Efficacy and safety of newly developed preservativefree latanoprost 0.005% eye drops versus preserved latanoprost 0.005% in open angle glaucoma and ocular hypertension: 12-week results of a randomized,multicenter, controlled phase III trial
Joon Mo Kim, Kyung Rim Sung, Ji Woong Lee, Haksu Kyung, Seungsoo Rho, Chan Yun Kim
1Department of Ophthalmology, Kangbuk Samsung Hospital,Sungkyunkwan University School of Medicine, Seoul 03181,Republic of Korea
2Department of Ophthalmology, Seoul Asan Medical Center,Ulsan University College of Medicine, Seoul 05505, Republic of Korea
3Department of Ophthalmology, Pusan National University Hospital, Pusan National University Medical School, Busan 49241, Republic of Korea
4Department of Ophthalmology, National Medical Center,Seoul 04564, Republic of Korea
5Department of Ophthalmology, CHA Bundang Medical Center, CHA University, Seongnam 13496, Republic of Korea
6Department of Ophthalmology, Severance Hospital, Yonsei University College of Medicine, Seoul 03722, Republic of Korea
Abstract
● KEYWORDS: latanoprost; benzalkonium chloride;intraocular pressure; preservative-free;
INTRODUCTION
Glaucoma is a major cause of irreversible blindness worldwide, and 111.8 million patients are expected globally by 2040[1]. Although many factors have been suggested as causes of glaucoma development, intraocular pressure (IOP)is still thought to be a major factor in the development and progression of glaucoma[2-3]. Many studies have reported that treatments that lower IOP decrease glaucoma progression[4-8].To date, control of IOP is the only proven way to suppress the progression of glaucoma. Therefore, IOP reduction remains the cornerstone of glaucoma management[9].
Prostaglandin analogue (PGA) has been used more and more frequently as it is preferred as a first-time glaucoma drug,which is effective and has less severe systemic side effects and requires only one dose per day[10]. Among the various PGAs,latanoprost, which was first developed, is the most widely used in ocular hypertension (OHT) and primary open angle glaucoma (POAG) due to its good effect and less side effects such as conjunctival hyperemia compared to other PGAs[10-12].However, latanoprost eye drops currently commonly used have a high concentration of benzalkonium chloride (BAK)and contain sodium phosphate, which could cause side effects such as conjunctivitis and corneal surface epithelial toxicity when administered for a long time[13-14]. Ocular surface changes that occur using prostaglandin eye drops with BAK can be significantly related to the concentration-dependent cytotoxicity of these preservatives[15]. In addition, the side effects of BAK may have a greater impact on glaucoma patients who need to use their medicine for life. On the other hand, in the case of preservative-free (PF) latanoprost, few apoptosis cells were found in the superficial layer of the corneal epithelium in human and toxic animal models[15-16]. Therefore, in the 2009 EMA guideline, the European Glaucoma Society recommends PF products for patients with glaucoma who have dry eye or ocular surface diseases[17].
Recently, a PF latanoprost generic eye drop was developed,TJO-002 (Xalost?S in Korea). TJO-002 has been formulated to have several presumed advantages over the conventional latanoprost preparation, which contains BAK. Polyoxyl 40 hydrogenated castor oil, carbomer (mucoadhesive polymer),and high-concentration sorbitol were used to promote substance stabilization and penetration into the eyeball instead of BAK and sodium phosphate. In order to improve the tolerability,instead of having a pH of 5.5 like the conventional latanoprost formulation [Xalatan?], TJO-002 has a physiologically active pH range of 7.0-7.3. This new formulation focuses on high stability, tolerability and non-inferior efficacy compared with the conventional formulation. This study aimed to compare TJO-002 with BAK-preserved latanoprost for IOP-lowering efficacy, safety and tolerability in patients with POAG/OHT.
SUBJECTS AND METHODS
Ethical Approval This study was approved by the Institutional Review Board of each center. This study was performed according to the tenets of the Helsinki Declaration and compliance with the International Conference on Harmonization Good Clinical Practice guidelines and Korean regulations. All patients were fully informed and provided written consent for participation before enrollment.
Study Design and Patients The study was a multicenter,randomized, investigator-masked, active control, and parallelgroup phase III clinical trial (NCT03419975). It was conducted in 17 clinical sites from 3 December 2015 to 5 March 2018.This study compared the newly developed PF latanoprost formulation TJO-002 (Taejoon Pharmaceutical Co., Ltd.,Yongin, Republic of Korea) with BAK-preserved latanoprost(Xalatan?, Pfizer Inc., Belgium NV Puurs, Belgium) during a 3-month treatment period. Given that TJO-002 is supplied in single dose units and BAK latanoprost in bottles, the investigational drug was managed by dividing the blind part and the unblind part and the investigator was blind part so that they could not know which eyedrop to be administered, only the investigator measuring IOP during the ophthalmological examination was masked to the study medication.
