Effect of Relative Humidity on Catalytic Combustion of Toluene over Copper Based Catalysts with Different Supports*
FANG Jiancai (方健才), CHEN Xiao (陳曉), XIA Qibin (夏啟斌), XI Hongxia (奚紅霞) and LI Zhong (李忠)**
?
Effect of Relative Humidity on Catalytic Combustion of Toluene over Copper Based Catalysts with Different Supports*
FANG Jiancai (方健才), CHEN Xiao (陳曉), XIA Qibin (夏啟斌), XI Hongxia (奚紅霞) and LI Zhong (李忠)**
State Key Lab of Subtropical Building Science, Institute of Chemical Engineering, South China University of Technology, Guangzhou 510640, China
The copper based catalysts, CuO/γ-Al2O3, CuO/γ-Al2O3-cordierite (Cord) and CuO/Cord, were prepared by impregnation method. The catalytic activity of the catalysts was tested in absence and presence of water vapor, and the catalysts were characterized. Temperature program desorption (TPD) experiments of toluene and water on the catalysts were carried out. The influence of water vapor on the activity of the catalysts was discussed. Results showed that addition of the water vapor has a significant negative effect on the catalytic activity of the catalysts. The higher the concentration of the water vapor in feed steam was, the lower the catalytic activity of the copper based catalysts became, which could be mainly ascribed to the competition of water molecules with toluene molecules for adsorption on the catalyst surfaces. TPD experiments showed that the strength of the interaction between water molecules and three catalysts followed the order: CuO/γ-Al2O3>CuO/γ-Al2O3-Cord>CuO/Cord. As a consequence of that, the degree of degradation in the catalytic activity of these three catalysts by the water vapor followed the order: CuO/γ-Al2O3>CuO/γ-Al2O3-Cord>CuO/Cord. However, the negative effect of the water vapor was reversible.
copper based catalysts, relative humidity, toluene, catalytic combustion, water inhibition
1 INTRODUCTION
Volatile organic compounds (VOCs) are recognized as major contributors to air pollution. These VOCs [1-4], including alcohols, alkanes and aromatics, may pollute the atmosphere and jeopardize human health. Several technologies have been developed for the destruction of VOCs [5, 6], of which catalytic combustion is the most feasible and economic approach due to its low destruction temperature and excellent selectivity toward the formation of harmless products. Precious metals and base metal oxides are commonly used for the catalytic combustion of VOCs [7-9]. The noble metals, especially, platinum and palladium, are recognized as the most typical species of precious metal catalysts due to their higher activity. However, their widely application was limited because they were rather expensive and susceptible to deactivation by poisoning, particularly in the presence of compounds containing chlorine, sulphur, or in presence of water [10, 11]. Recent years, many efforts were made to the design of transition metal based catalysts as well as perovskite catalysts with high catalytic activity. Wang [12] investigated the catalytic incineration of toluene over γ-Al2O3-supported transition-metal oxide catalysts by employing a fixed bed flow reactor, and reported that CuO/γ-Al2O3was the most active of seven catalysts tested. Wang and Chou [13] used chromium oxide (Cr2O3) catalyst for catalytic oxidation of-hexane, benzene, and an emission air/vapor mixture collected from an oil/water separator of a refinery, and found that it was efficient for catalytic combustion of these organic pollutants.
Although the use of metal oxide catalysts for incineration process is widely accepted due to its cheapness and duration, it was found that the removal efficiency of VOCs on the catalysts was negatively affected by high concentration of water vapor of feed steam in practical applications. Several authors have dealt with the inhibiting effect of water. Wang [12] reported that the presence of water vapor reduced the conversion of toluene on CuO/γ-Al2O3. Abdullah. [14] studied catalytic combustion of ethyl acetate and benzene over Cr-ZSM-5 and reported the inhibition effect of water due to a competitive adsorption. Pan. [15] reported that the existence of water vapor had a significant negative effect on the catalytic activity of copper based catalysts. Thevenet. [16] studied the influence of water vapor on the efficiency of plasma/ photocatalytic oxidation of acetylene, and found that the presence of water vapor in the gas stream induced a strong decrease in the photocatalytic oxidation of acetylene. These results above indicated that the presence of water vapor had negative influence on activity of catalysts for catalytic oxidation of VOCs. Therefore, it is necessary to develop catalysts with higher catalytic activity in presence of relatively higher humidity.
