Mutation-based therapies for intrahepatic cholangiocarcinoma: new options on the horizon
Si-yuan Pan, Yu-Hang Ye, Zheng-Jun Zhou, Jia Fan1,, Jian Zhou1,, Shao-Lai Zhou1,
1Department of Liver Surgery and Transplantation, Zhongshan Hospital, Fudan University, Shanghai 200032, China.
2Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai 200032, China.
#Authors contributed equally.
Abstract Intrahepatic cholangiocarcinoma (ICC), a rare but rising global malignancy originating from the bile ducts, poses significant challenges in terms of effective treatment and patient outcomes.While surgical excision remains the curative option, its limited efficacy necessitates more therapeutic strategies, including systemic therapies.The management of ICC involves a multidisciplinary approach, with treatment decisions guided by patient-specific and tumor-specific factors.Gemcitabine-cisplatin (GEMCIS) chemotherapy has been a standard first-line therapy, but recent advancements in immunotherapy, particularly the introduction of durvalumab, have provided new hope.Additionally, gene mutation-based therapies, targeting fibroblast growth factor receptors (FGFRs), isocitrate dehydrogenase-1 (IDH1), human epidermal growth factor receptor-2 (HER2), and B-RAF proto-oncogene (BRAF),offer promising prospects for personalized treatment.High-throughput genomic profiling technologies have facilitated the identification of actionable targets and the development of innovative therapeutic approaches.This review summarizes the mutation-based therapies in ICC, including FDA-approved targeted drugs and ongoing clinical trials, highlighting the evolving landscape of ICC treatment.
Keywords: Intrahepatic cholangiocarcinoma, mutation-based therapy, precision medicine
INTRODUCTION
Cholangiocarcinoma, known as bile duct cancer, is a malignancy originating from the epithelial cells lining the bile ducts.It is a rare and aggressive disease that can develop in various locations along the biliary tract.
In clinical practice, magnetic resonance imaging (MRI) plain scans and dynamic contrast-enhanced scans are commonly employed for the diagnosis of hepatocellular carcinoma (HCC) and cholangiocarcinoma.Specifically, during the arterial phase, hepatocellular carcinoma typically exhibits uniform hypervascularity,accompanied by a significant reduction in enhancement during the portal vein phase, which is lower than that of the normal liver parenchyma[1].In contrast, ICC manifests as heterogeneous hypervascularity in the arterial phase, with persistent and non-uniform enhancement observed throughout the portal vein and delayed phases, surpassing the enhancement seen in the liver parenchyma[1,2].It is important to note,however, that at the imaging level, ICC cannot be reliably differentiated from HCC[1].Categorized into three types based on anatomical location (intrahepatic, perihilar, and distal), cholangiocarcinoma exhibits heterogeneity[3,4].Among them, intrahepatic cholangiocarcinoma (ICC) exhibits notable heterogeneity, with tumors resembling either conventional mucinous adenocarcinomas (large duct type) or transformed interlobular bile ducts (small duct type)[5].While the most common risk factors for ICC differ between Western regions, where primary sclerosing cholangitis predominates, and Eastern regions, where parasitic infections are prevalent, the incidence has been similarly increasing in recent years[4,6].Although radical surgical excision remains the only curative option for ICC, its efficacy is limited.The 5-year post-surgery survival rate for patients is approximately 30%, with a high recurrence rate of 60%-70% within five years post-surgery[7-9].What is worse, over 70% of patients with ICC are diagnosed with advanced-stage ICC,characterized by either local infiltration or distant metastasis[10,11].This subgroup of patients has a poor prognosis and does not meet the criteria for surgery, compelling a shift towards systematic therapy[11].