This study enrolled adult patients (≥19 years of age) with POAG/OHT. Patients with an IOP of 21 to 35 mm Hg at 9a.m.(±1h) in eligible eyes after a run-in period were randomized 1:1 and assigned the treatment schedule with TJO-002 or BAK latanoprost administered as one drop daily in each eye.We excluded the patients who had 20/80 or below of bestcorrected visual acuity on the Snellen chart and medical history of chronic intraocular inflammation in progress or within 3mo prior to screening. Patients who needed to use contact lenses during the clinical study and women who were pregnant, planning to become pregnant, currently nursing,of childbearing potential, or not using a reliable form of contraception were also excluded. Patients were randomized if IOP was >21 mm Hg in the eligible eye(s). If both eyes met the criteria, the eye with the higher IOP was selected. If the IOP was equal, the right eye was selected. Patients were instructed to instill one drop in each eye once daily in the evening(9p.m.±1h) and were scheduled for follow-up visits at 4, 8,and 12wk. The subjects were asked to keep a daily indication of whether or not to take an investigational drug in their diary table every time they administered, and were asked to answer the symptoms that they felt bad for the last week before the visit.
Assessment Parameters The primary efficacy variable was the change in IOP between baseline and 12wk in the study eye.Diurnal IOP (average of 2 consecutive IOP measurements)was measured at the same hour (9a.m.±1h and 5p.m.±1h)at each visit using a calibrated Goldmann applanation tonometer(Figure 1). All patients underwent ocular examinations, including visual acuity assessment, slit lamp biomicroscopy, gonioscopy,standard automated perimetry, and ophthalmoscopy. IOP was measured (at 9a.m.and 5p.m.) during the baseline visit and at the 8-week and 12-week visits after eye-drop instillation. At the 4-week visit after instillation, IOP was measured only at 9a.m. Safety outcome measures included adverse events (AEs) reporting, visual acuity, and tolerability.

Table 1 Patient demographics
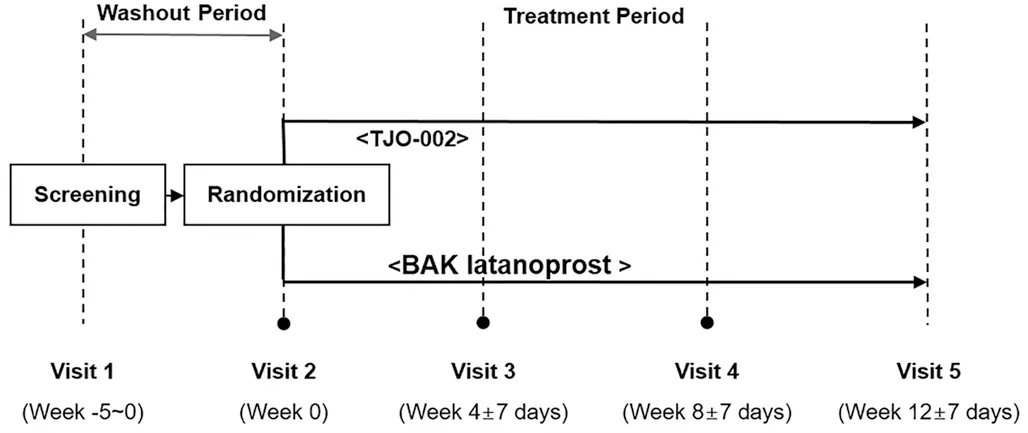
Figure 1 Study schedule Eligible patients were randomized to either the TJO-002 group or the BAK-preserved latanoprost group.
Tolerability was evaluated with the frequency and percentage of distributions of severity level by symptoms in each group after administration at 4, 8, and 12wk with questionnaire in the blind part (investigator). The symptoms checked during follow-up visits were pruritus, burning/stinging, blurred vision,sticky eye sensation, eye dryness sensation, and foreign body sensation. Each of symptoms was written by investigator about the symptoms subjects feel after instilling investigational drugs. The tolerability was evaluated by checking how the symptoms were changed based on the symptoms of the worst degree among the records written about the symptoms of the investigational drug administration for a week before visit.