The main objective of this work is to prepare copper based catalyst with different supports and study the effects of water vapor on their catalytic activity for catalytic combustion of toluene. The catalysts CuO/Cord, CuO/γ-Al2O3and CuO/γ-Al2O3-Cord were prepared by impregnation method, and then characterized by X-ray diffraction (XRD) and N2adsorption. The catalytic activity of the prepared catalysts for toluene combustion was tested under the condition of relatively high humidity feed steam with humidity range of 0-90%. Temperature program desorption (TPD) experiments of toluene and water on the catalysts were carried out. The influence of water vapor on the activity of the catalysts was discussed and reported here.
2 EXPERIMENTAL
2.1 Materials and instrumentation
Toluene (analytical grade) was purchased from the Chemical Plants of Tianjin (Tianjin, China). γ-Al2O3was supplied by Jiangxi Weihua Chemical Company and cordierite was provided by Jingdezhen Jiayi New Material Company. Cu (NO3)2was analytical reagents.
GC9160 gas chromatograph was supplied by Shanghai Chromatograph Company. Gas Generator GX300A was purchased from Beijing Zhongxing Huili Science and Technology Company. Mass flow rate controller (MFC) was supplied by Beijing Sevenstar Electronic Company. Temperature-programmed controller AI-708 was supplied by Xiamen Yudian Automatization Company, and its temperature could be controlled to an accuracy of±0.2°C. CENTER 311 humidity/temperature meter was provided by Center Technology Group Company (Taiwan, China).
2.2 γ-Al2O3-Cord substrate preparation
In this work, γ-Al2O3, γ-Al2O3-Cord, and cordierite (MgO·Al2O3·SiO2) with 0.25-0.42 mm in diameter were separately used as the substrates. Commercial γ-Al2O3and cordierite (MgO·Al2O3·SiO2) can be purchased, and the γ-Al2O3-Cord needed to be prepared. Firstly, 500 ml of deionized water was added to a round bottom flask equipped with stirrer, reflux condenser and thermometer, and heated up to 85°C by thermostat. Then, aluminum isopropylate (the molar ratio of aluminum isopropylate to distilled water was 1︰150) was slowly added, hydrolyzed for 1.5 h at 85°C, followed by stirring for 2 h at 95°C, and then added 2 mol·L-1dilute nitric acid to adjust the pH value of the solution to 3.5-4.0 for peptization. After that, the alumina suspension was obtained by refluxing for 24 h at 95°C. Secondly, the cordierite was impregnated in the prepared suspension above for 1 h, then dried at 120°C for 4 h in air, and followed with calcination at 500°C for 2 h. The dipping procedure above was repeated twice and thus the γ-Al2O3-Cord was available. The as-prepared substrate was designated as γ-Al2O3-Cord. The mass content of a γ-Al2O3layer was about 12% in the γ-Al2O3-Cord substrate.
2.3 Catalyst preparation
γ-Al2O3, γ-Al2O3-Cord, and cordierite (MgO·Al2O3·SiO2) with 0.25-0.42 mm in diameter were separately used as the substrates. Firstly, copper oxide catalysts supported separately on those supports were prepared by incipient wetness impregnation method. For example, CuO/γ-Al2O3, CuO/Cord and CuO/γ-Al2O3-Cord samples with one-step impregnation were prepared by impregnating supports with an aqueous solution containing the requisite amount of Cu(NO3)2, followed by stirring for 12 h at room temperature, and then dried at 80°C for 10 h. After that, they were calcined in air at 550°C for 5 h. Secondly, the obtained samples were reduced at 350°C in hydrogen atmosphere for 10 min and then calcined at 300°C in air atmosphere for another 5 h. Thus, the copper based catalysts were obtained and designated as CuO/γ-Al2O3, CuO/Cord and CuO/γ-Al2O3-Cord, respectively. The loading of copper oxide was fixed at 10% for all of the catalysts prepared.
2.4 Catalyst characterization
2.4.1
Nitrogen adsorption carried out at 77 K using an Accelerated Surface Area and Porosimetry System (ASAP 2010, Micromeritics) [17] was used to determine the textural properties of the catalysts. The BET surface area was calculated from the adsorption isotherms using the standard Brunauer-Emmett-Teller (BET) equation. The Barrett-Joyner-Halenda (BJH) method was used to calculate the mesopore volume.
2.4.2
XRD measurements were conducted using X’Pert Pro (PANalytical, Netherlands) diffraction meter employing Ni-filtered Cu Kαradiation (0.15418 nm) at room temperature. The X-ray tube was operated at 40 kV and 40 mA. The scanning rate was 0.04 (°)·s-1.