The management of ICC typically relies on patient-specific and tumor-specific factors, and treatment decisions often involve multimodal therapy ideally determined by a multidisciplinary team of experts.This team selects patients for surgical resection, perioperative chemotherapy, transplantation, systemic therapies,and so on.While gemcitabine-cisplatin (GEMCIS) chemotherapy has provided significant relief as a firstline treatment in systemic therapies[12], single GEMICS therapy showed limitations in the coming age of precision medicine.The introduction of immunotherapy has significantly transformed the treatment approach for cholangiocarcinoma over the years, providing renewed hope[10].One notable advancement is the introduction of durvalumab, a powerful antibody that inhibits the programmed cell death-ligand 1 (PDL1), into the realm of first-line treatments[13,14].This therapy has shown significant efficacy and offers new possibilities for patients with advanced ICC.Additionally, in contrast to HCC, the study of gene mutationbased therapies in ICC has progressed rapidly.Several drugs targeting specific gene mutations have received FDA approval as second-line treatment options[15-17].These options provide a fresh outlook for patients who cannot tolerate first-line chemotherapy or have developed resistance, especially for those with specific gene mutations.
The gold standard for confirming ICC typically involves conducting a pathology examination after a biopsy,and treatment is initiated upon confirmation of the diagnosis.However, given the absence of histologic markers indicating specific biological behavior, such as aggressiveness, the utilization of genetic testing to identify targeted drugs becomes crucial for achieving efficient personalized treatment[18].The introduction of next-generation sequencing (NGS) has revolutionized genomic profiling technologies, allowing for advanced analysis of genetic information[19], providing valuable insights into initiation, progression, and treatment resistance mechanisms in ICC.These technologies have enabled the identification of specific genetic alterations, unveiling new possibilities for the progression of innovative intervention methods.In ICC, several actionable targets were discovered, with fibroblast growth factor receptors (FGFRs) being of particular interest[10,20,21].Numerous tyrosine kinase inhibitors targeting FGFRs are currently undergoing assessment in prospective investigations.Moreover, other potential therapeutic targets, including isocitrate dehydrogenase-1 (IDH1)[22,23], human epidermal growth factor receptor-2 (HER2)[24,25], and the BRAF protooncogene (BRAF)[25], are also being investigated, showing promising outcomes [Figure 1].A summary of top mutation targets and corresponding drugs in ICC are listed in Table 1
In this review article, we elegantly delineated a series of targeted drugs that were either proved by the FDA or still in clinical trials.
Fibroblast growth factor receptor inhibitors
FGFR2 gene variations are prevalent in ICC, with an incidence ranging from 11% to 45%[17,20,26].Specifically,FGFR2 rearrangements and aberrations are detected in approximately 15% of ICC cases[27].In the transformative journey from normal cells to tumor cells in ICC, these genetic mutations play an irreplaceable role by influencing crucial pathways.This impact occurs especially when FGFR2 fusions and rearrangements affect key pathways, ultimately leading to the transition from normal cells to tumor cells[20].As a result, they have been prospectively assessed and recognized as the targets amenable to intervention[20].This highlights the potential therapeutic significance of targeting FGFR2 gene variations in ICC management.
Pemigatinib, an FDA-approved selective inhibitor of FGFR2, has demonstrated efficacy in the treatment of ICC with FGFR2 fusion or rearrangement with local infiltration or distant metastasis.The phase II trial,FIGHT-202, provided valuable insights into the effectiveness of pemigatinib in advanced-stage ICC patients[28].Among those with FGFR2 fusion or rearrangement, the treatment demonstrated a 37.0%objective response rate (ORR) and an 82.4% disease control rate (DCR).The median relapse-free survival(median RFS) and overall survival (OS) were 7.0 months and 17.5 months, respectively, while the median overall survival (median OS) of treated responders reached 30.1 months.Moreover, another study involving 30 subjects assessed the treatment's efficacy[29].Among them, 15 patients achieved a partial response (PR),resulting in an ORR of 50.0% (95%CI: 31.3-68.7).In addition, it is worth noting that among the 31 patients,a remarkable DCR of 100% (95%CI: 88.4-100) was achieved, with 15 patients demonstrating stable disease(SD).The median time to response was 1.4 months (95%CI: 1.3-1.4), although the duration of response(DOR) remains undetermined.In this study, the median progression-free survival (median PRF) was 6.3 months [95%CI: 4.9-not estimable (NE)].It is important to mention that out of the total patients, eight individuals (25.8%) experienced grade 3 or higher treatment-emergent adverse events, with the most commonly observed adverse events including hyperphosphatemia, hypophosphatasemia, and so on.These promising findings highlight the significant antitumor activity and positive safety characteristics of pemigatinib, supporting it as a treatment option for patients of ICC with FGFR2 rearrangements.The ongoing phase III randomized trial, FIGHT-302, is presently enlisting participants to investigate and determine the effectiveness of pemigatinib compared to the combination of gemcitabine and cisplatin chemotherapy.This trial focuses on individuals with unresectable or metastatic ICC harboring FGFR2 fusions or rearrangements, serving as a primary treatment option.The overarching goal is to scrutinize and establish the comparative advantages and results of these two distinct treatment modalities[30].