Statistical Analysis The primary objective was to demonstrate the non-inferiority of the trial drug to the control drug in terms of diurnal IOP variation after the administration of the drugs for 12wk. If the maximum value of the confidence intervals was less than 1.5 mm Hg, the trial group was judged to be non-inferior to the control group[18]. The upper limit of noninferiority was set at 1.5 mm Hg as this is the standard acceptance level for noninferiority in glaucoma studies[19-21].The adjusted average and standard error of the IOP variations in the trial and control groups, the difference between the average and adjusted average, 95% two-tailed confidence intervals of adjusted average difference andP-values were calculated by conducting an analysis of covariation(ANCOVA) with baseline IOP as covariate and treatment as a parameter for IOP variations. Additionally, if there were any statistically significant variables among sex, ages, and BMI distribution, an ANCOVA was performed that corrected for these variables as a sensitivity analysis. Statistical analyses were performed using SAS v9.2 (SAS Institute, Cary, NC,USA). Tolerability was evaluated with the frequency and percentage of severity level distribution for each symptom in each group at 4, 8, and 12wk, and those differences between the groups were evaluated through Chi-square tests or Fisher’s exact tests. When there was a missing value, the last observation carried forward method was used.
RESULTS
Among 196 consenting subjects, 52 people were excluded(38 patients with “Deviation of inclusion/exclusion criteria”,13 with “Consent withdrawal” and 1 with “Other”), and 144 people were randomized. The full description of the inclusion and exclusion steps is outlined in Figure 2.
