the recipients of groups D and F [Table 2, 24/8 (group E) vs 17/8 (group D) and 24/11 (group F) respectively; P=0.011 and P=0.013]. But there was no significant difference between the recipients of groups D and F (P=0.912).
Weight change after transplantation is one of the objective indicators related to recipients' quality of life. All recipients of spontaneous tolerance model suffered from weight loss early after LTx, and then they started a weight gain process (Fig. 1A). The time for recovering from weight loss and the speed of weight increase were different in the recipients in the three groups. The longest time for weight recovery and the slowest weight gain process were found in the recipients of group B after depletion of passenger leukocytes (Table 3 and Fig. 1A), and the mean time for weight recovery was 60.0±3.3 days. However, the time for weight recovery was significantly reduced and the weight gain process was accelerated in the recipients of group C after transfusion ofdtlNKs, and the mean time of weight recovery was 32.3±2.5 days (Table 3). In the recipients of LEW→BN liver allograft combination, all BN recipients suffered from weight loss. Weight loss in the recipients of group E was most severe among the three groups (Fig. 1B). Significantly, a slower weight loss was seen in the BN recipients of group F after transfusion ofdtlNKs (group F vs. group E, P<0.05).
dtlNKs transfusion for alleviating liver allograft acute rejection
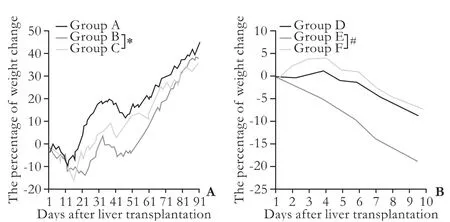
Fig. 1. The percentage of post-transplant weight change in the recipients after rat LTx (n=8-12). A: Post-transplant weight in LEW recipients of BN→LEW liver allografts combination (groups A, B and C); B: Change of post-transplant weight in BN recipients of LEW→BN liver allograft combination (groups D, E and F). *: P<0.05, group B vs group C; #: P<0.05, group E vs group F.
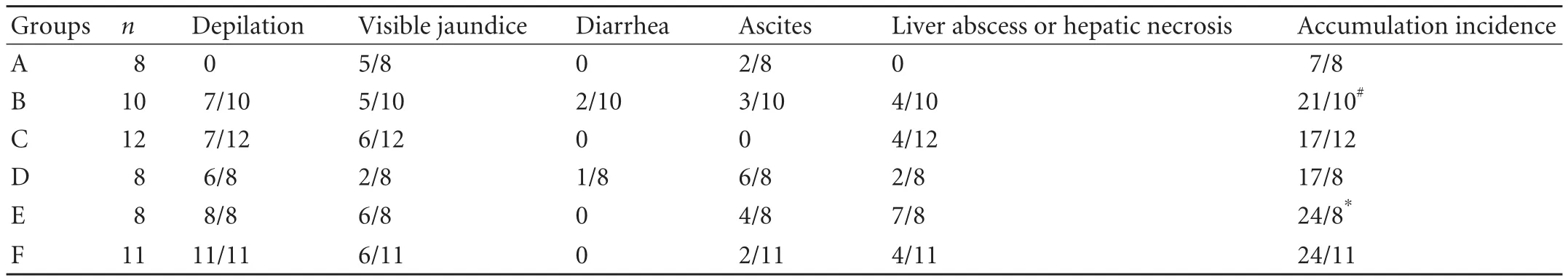
Table 2. Incidence of adverse life events in recipients after liver transplantation
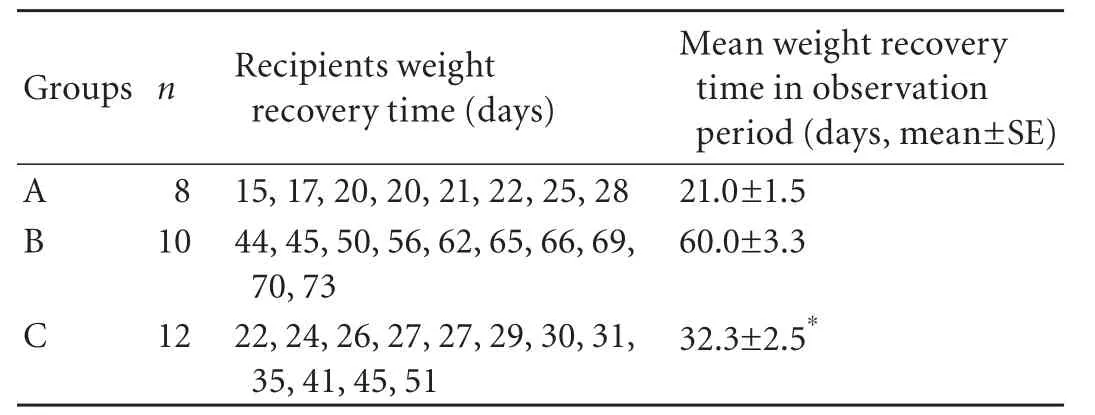
Table 3. The weight recovery time in recipients of spontaneous tolerance model after rat liver transplantation
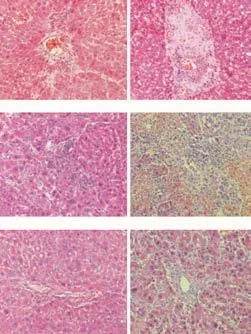
Fig. 2. Representative hematoxylin-eosin stained liver sections from the recipients with or without transfusion of liver NK cells (original magnification ×200). A-C: Representative liver sections on 100 days post-transplantation in LEW recipients of groups A, B and C; D-F: Representative liver sections on 42 (group D), 11 (group E) and 17 (group F) days post-transplantation in BN recipients of LEW→BN liver allografts combination.