Infigratinib, another FDA-approved FGFR2 inhibitor, has shown promising results in the treatment of advanced ICC with FGFR2 genomic aberrations.A prospective cohort study evaluated infigratinib in 108 patients with advanced ICC, where they received infigratinib (BGJ398)[31,32].In the subset of individuals with FGFR2 gene fusions or rearrangements (n= 83) in which over half of the patient cohort has received treatments from two or more therapeutic regimens., the ORR, as assessed through centralized review, stands at 23%, accompanied by a median PFS of 7.3 months.Utilized as a second-line intervention, the ORRescalates to 34%, while in the context of third-line and subsequent therapeutic modalities, it diminishes to 13.8%, signifying heightened therapeutic effectiveness when instituting systemic treatment during the advanced stages of the disease.Frequently encountered adverse events during the course of treatment encompass hyperphosphatemia, oral inflammation, and ocular maladies.Given these favorable findings,infigratinib is slated for further examination in a randomized phase III clinical trial denoted as PROOF 301[33].The PROOF 301 trial aims to provide additional evidence and comparison of infigratinib with other treatment options, further elucidating its efficacy and safety profile.
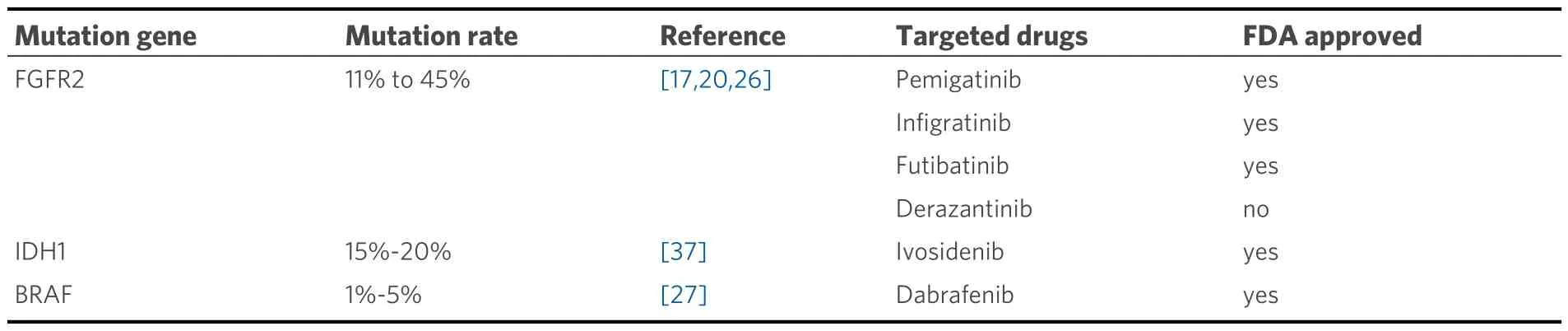
Table 1.Top actionable mutation targets and corresponding drugs in ICC
Figure 1.The mutated genes and potential targets in cholangiocarcinoma.