Demographic Characteristics There were 78.38% menvs21.62% women in the TJO-002 group and 60.00% men vs 40.00% women in the BAK latanoprost group. The sex ratios between the two groups were statistically significantly different(P=0.0167). There were no differences in other characteristics between the two groups (P>0.05; Table 1).
Efficacy Twelve weeks after initiation of drug administration,the mean diurnal IOP change was -7.21±3.10 mm Hg in the TJO-002 group and -7.02±3.17 mm Hg in the BAK latanoprost group. Both groups showed a statistically significant decrease of average diurnal IOP (P<0.0001) compared with baseline,but there was no significant difference in the follow-up IOPs and IOP changes between the two groups (Table 2).
Table 3 shows the change in the mean IOP at 9a.m.of each follow-up visit after drug administration compared to baseline.Both groups showed a statistically significant decrease in IOP at each follow-up visit (P<0.0001 each) from the baseline IOPs, but there was no statistically significant difference in the follow-up IOPs between the two groups.
Although the IOP in the TJO-002 group was less than that of the BAK latanoprost group at 9a.m.at 8wk after the beginning of the instillation, the difference was not statistically significant(P=0.06). Table 4 shows diurnal IOP fluctuation from 9a.m.to 5p.m.at 8 and 12wk of drug administration compared to that of baseline. The IOP fluctuations of the TJO-002 group were less than those of the BAK latanoprost group during the entire study period. However, the difference did not reach statistical significance except by 8wk after instillation (P<0.0342).

Figure 2 Study progress diagram FAS: Full analysis set; PPS: Per protocol set.
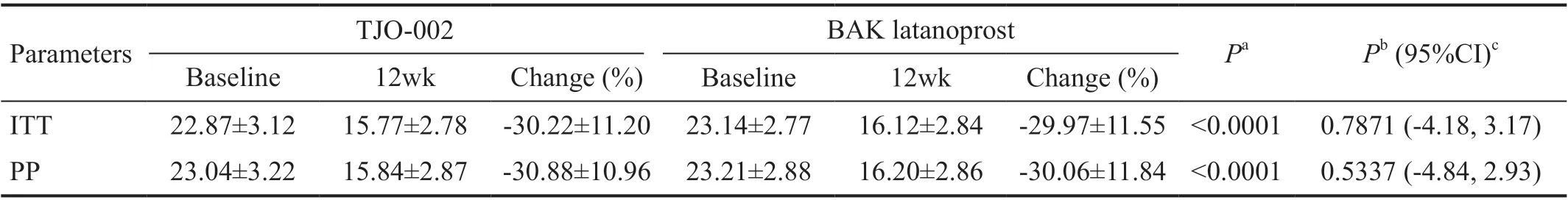
Table 2 Diurnal IOP by measurement time at baseline and 12wk of the treatment groups by ITT and PP mean±SD, mm Hg
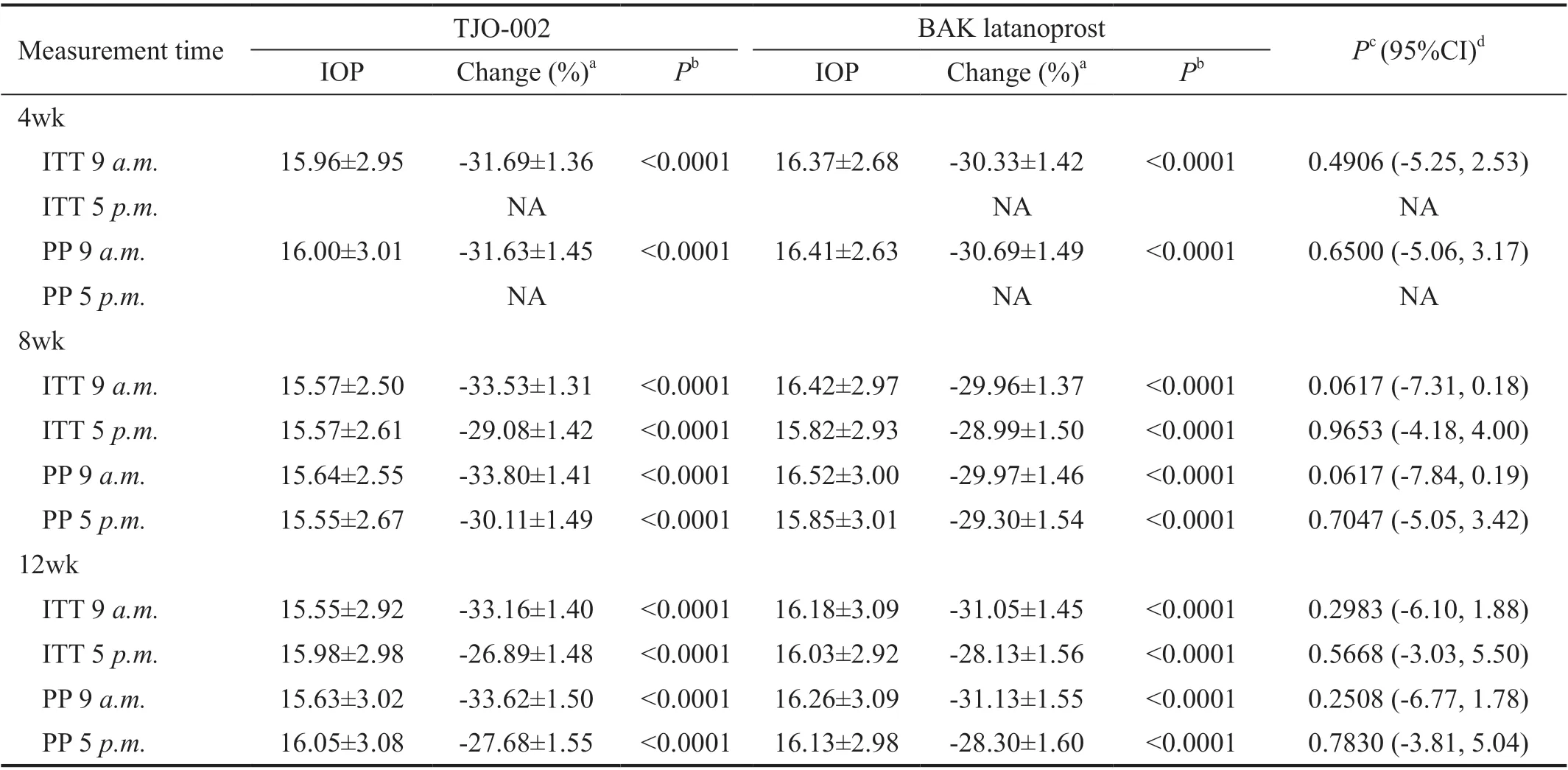