Representative images of liver allografts at 100 days in tolerant recipients (groups A, B, C) showed that the histological structure of liver grafts was well preserved except for disordered lamellar structure of hepatocyte arrangement (Fig. 2A-C). Nevertheless, atypical hyperplasia and moderate mononuclear cell infiltration were also detected in the liver grafts in which leukocytes derived from the donor liver were depleted via WBI (Fig. 2B). Surprisingly, less mononuclear cell infiltration was found in the allograft containing donor liver NK cells (Fig. 2A, C), indicating that the cells do not exacerbate liver allograft rejection.
Representative images of liver allografts on the day that the rejected recipients died from acute rejection (groups D, E, and F) showed that expansion of the portal area caused by infiltration of mononuclear cells was marked by inflammatory spillover into the periportal parenchyma (Fig. 2D-F). In the allografts of group D, extensive mononuclear cell infiltration, fibrosis and destruction of hepatic lobules were also seen around the portal tract (Fig. 2D), indicating the occurrence of a relatively chronic rejection. In the allografts depleted of donor-derived leukocytes, severe hepatocyte necrosis with extensive mononuclear cell infiltration and destruction of hepatic lobules without fibrosis were obseved (Fig. 2E). In contrast to histopathological changes in the allografts of group E, less infiltration of erythrocytes and minor destruction of hepatic lobules were detected in the recipients transfused with purifieddtlNKs (Fig. 2F), although mononuclear cell infiltration and fibrosis were not significantly different. Thus donor liver NK cells may alleviate acute rejection of liver allograft.
Discussion
WBI of donor with a lethal dose of 10 Gy could deplete most leukocytes including T, B, dendritic, and monocyte/macrophage cells.[2,20]In the present study, peripheral blood leukocytes of the rat after WBI were less than 3% of leukocytes count of normal rat, and the residual leukocytes in the liver were hardly detected after sufficient perfusion. Hence it is feasible to investigate the roles of a single population of donor liver NK cells in rat LTx via transfusion ofdtlNKs immediately after grafting of the irradiated liver.
Liver allograft tolerance is specifically associated with "passenger leukocytes" derived from a donor. It was reported that injection of leukocytes derived from donor could down-regulate recipient's alloreativity to donor antigen, which was associated with the recirculation of donor leukocytes in a recipient.[21]Others[3]confirmed that injection of donor liver leukocytes after grafting of the irradiated liver depleted of donor leukocytes could prolong the graft survival, suggesting that donor liver leukocytes including liver NK cells might be essential to liver allograft tolerance. In our study, transfusion ofdtlNKs did not significantly shorten the MdST in the LEW recipients of liver allograft combination BN→LEW. Conversely, it could prolong the MaST and MdST in theBN recipients of liver allograft combination LEW→BN. These findings indicate that donor liver NK cells play a role in liver transplant immunity, but do not exacerbate liver allograft rejection.
Donor liver NK cells migrate into the recipient after LTx and generate various interactions with the allogeneic elements of the recipient. We found that CFSE-labeled (CFSE: carboxyflourescein diacetatesuccinimidyl ester) donor liver NK cells (more than 5×105) transfused into the recipient via the portal vein could be detected in the liver for more than 2 weeks,[22]which was consistent with the report that hepatic NK cells (more than 1.0×106) derived from liver graft could be detected in the recipient circulation for approximately 2 weeks.[9]These findings suggest that alloreactive liver NK cells derived from liver graft can be transferred into the recipient and survive, even play a role in regulating immunological response to liver graft. After revascularization of the liver graft, the abundant and higher-activity of liver NK cells derived from the graft may migrate into the recipient in a way of "passenger leukocytes". In the process of migration, donor liver NK cells interact with allogeneic cells of the recipient, which may be beneficial to the acceptance of liver allograft. NK cells have been wildly used for adoptive immunotherapy against cancer at present. And the alloreactivity of NK cells has been confirmed to eliminate the relapse of acute myeloid leukemia and protect patients from graft-verus-host disease in HLA haplotype-mismatched hematopoietic transplantation.[22,23]Thus, allogeneic NK cells derived from donor liver could modulate recipient's immune response to the graft.