2.4.3
TPD is a surface analysis technique. It is usually used to estimate binding energy between the adsorbate and the adsorbent or the interaction between water molecule and adsorbent surfaces [18, 19]. In this work, TPD experiments were conducted separately to estimate the ability of catalysts for adsorption of toluene in the presence of water vapor and the interaction between water molecule and catalyst surfaces.
Firstly, the sample of catalysts adsorbed toluene in a gas flow with toluene concentration of 3.5 g·m-3and the relative humidity of 90% for 4 h. Then, the sample that had adsorbed the toluene vapor was packed in a stainless reaction tube with inner diameter of 0.2 cm and length of 0.5 cm. Subsequently, the stainless tube was placed in a reaction furnace and then heated in the high-purity N2flow at a constant rate of 30 ml·min-1and a heating rate of 10°C·min-1. The desorbed toluene was measured online by using gas chromatography [Hua’ai GC 9160 gas chromatograph equipped with flame ionization detector (FID)] at the outlet of the stainless tube, and effluent curves were recorded, which were called the TPD curves of toluene desorption from the catalyst.
H2O-TPD experiments were conducted at a heating rate of 3°C·min-1. In each experiment, the sample that had adsorbed the water vapor was packed in a fixed-bed quartz flow reactor at a N2flow rate of 10 ml·min-1. The desorbed water vapor was measured by using the Autochem 2920 with a thermal conductivity detector (TCD) at the outlet of the quartz tube, and effluent curves were recorded, which were called the H2O-TPD curves.
2.5 Catalytic evaluation
Catalytic activity tests were carried out at atmospheric pressure in a continuous flow tubular stainless steel reactor (length of 25 mm and i.d. of 4 mm) with an internal catalyst bed. A catalyst sample of 0.1 g was loaded in the middle of the reactor supported by quartz wool. Reactant gas was the mixtures of gases containing toluene vapor, water vapor and dried air. The reactant gas flow (90 ml·min-1with 3.5 g·m-3toluene in air) was controlled by mass flow controllers, wherein the relative humidity of the feed stream was measured by electrical hygrometer with an accuracy of±3% and controlled automatically by using a humidifier. The effluent gases were analyzed online by using gas chromatography (Hua’ai GC 9160 gas chromatograph equipped with FID).
3 RESULTS AND DISCUSSION
3.1 Catalyst characterization
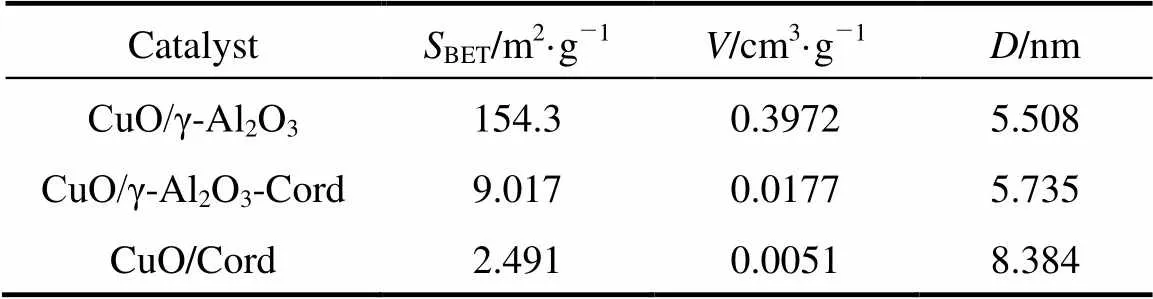
Table 1 summarizes the textural parameters of the catalysts studied. The data in Table 1 indicate the specific surface areas, pore volumes and average pore diameters of the catalysts CuO/γ-Al2O3, CuO/γ-Al2O3- Cord and CuO/Cord.
Note:BETis the BET surface area,represents pore volume andstands for average pore diameter.
Figure 1 shows the XRD patterns of the copper based catalysts studied. For the catalysts CuO/γ-Al2O3, CuO/Cord and CuO/γ-Al2O3-Cord, the CuO (JCPDS45- 0937) diffraction peaks at 2values of 35.5° and 38.8° could be observed clearly. The crystallite size of CuO can be estimated using the XRD data by Scherrer equation. The mean diameter of the CuO crystallites on the catalysts CuO/γ-Al2O3, CuO/Cord and CuO/γ- Al2O3-Cord, calculated from the X-ray line broadening according to the Scherrer equation, were about 22.3, 22.8 and 23 nm, respectively.