Table 3 IOP by measurement time at baseline, 4, 8 and 12wk of the treatment groups by ITT and PP mean±SD, mm Hg
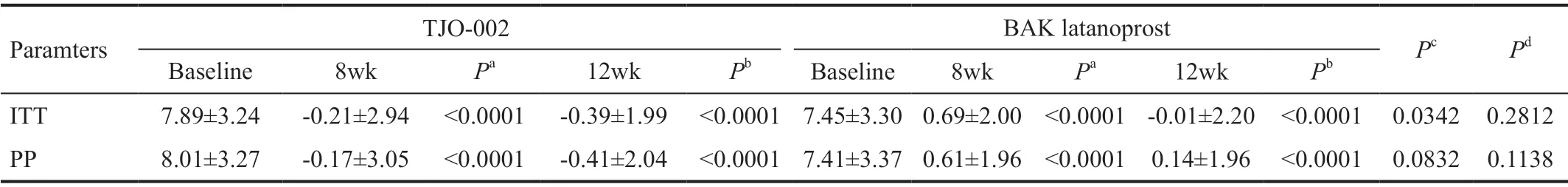
Table 4 Diurnal IOP fluctuation from 9 a.m. to 5 p.m. at baseline, 8 and 12wk of the treatment groups by ITT and PP mean±SD, mm Hg
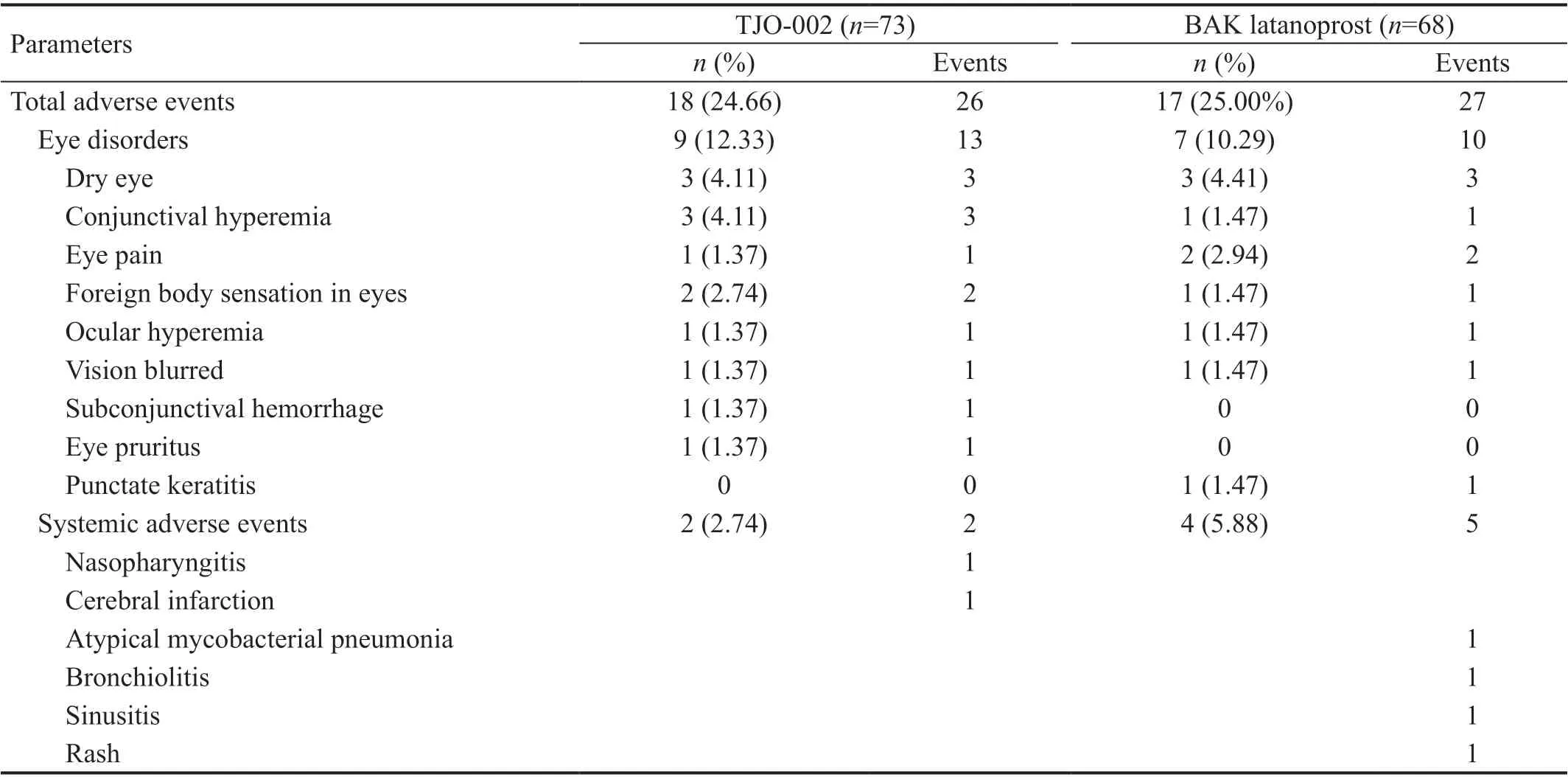
Table 5 Number of patients with ocular adverse events and drug-associated systemic adverse events
Safety Table 5 shows ocular and systemic AEs in both groups. The incidence of AEs regardless of relationship with the study medications was 24.66% (18/73 people, 26 cases)in the TJO-002 group and 25.00% (17/68, 27 cases) in the BAK latanoprost group; there was no statistically significant difference between the groups (P=0.9625). The incidence of“Eye disorders” in the BAK latanoprost group was 10.29%(7/68 people, 10 cases), and in the TJO-002 group, it was 12.33% (9/73, 13 cases). The difference in incidence between the groups was not statistically significant (P=0.7035). Drugassociated systemic adverse events other than ocular adverse events included nasopharyngitis (1) and cerebral infarction (1)in the TJO-002 group and atypical mycobacterial pneumonia(1), bronchiolitis (1), sinusitis (1), acute myeloid leukemia (1)and rash (1) in the BAK latanoprost group. However, those AE did not appear to be associated with the study medications.
Tolerability Severity of pruritus, burning/stinging, blurred vision, sticky eye sensation, eye dryness sensation, and foreign body sensation were compared between the two groups at 4-, 8-, and 12-week visits in the PP population among them,the severity of pruritus (12wk:P=0.0117), burning/stinging(4wk:P=0.0256, 8wk:P=0.0003, 12wk:P<0.0001), and sticky eye sensation (8wk:P=0.0010) were significantly different between the groups. TJO-002 showed a statistically significantly better tolerability than BAK latanoprost in three categories (Table 6).