Treatment for end-stage liver disease is to improve the life quality of the patient. In the present study, changes of body weight and general status in rats after transplant were used to evaluate their quality of life. The results showed that transfusion ofdtlNKs immediately after transplantation of the irradiated liver could diminish the incidence of adverse events in both LEW and BN recipients, promote the recovery or growth of body weight in Lewis recipients after transplant, and retard the weight loss in BN recipients as well. These findings indicate that donor liver NK cells are helpful to the improvement of recipient survival. We suppose that a considerable number of damaged cells due to irradiation or ischemia-reperfusion are still in the liver. The damaged cells as an important source of alloantigens might recruit the immune cells of the recipient into the liver graft and promote rejection. However, transfusedpdlNKs might remove these damaged cells by their selfimmune surveillance, which may down-regulate the acute rejection caused by indirect recognition. Thus, the recipient with liver NK cells derived from liver graft would have a better survival after LTx.
The accumulation of activated lymphocytes into the allograft is recognized essential to the pathogenesis of tissue injury, and acute liver allograft rejection is characterized by a mixed portal tract infiltration containing mononuclear cells. In our study, transfusion ofdtlNKs immediately after grafting of the irradiated liver could mitigate the damage of liver graft in both LEW and BN recipients compared with those rats that received the graft depleted of donor leukocytes. All these indicate that donor liver NK cells play a role in down-regulating acute rejection. It has been reported that NK cell infiltration with the destruction of CD8+T lymphocytes co-exists in the tolerant liver allograft, which may be relative to the donor liver NK cells transmitting inhibition signal and inducing apoptosis of CD8+lymphocytes via KIR receptor.[24]Zhao et al[25]reported that NK cells in BALB/c→B6 fully MHC-mismatched mixed chimeric mice were specifically tolerant to the antigens of donors (bone marrow cells of BALB/c mice) and hosts (bone marrow cells of B6 mice). Therefore, it appears that donor liver NK cells are not really unnecessary for liver tranplant immunity. Conversely, they might mitigate the damage by immune rejection and even co-regulate liver allograft tolerance together with other NPCs of liver graft.
Surprisingly, depletion of passenger leukocytes via donor WBI did not alter original tolerance to acute rejection in the majority of tolerant recipients after LTx, which seems to be ambivalent to the long-held dictum that passenger leukocytes derived from the liver graft are essential to liver allograft tolerance in some strains of rats.[2,3]We ascribe the different strain combinations used in our study to the root cause that generated the difference mentioned above. The fate of liver graft is strictly dependent on the genetic background of the donor and recipient.[26]Thus the different strain combinations of the donor and recipient may account for the different immune responses of recipients to the allograft after depletion of donor derived passenger leukocytes. In addition, the underlying mechanisms of liver allograft tolerance include a series of molecular and cellular mechanisms.[27]Except donor liver NK cells, thee are lots of other cells and molecules (hepatocytes, NPCs, MHC, etc) in the liver graft from the donor. The interaction between donor liver NK cells and other donor derived cells or molecules may also regulate the immune response after LTx.
The present study has demonstrated a novel property of donor liver NK cells in LTx, which could alleviate liver allograft acute rejection, even in liverallograft acceptance. This gives some insights into the mechanism of liver allograft tolerance.
Acknowledgement
We heartily thank Doctor Shao-Min Huang from Cancer Center of Sun Yat-Sen University for experimental animals WBI.
Funding:This research was supported by a grant from the National Natural Science Foundation of China (No. 30671987).
Ethical approval:The rats were treated in accordance with the guidelines of the European Community Standards on the Care and Use of Laboratory Animals (No. 28871-22A9).
Contributors:YJD and LTZ contributed equally to this study. WYL proposed the study and wrote the first draft. LGL and LLH performed the research and analyzed the data. All authors contributed to the design and interpretation of the study and to the further draft. WYL is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis 2007;27:194-213.
2 Sun J, McCaughan GW, Gallagher ND, Sheil AG, Bishop GA. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation 1995;60:233-236.
3 Sun J, Sheil AG, Wang C, Wang L, Rokahr K, Sharland A, et al. Tolerance to rat liver allografts: IV. Acceptance depends on the quantity of donor tissue and on donor leukocytes. Transplantation 1996;62:1725-1730.
4 Hamerman JA, Ogasawara K, Lanier LL. NK cells in innate immunity. Curr Opin Immunol 2005;17:29-35.
5 Ljunggren HG, Karre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today 1990;11: 237-244.
6 Multhoff G, Botzler C, Wiesnet M, Eissner G, Issels R. CD3-large granular lymphocytes recognize a heat-inducible immunogenic determinant associated with the 72-kD heat shock protein on human sarcoma cells. Blood 1995;86:1374-1382.