Figure 1 XRD profiles of the copper based catalysts studied
3.2 Effect of water vapor on the catalytic activity
Figure 2 shows the effects of water vapor concentration on the evolution of toluene conversion over the catalysts as a function of reaction temperature. It can be seen that all of the profiles of toluene conversion shift slightly to higher temperatures with increasing water vapor concentration. It meant that the presence of water vapor has a significant negative effect on the catalytic activity of the copper based catalysts. The higher the concentration of the water vapor was, the lower the catalytic activity of the copper based catalysts became. It can be ascribed to the competition of water molecules with toluene molecules for adsorption on the active sites [15, 20]. The higher coverage of the active sites by the water vapor lead to a reduction in the number of active sites available for the reaction of toluene, and thus resulted in the reduction of catalytic activity of the copper based catalysts with increasing water vapor concentration.
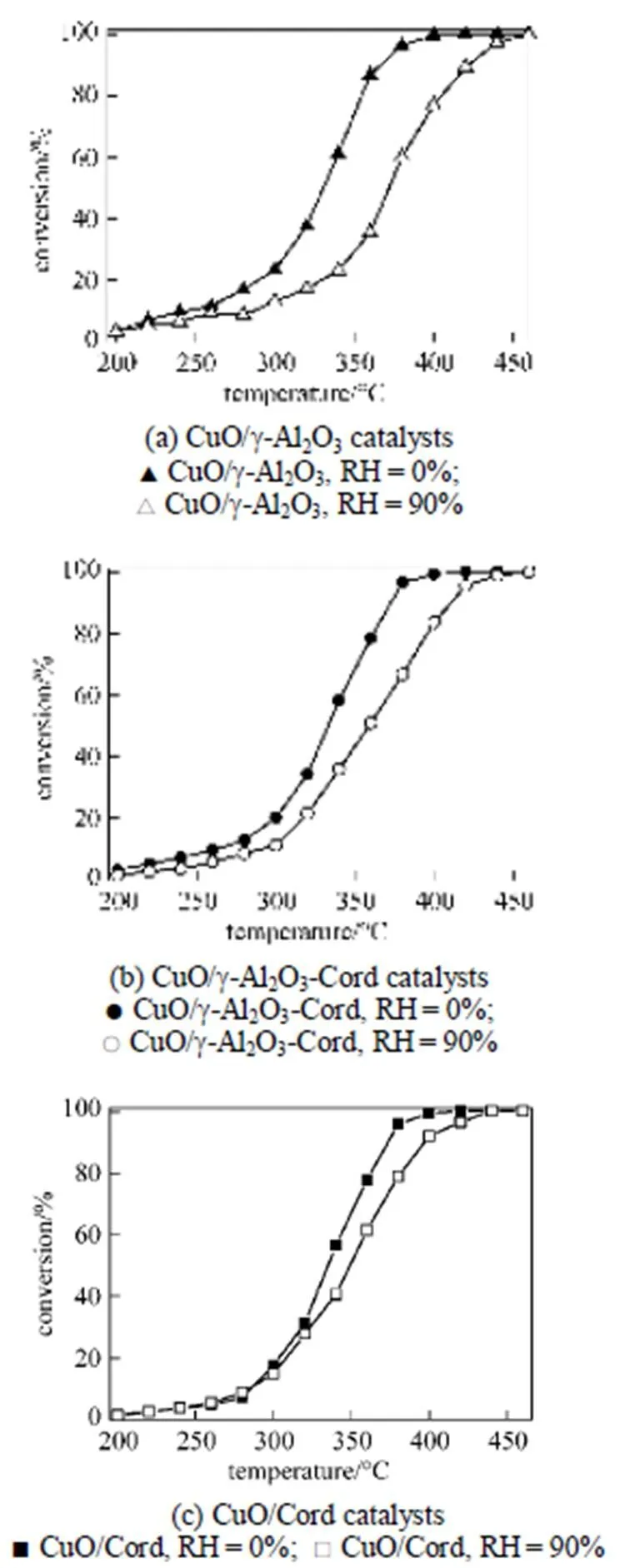
Figure 2 Conversion of toluene over the catalysts in the absence and presence of water (Toluene concentration is 3.5 g·m3and SV is 40000 h-1)
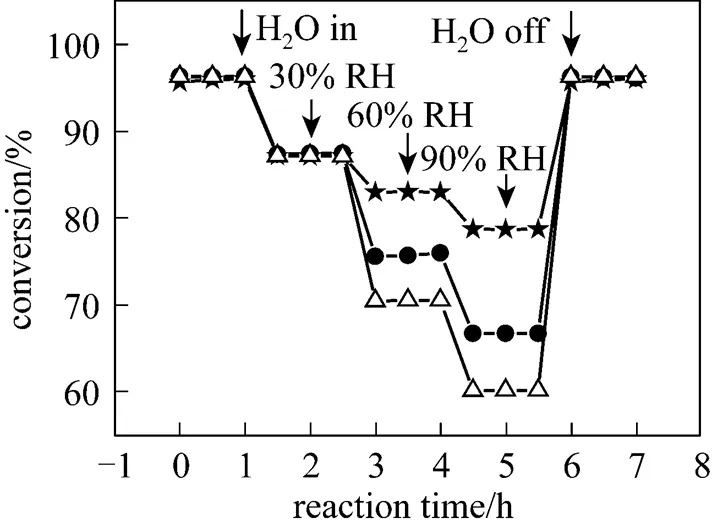
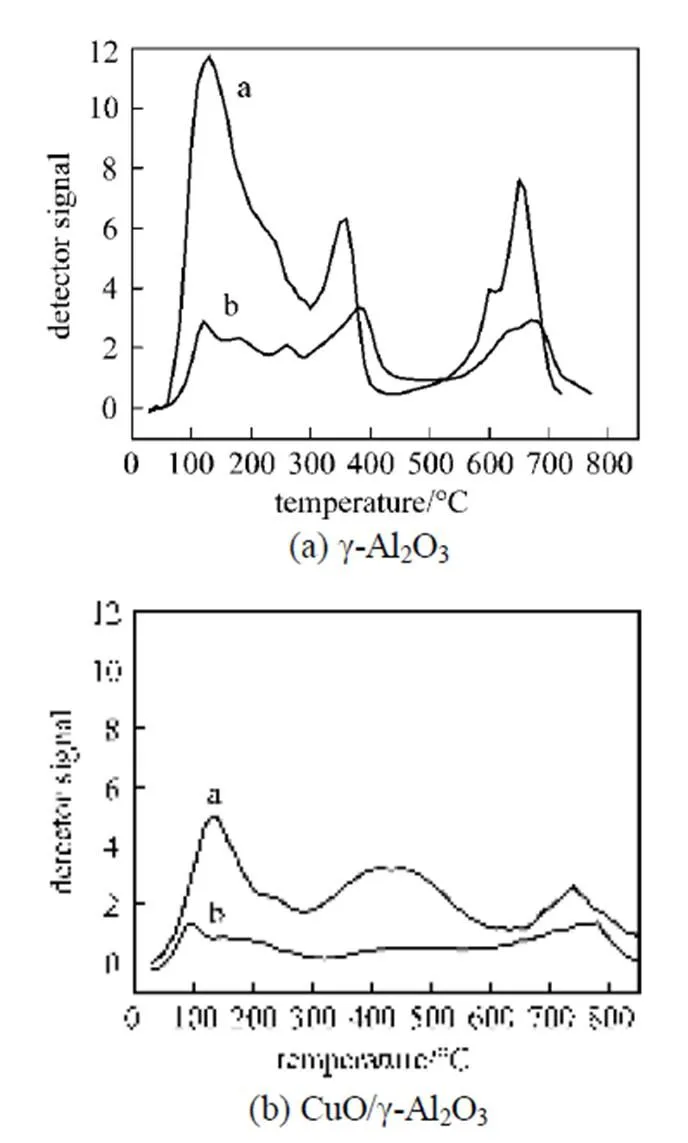
In addition, it was also noticed that the degradation degree of the catalytic activity of these copper based catalysts with various supports was different. In this work, the90(reaction temperature required for 90% conversion of toluene) was used as criteria to evaluate the catalytic activity of the catalysts. Table 2 shows the90values of the catalysts in the absence and presence of water vapor. The data in Table 2 indicate that although the90values of the catalyst CuO/γ-Al2O3in the absence of water vapor was the lowest, its90values became the highest when relative humidity of feed steam increased from 0 to 90%. In other words, the90value of the CuO/γ-Al2O3was changed from 367°C to 422°C due to an increase of the water vapor concentration, and the variation percentage of the90value was up to 15%. For the catalysts CuO/γ-Al2O3-Cord and CuO/Cord, when the relative humidity of feed steam increased from 0 to 90%, their90values varied from 372°C to 411°C and from 374°C to 398°C, and the percentage of increase in their90values was 10.5% and 6.4%, respectively, which were much lower than that in the90value of CuO/γ-Al2O3. It meant that although the catalyst CuO/γ-Al2O3had higher catalytic activity in the absence of water vapor, its durability