DISCUSSION
In this randomized, investigator-masked multicenter trial in patients with POAG/OHT, the newly formulated PF latanoprost, TJO-002, showed similar efficacy and better tolerability compared with BAK latanoprost. In terms of efficacy, TJO-002 was non-inferior to BAK latanoprost in lowering IOP at all study follow-up assessment points (week 4, 8, and 12). In terms of tolerability, TJO-002 showed lower incidence of pruritus, burning/stinging, and sticky eye sensation than BAK latanoprost for the study duration.There was no difference in systemic side effects between the two groups. TJO-002 appeared to have better efficacy and tolerability compared with BAK latanoprost eyedrops.
Measured IOPs were significantly reduced at all follow-up periods from baseline in the groups, and neither the magnitudenor the distribution of the IOP reduction at any visits were statistically different between the two groups. When the IOP measured at 9a.m.was analyzed separately, as it approximates the time of maximal IOP reduction by both medications, it was decreased and maintained for the entire duration of the study.This means that TJO-002 was at least non-inferior to BAK latanoprost in terms of the ability to lower IOP. The reduction of mean IOP at 9a.m.of the last visit compared with baseline was -8.13 mm Hg (33.16%) for TJO-002 and -7.43 mm Hg(31.05%) for BAK latanoprost, which was consistent with the range of the optimal IOP reduction associated with latanoprost 0.005% (approximately 28%-31%) reported previously[22-24].Aspberget al[22]reported that latanoprost was associated with a 28% decrease from the baseline IOP. These results are in agreement with the result of a study in which the IOPlowering efficacy of latanoprost was not dependent on the presence of BAK. Pellinen and Lokkila[25]demonstrated comparable corneal penetration of preserved and PF tafluprost in the aqueous humor of rabbits. Aiharaet al[26]reported that fewer ocular surface complications without significant IOP changes were observed with BAK-free travoprost than with BAK latanoprost, with a reduced prevalence of superficial punctate keratitis and less hyperemia, in a long-term 12-month prospective study. Harasymowyczet al[23]reported that PF latanoprost showed the same efficacy, along with improved local tolerance, compared with BAK latanoprost.
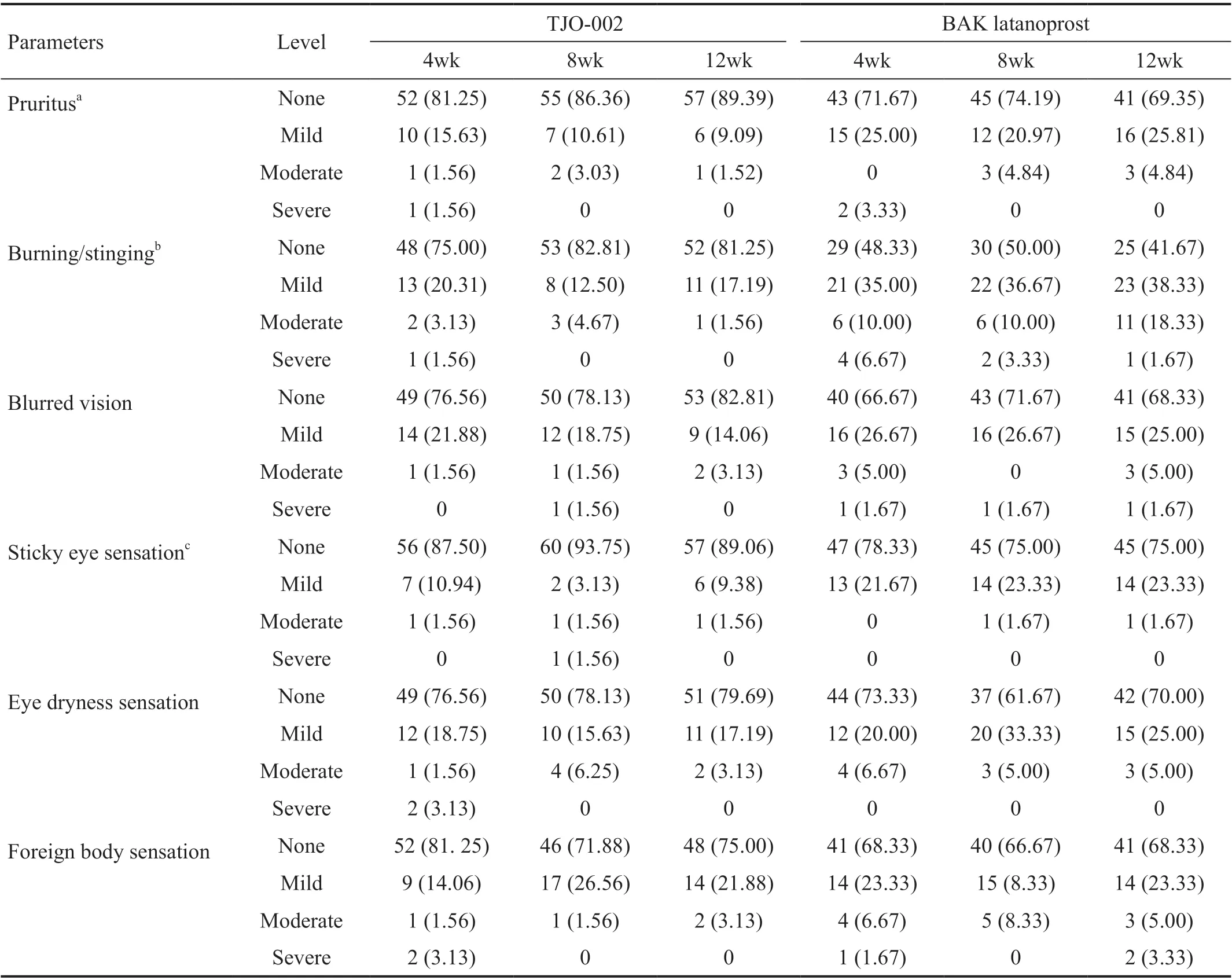
Table 6 Number of patients with symptoms categorized by severity level comparing the TJO-002 group and the BAK latanoprost group n (%)
Considering the importance of adherence and the fact that glaucoma requires long-term treatment, local ocular tolerability as well as efficacy is an important factor in preserving the quality of life in patients with glaucoma. In this study, TJO-002 showed better tolerability compared with BAK latanoprost, in terms of pruritus, burning/stinging, and sticky eye sensation.
There are several presumed reasons for the favorable tolerability of TJO-002. One may be the absence of BAK. While BAK is a commonly used preservative in ophthalmic eye drops,its ocular toxicity is well known. Some studies have shown ocular surface damage, including inflammatory and toxic effects, associated with BAK[14,27-28]. Martinez-de-la-Casaet al[29]reported that the preservative appeared to have an impact on tear cytokine levels. Latanoprost with BAK increased the levels of interleukin, basic fibroblast growth factor, plateletderived growth factor, and tumor necrosis factor-α in tear film. Baudouinet al[28]also suggested that BAK in topical eye drops induces tear film instability, conjunctival inflammation,subconjunctival fibrosis, epithelial apoptosis, and corneal surface impairment. Long-term use of BAK could lead to apoptosis of conjunctival cells and chronic conjunctival inflammation[30]. Furthermore, Desbenoitet al[31]reported that BAK