7 Hata K, Zhang XR, Iwatsuki S, Van Thiel DH, Herberman RB, Whiteside TL. Isolation, phenotyping, and functional analysis of lymphocytes from human liver. Clin Immunol Immunopathol 1990;56:401-419.
8 Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol 1998;28:84-90.
9 Moroso V, Metselaar HJ, Mancham S, Tilanus HW, Eissens D, van der Meer A, et al. Liver grafts contain a unique subset of natural killer cells that are transferred into the recipient after liver transplantation. Liver Transpl 2010;16:895-908.
10 Claerhout I, Kestelyn P, Debacker V, Beele H, Leclercq G. Role of natural killer cells in the rejection process of corneal allografts in rats. Transplantation 2004;77:676-682.
11 Kitchens WH, Uehara S, Chase CM, Colvin RB, Russell PS, Madsen JC. The changing role of natural killer cells in solid organ rejection and tolerance. Transplantation 2006;81:811-817.
12 van der Touw W, Bromberg JS. Natural killer cells and the immune response in solid organ transplantation. Am J Transplant 2010;10:1354-1358.
13 Maier S, Tertilt C, Chambron N, Gerauer K, Hüser N, Heidecke CD, et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28-/- mice. Nat Med 2001;7:557-562.
14 Kondo T, Morita K, Watarai Y, Auerbach MB, Taub DD, Novick AC, et al. Early increased chemokine expression and production in murine allogeneic skin grafts is mediated by natural killer cells. Transplantation 2000;69:969-977.
15 Beilke JN, Kuhl NR, Van Kaer L, Gill RG. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med 2005;11:1059-1065.
16 Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med 2006;203:1851-1858.
17 Bouwens L, Remels L, Baekeland M, Van Bossuyt H, Wisse E. Large granular lymphocytes or "pit cells" from rat liver: isolation, ultrastructural characterization and natural killer activity. Eur J Immunol 1987;17:37-42.
18 Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation 1979;28:47-50.
19 Dresske B, Lin X, Huang DS, Zhou X, Fandrich F. Spontaneous tolerance: experience with the rat liver transplant model. Hum Immunol 2002;63:853-861.
20 Takata N, Yamaguchi Y, Mori K, Misumi M, Katsumori T, Goto M, et al. Prolonged survival of rat hepatic allografts after total-body irradiation of the donors. Transplantation 1992;54:215-218.
21 Sheng-Tanner X, Miller RG. Correlation between lymphocyteinduced donor-specific tolerance and donor cell recirculation. J Exp Med 1992;176:407-413.
22 Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002;295:2097-2100.
23 Guo H, Qian X. Clinical applications of adoptive natural killer cell immunotherapy for cancer: current status and future prospects. Onkologie 2010;33:389-395.
24 Navarro F, Portalès P, Candon S, Pruvot FR, Pageaux G, Fabre JM, et al. Natural killer cell and alphabeta and gammadelta lymphocyte traffic into the liver graft immediately after liver transplantation. Transplantation 2000;69:633-639.
25 Zhao Y, Ohdan H, Manilay JO, Sykes M. NK cell tolerance in mixed allogeneic chimeras. J Immunol 2003;170:5398-5405.
26 Kamada N. The immunology of experimental liver transplantation in the rat. Immunology 1985;55:369-389.
27 Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev 2006;213:101-118.
Received November 1, 2010
Accepted after revision April 18, 2011
Author Affiliations: Department of Hepatobiliary Surgery, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China (Yu JD, Long TZ, Li GL, Lv LH, Lin HM, Huang YH, Chen YJ and Wan YL); Department of Breast Surgery, Guangzhou Women and Children's Medical Center, Guangzhou 510120, China (Long TZ)
Yun-Le Wan, MD, Department of Hepatobiliary Surgery, Sun Yat-Sen, Memorial Hospital, Sun Yat-Sen University, 107W, Yanjiang Road, Guangzhou 510120, China (Tel: 86-20-34071173; Email: wanyldr@ 163.com)
? 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
 Hepatobiliary & Pancreatic Diseases International2011年4期
Hepatobiliary & Pancreatic Diseases International2011年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Surgical treatment of Budd-Chiari syndrome: analysis of 221 cases
- Hepatitis C virus infection and biological falsepositive syphilis test: a single-center experience
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Success rate and complications of endoscopic extraction of common bile duct stones over 2 cm in diameter
- Enteral supplementation with glycyl-glutamine improves intestinal barrier function after liver transplantation in rats
- Noninvasive indocyanine green plasma disappearance rate predicts early complications, graft failure or death after liver transplantation