to water vapor was low, resulting in a significant degradation of its catalytic activity at higher water vapor concentration. A comparison of the90values and the percentage of90increase at high water vapor concentration show that the degree of degradation in the catalytic activity of these three catalysts by water vapor followed the order: CuO/γ-Al2O3>CuO/γ-Al2O3-Cord>CuO/Cord, which was ascribed to difference in adsorption ability of these three catalysts towards water vapor. In other words, the durability towards water vapor poisoning of these catalysts followed the order: CuO/γ-Al2O3< CuO/γ-Al2O3-Cord Table 2 Effect of the water vapor on the temperature of T90① ① Temperature at which toluene conversion reaches 90%. Figure 3 shows the toluene conversiontime at 380°C on the three catalysts when water vapor was periodically added to the feed stream (water vapor was periodically added to the feed stream every 60 min as shown by the striped areas). It can be seen that when water vapor was added to the feed stream, the activity of the catalyst CuO/γ-Al2O3decreased abruptly to a lower level, and then kept constant during the presence of water vapor. The higher the concentration of water vapor added to the feed stream, the lower the activity of the catalysts became. Nevertheless, it was found that once the water vapor was removed, the catalytic activity of these catalysts was wholly recovered to their initial activity. That is to say, the negative effect of the water vapor is reversible, and the catalytic activity recovers upon the water vapor is withdrawn. This alternative deactivation and recovery of the catalytic activity were observed for the entire cycle from Fig. 3. Consequently, it can be stated that deactivation of the catalysts by the water vapor is temporary, and once water vapor no longer existed in the feed stream, the initial activity of the catalyst is easily recoverable due to no competition adsorption of water molecules. This behavior could be attributed to a competitive adsorption of water and toluene on the active sites. Figure 3 Effect of the water vapor on toluene conversion over three prepared catalysts at 380°C (Toluene concentration is 3.5 g·m-3and SV is 40000 h-1) ★?CuO/Cord; ●?CuO/γ-Al2O3-Cord; △?CuO/γ-Al2O3 Figure 4 shows the TPD spectrums of water desorption from the catalysts CuO/γ-Al2O3, CuO/γ-Al2O3-Cord and CuO/Cord. It can be seen that there was an obvious peak in each TPD spectrum due to desorption of the water vapor on the catalysts. A comparison of three TPD spectrums of water desorption indicates that the desorption of the water from CuO/Cord started at the earliest and the peak temperature of its TPD curve was the lowest,.. 