was found in the iris, lens capsule, and trabecular meshwork tissue of rabbits after topical exposure, thus suggesting the penetration of BAK into deep ocular structures.Pisellaet al[32]demonstrated that removal of preservative from timolol ophthalmic solution was associated with improvement of corneal epithelial barrier function, prevention of ocular surface inflammation, and reduction of complaints. Yanget al[33]suggested that topical latanoprost treatment itself could induce dry eyeviainflammation. They reported the effects of latanoprost in mice: it decreased tear production,induced conjunctival goblet cell loss, disrupted the corneal epithelial barrier, and promoted cell apoptosis in the ocular surface. Therefore, latanoprost itself may cause ocular surface problems, and BAK can further aggravate that problem. The new BAK-free formulation of latanoprost in this study, TJO-002,appeared to minimize the discomfort by eliminating BAK toxicity.Another reason may be the ocular tissue-friendly composition of TJO-002, which includes carbomer and sorbitol as the excipient. Carbomer has been widely used for artificial tears[30].Carbomers are anionic polymers and strongly interact with anionic mucin[34]. This mucoadhesive interaction causes carbomer-based formulations to bind with the mucin layer to prolong adhesion[35]. Reports have demonstrated that the ocular retention time of carbomer gel was significantly longer than that of other low-viscosity eye drops[36-37]. In a previous study,when compared to sodium hyaluronate, carbomer showed equivalent therapeutic effects on symptom severity in moderate dry eye[37]. The properties of carbomer seem to play a role in reducing ocular AEs. Furthermore, due to the characteristics of the carbomer, latanoprost may stay on the surface of the eye longer, possibly resulting in a better IOP reduction. The IOP at 9a.m.after 8wk in the TJO-002 group was lower than that in the BAK latanoprost group. Sorbitol is used to enhance the stability of the topical composition in TJO-002.In a 4-week test of stability under severe conditions (55°C,relative humidity 75%), the main ingredient, latanoprost,was maintained without loss. This result showed that the latanoprost preparation containing sorbitol was kept more stable than the preparation without sorbitol (data not shown here). Sorbitol appeared to maintain the stability of TJO-002 at room temperature for 3y. In addition, the appropriate pH for activation and maintenance of TJO-002 is pH 7.0-7.3, at which TJO-002 is neutral, while that of BAK-preserved latanoprost used in this study (Xalatan?, Pfizer Inc., Belgium NV Puurs,Belgium) is pH 5.5. This may be one of the reasons why there is less tingling sensation with TJO-002 than with BAK latanoprost.Gonneringet al[38]showed that the optimal pH range to prevent corneal damage is 6.5 to 8.5, which includes the pH of lacrimal fluid (approximately pH 7.4). Although corneas perfused at pH 5.5 showed changes in endothelial morphology, those perfused at pH values of 7.0, 8.0, and 8.5 maintained normal endothelial morphology[38]. While conjunctival hyperemia was more common in TJO-002 (3vs1). Considering the absence of stimulation by BAK, it was expected to appear less, but the opposite result was obtained. The exact reason for this is not known. In our opinion, the carbomer contained in TJO-002 may be the result of prolonging the hyperemia effect of latanoprost by causing latanoprost to stay in the conjunctival sac for a long time. Severe foreign body sensation was shown in 2 cases of TJO-002 at 4wk after instillation and were lost over time, but in BAK latanoprost, severe case was shown at 12wk. Further study is needed.