50.5°C, while that from CuO/Cord began at the latest and the peak temperature was the highest,.. 90.1°C. It indicated that desorption of the water from the catalyst CuO/Cord was the easiest, while from the catalyst CuO/γ-Al2O3it was the hardest. It was suggested that interaction of water molecular with surfaces of the catalyst CuO/Cord was the weakest, while it was the strongest for the catalyst CuO/γ-Al2O3. Therefore, it can be deduced from these TPD spectrums in Fig. 4 that the strength of the interaction between water molecules and three catalysts with different supports followed the order: CuO/γ-Al2O3>CuO/γ-Al2O3-Cord>CuO/Cord. Figure 4 TPD profiles of H2O on three catalysts prepared a—CuO/γ-Al2O3; b—CuO/γ-Al2O3-Cord; c—CuO/Cord It can be seen from Fig. 5 that the peak areas of the curves a were much larger than those of the curve b, indicating that the amounts adsorbed of toluene on the prepared catalysts in the absence of water vapor were higher than those in the presence of water vapor due to competition adsorption of water molecules. For comparison of peak areas, the profiles of the TPD curves were separately integrated by means of numerical method to obtain the peak areas of these curves. It was found that for the catalyst CuO/γ-Al2O3, the peak area of the curve b was 26% of that of the curve a; for the catalyst CuO/γ-Al2O3-Cord, the peak area of the curve b was 42% of that of the curve a; and for the catalyst CuO/Cord, the peak area of the curve b was 63% of that of the curve a. It indicated that the differences in peak area between the curves a and curves b for each of the catalysts followed the order: CuO/γ-Al2O3>CuO/γ-Al2O3-Cord>CuO/Cord. It meant that the durability of the CuO/Cord towards the competition adsorption of water vapor was the best, while the durability of the CuO/γ-Al2O3towards water vapor competition adsorption was the worst. The presence of water vapor has a significant negative effect on the catalytic activity of the copper based catalysts. The higher the concentration of the water vapor in feed steam was, the lower the catalytic activity of the copper based catalysts became. The degree of degradation in the catalytic activity of these three catalysts by water vapor followed the order: CuO/γ-Al2O3>CuO/γ-Al2O3-Cord>CuO/Cord. It can be mainly ascribed to the competition of water molecules with toluene molecules for adsorption on the active sites. The strength of the interaction between water molecules and three catalysts followed the order: CuO/γ-Al2O3>CuO/γ-Al2O3-Cord>CuO/Cord. The durability of three kinds of catalysts towards the water vapor competition adsorption followed the order: CuO/Cord>CuO/γ-Al2O3-Cord>CuO/γ-Al2O3. The negative effect of the water vapor is reversible and the catalytic activity can be recovered when the water vapor is withdrawn. Figure 5 TPD profiles of toluene adsorbed on the catalysts which adsorbed previously toluene under the condition of RH = 0% or RH = 90% a—RH=0%; b—RH=90% 1 Jones, A.P., “Indoor air quality and health”,, 33 (28), 4535-4564 (1999). 2 Carpentier, J., Lemonier, J.F., Siffert, S., Zhilinskaya, E.A., Aboukais, A., “Characterisation of Mg/Al hydrotalcite with interlayer palladium complex for catalytic oxidation of toluene”,..:., 234 (1/2), 91-101 (2002). 3 Paulis, M., Gandia, L.M., Gil, A., Sambeth, J., Odriozola, J.A., Montes, M., “Influence of the surface adsorption-desorption processes on the ignition curves of volatile organic compounds (VOCs) complete oxidation over supported catalysts”,..:., 26 (1), 37-46 (2000). 4 Chu, H., Lee, W.T., “The effect of sulfur poisoning of diethyl disulfide on the catalytic incineration over a Pt/Al2O3catalyst”,, 209 (2/3), 217-224 (1998). 5 Wang, B.Q., Ma, G.D., Chen, J.N., “Development in research of technology for purifying volatile organic compounds from exhaust gases”,, 4 (5), 47-51 (2003). 6 Wu, Y.W., Li, Z., Xi, H.X., Xu, K.F., Han, J.L., Guo, J.G., “The technologiesand materials of adsorbents & catalysts for the control of VOCspollution”,, 19 (1), 88-95 (2003). 7 Salvador, O., Lisardo, B., Herminio, S., “Kinetics of the deep combustion of benzene, toluene-hexane and their binary mixtures over a platinum on γ-alumina catalyst”,..:., 38 (2), 139-149 (2002). 8 Yazawa, Y., Takagi, N., Yoshida, H., “The support effect on propane combustion over platinum catalyst: Control of the combustion-resistance of platinum by the acid strength of support materials”,..:., 233 (1/2), 103-112 (2002). 9 Luo, M.F., He, M., Xie, Y.L., Fang, P., Jin, L.Y., “Toluene oxidation on Pd catalysts supported by CeO2-Y2O3washcoated cordierite honeycomb”,..:., 214 (69), 213-218 (2007). 10 Kim, H.S., Kim, T.W., Kolb, H.L., “Complete benzene combustion over Pt-Pd bimetal catalyst supposed on γ-alumina: Influence of Pt-Pd ratio on the catalytic activity”,..:., 280 (2), 125-131 (2005). 11 Bertinchamps, F., Attianese, A., Mestdagh, M.M., Gaigneaux, E.M., “Catalysts for chlorinated VOCs abatement: Multiple effects of water on the activity of VOx based catalysts for the combustion of chlorobenzene”,, 112, 165-168 (2006). 