Despite various efforts, this study has several limitations.First, there was no objective examination for ocular surface evaluation, such as tear film break-up testing and corneal/conjunctival staining evaluations. Second, the study was performed using data from one ethnic group; thus, results may not be applicable to other ethnic groups. Third, we did not evaluate all adverse effects of prostaglandin analogue(e.g.,lid pigmentation, deepening of upper eyelid sulcus, and growth of eyelashes) due to the relatively short follow-up duration. Fourth, other ingredients in addition to BAK may have been involved, but comparisons were not made. Since not all of the component of two drugs are the same except for BAK, all other ingredients in the drug may be involved. Fifth,our study conducted a relatively short follow-up duration,12wk. Considering that responses may vary from person to person, the duration of the study may not be appropriate.Further longterm study is needed. Sixth, we did not measure the 24-hour IOP variation, but only examined IOP twice in a day to estimate a certain daily change. However, despite the above limitations, we consider that we sufficiently evaluated and compared TJO-002, PF latanoprost, with conventional latanoprost containing preservative in terms of IOP reduction and ocular surface adverse effects. Finally, compared to previous studies, the subjects in our study are more male and have a relatively young average age. Other similar studies show that the average age is mostly over 60, with similar sex ratios or more female than male[39-42]. However, since our study is a multicenter study, and we have not tried to control the sex ratio of patients, it is not known why this structure was established. Considering the possible reasons, our study was performed in tertiary hospital and general hospitals. These hospitals in Korea are located in large cities, and residents of large cities and office workers around them participated in the study, so it seems that there were relatively more males and younger people than other studies. In addition, male have a higher prevalence of glaucoma than female in Korea[43]. It will be difficult to put our study on the same line with other existing studies and compare it, but it will be a good reference considering age and gender.
In conclusion, PF latanoprost generic, TJO-002, offers a useful alternative to the available prostaglandin analogues containing BAK for the treatment of POAG/OHT and is likely to be associated with fewer ocular surface problems, without any reduction in efficacy. On the basis of our result, PF-latanoprost could be considered as an alternative to conventional latanoprost, especially in patients suffering from pre-existing or concomitant ocular surface diseases. In the future, it is also of interest to study the comparison of the difference between efficacy and safety with and without preservatives in three different prostaglandin analogues in relation to the surface eye effect of PF.
ACKNOWLEDGEMENTS
Foundation:Supported by Taejoon Pharmaceutical.
Conflicts of Interest:Kim JM, None; Sung KR, None;Lee JW, None; Kyung H, None; Rho S, None; Kim CY,None.
 International Journal of Ophthalmology2021年10期
International Journal of Ophthalmology2021年10期
- International Journal of Ophthalmology的其它文章
- lmpact of intraocular pressure fluctuations on progression of normal tension glaucoma
- Effective treatment for secondary angle-closure glaucoma caused by traumatic lens subluxation:phacoemulsification with capsular-tension-ring implantation combined with ophthalmic endoscopecontrolled goniosynechialysis
- Progressive restrictive strabismus in an infant
- Association of peripheral anterior synechia, intraocular pressure, and glaucomatous optic neuropathy in primary angle-closure diseases
- Protective effect of LIF-huMSCs on the retina of diabetic model rats
- Therapeutic effect of a traditional Chinese medicine formulation on experimental choroidal neovascularization in mouse