12 Wang, C.H., “Al2O3-supported transition-metal oxide catalysts for catalytic incineration of toluene”,, 55 (1), 11-17 (2004). 13 Wang, J.B., Chou, M.S., “Kinetics of catalytic oxidation of benzene,-hexane, and emission gas from a refinery oil/water separator over a chromium oxide catalyst”,, 50 (1), 227-233 (2000). 14 Abdullah, Z.A., AbuBakar, M.Z., Bhatia, S., “A kinetic study of catalytic combustion of ethyl acetate and benzene in air stream over Cr-ZSM-5 catalyst”,, 42 (24), 6059-6067 (2003). 15 Pan, H.Y., Xu M.Y., Li, Z., Huang, S.S., He,C., “Catalytic combustion of styrene over copper based catalyst: Inhibitory effect of water vapor”,, 76 (5), 721-726 (2009). 16 Thevenet, F., Guaitella, O., Puzenat, E., Guillard, C., Rousseau, A., “Influence of water vapour on plasma/photocatalytic oxidation efficiency of acetylene”,..:., 84 (3/4), 813-820 (2008). 17 Wu, Y.W., Li, Z., Xi, H.X., “Influence of the microporosity and surface chemistry of polymeric resins on adsorptive properties toward phenol”,.., 113 (1-3), 131-135 (2004). 18 Li, X., Li, Z., Xia, Q.B., Xi, H.X., “Effects of pore sizes of porous silica gels on desorption activation energy of water vapor”,, 27 (5/6), 869-876 (2007). 19 Yu, M.X., Li, Z., Xi, H.X., Xia, Q., Wang, S.W., “Activation energy of desorption of dibenzothiophene on modified activated carbon by loading metal ions”,, 132 (1-3), 233-239 (2007). 20 Wang, C.H., Weng, H.S., “Al2O3-supported mixed-metal oxides for destructive oxidation of (CH3)2S2”,...., 36 (7), 2537-2542 (1997). 2009-01-13, 2009-08-09. the National Natural Science Foundation of China (20936001), the Natural Science Foundation of Guangdong Province, and the State Key Lab of Subtropical Building Science, South China University of Technology (x2yjC709028Z). ** To whom correspondence should be addressed. E-mail: cezhli@scut.edu.cn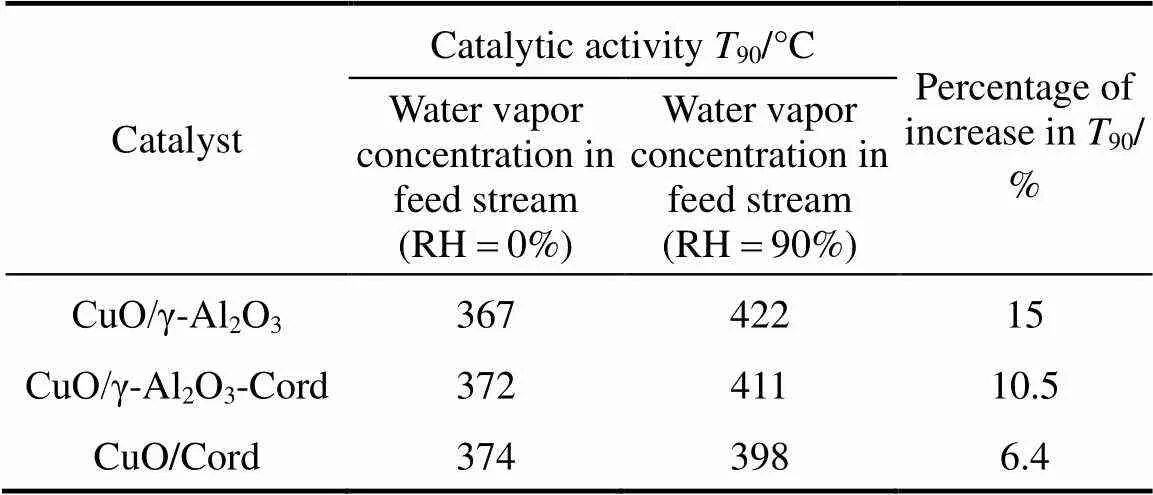
3.3 TPD spectrums of water desorption from three kinds of catalysts

3.4 TPD spectrums of toluene desorption from three kinds of catalysts


4 CONCLUSIONS
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Prediction of Critical Endpoints Based on the PR Equation of State*
- Kinetic Studies on Wheat Straw Hydrolysis to Levulinic Acid*
- Calculation of Transport Properties of CF4+Noble Gas Mixtures
- Synthesis of 4,4′-MDC in the Presence of Sulfonic Acid-functionalized Ionic Liquids*
- A New Study on Combustion Behavior of Pine Sawdust Characterized by the Weibull Distribution*
- Simulation of Liquid Argon Flow along a Nanochannel: Effect of Applied Force*