Futibatinib (TAS-120), a highly selective and irreversible FGFR1-4 inhibitor, has undergone evaluation in phase I and II studies specifically focused on ICC patients with FGFR2 fusions[34,35].Within the confines of the phase II FOENIX-CCA2 trial, a collective of 103 patients was recruited and subjected to a daily administration of 20 mg of futibatinib.Within this patient cohort, 22% exhibited rearrangements and 78%manifested FGFR2 fusions.The trial results demonstrated an ORR of 43% and a DCR of 85%, with a median DOR of 9.5 months.The median PFS was observed to be 8.9 months, while the median OS reached an impressive 20 months.Adverse effects associated with alternative FGFR inhibitors encompass a range of manifestations, such as hyperphosphatemia and alopecia.Notably, futibatinib has already secured approval from the FDA and will be further assessed in the PHASE III FOENIX-CCA3 TRIAL, specifically focusing on its use as a primary treatment modality for patients diagnosed with ICC and characterized by the presence of documented mutations[36].
Given the effectiveness of targeting FGFR2 fusions in ICC, three distinct randomized phase III trials are underway to compare FGFR inhibitors (pemigatinib, infigratinib, and futibatinib) with gemcitabine and cisplatin chemotherapy[30,33,36].
It provides a concise overview of the trials, medications, and effectiveness associated with the currently available FGFR inhibitors in Table 2.
Isocitrate dehydrogenase inhibitors
IDH1 mutations have been identified in a subset of ICC cases, estimated to be approximately 15%-20% of cases[37].These mutations result in the production of abnormal IDH enzymes that convert isocitrate to an abnormal metabolite, 2-hydroxyglutarate (2-HG), which accumulates in cancer cells and contributes to tumor growth and progression[38,39].
Ivosidenib, a small-molecule inhibitor targeting IDH1 mutant protein, has demonstrated favorable safety and long-term disease stability in patients with ICC[22,40,41].In 2021, it received approval from the US FDA as a second-line treatment for cholangiocarcinoma patients with local infiltration or distant metastasis who harbor IDH1 mutation.The results of the ClarIDHy trial revealed that the median RFS was 2.7 months in the ivosidenib group compared to 1.4 months in the placebo group[41].The ORR was 2.4%, with 50.8% of patients experiencing disease stabilization.Additionally, the median OS among patients treated with ivosidenib group was 10.3 months, while it was 7.5 months in the placebo group.In the study, ivosidenib showed a substantial enhancement in PFS compared to placebo, with a PFS of 2.7 months versus 1.4 months, respectively.The hazard ratio was 0.37, indicating a significant reduction in the risk of progression with theP-value, calculated one-sided, less than 0.0001.In a word, the findings revealed that ivosidenib was relatively well-tolerated, and targeting IDH1 mutations in advanced ICC offers notable clinical benefits.
Human epidermal growth factor receptor-2 inhibitors
In contrast to extrahepatic cholangiocarcinoma (ECC), ICC has relatively few mutations in HER2, only 5%-10%[42,43].Previous studies conducted on ICC patients using lapatinib and varlitinib against HER2 did not produce noteworthy outcomes[44,45].Nevertheless, it is crucial to emphasize that the lack of HER2 assessment in either drug may have contributed to this outcome.In particular, within pre-selected instances of advanced cholangiocarcinoma exhibiting HER2 genetic modifications, there have been indications of enhanced outcomes.
Neratinib underwent assessment in the SUMMIT trial, which involves diverse histological types[25].Among the nineteen participants with HER2 mutations who were administered the medication, the median OS was measured in individuals who had previously undergone at least one round of systematic therapy.The observed ORR stood at 10.5%, with a median PFS of 1.8 months.
Additionally, several monoclonal antibody agents targeting HER2 were examined in forward-looking study groups.The amalgamation of trastuzumab and pertuzumab was appraised in the phase II research initiative known as MyPathway[24].Among the 39 patients with HER2-positive biliary cancer (amplified or overexpressed HER2), nine achieved PR, with an ORR of 23%.
Zanidatamab, a dual-targeting antibody against HER2, was studied in a phase I trial involving 83 patients with tumors exhibiting HER2 amplification, including 22 patients with HER2-expressed biliary tract carcinoma (BTC)[46].The ORR reached 37% in this study, and there were no deaths attributed to the treatment.
Another experimental medication, trastuzumab deruxtecan (DS-8201), was assessed in the HERB trial[47].Among the 22 BTC patients who tested positive for HER2, the ORR was recorded as 36.4%.Furthermore,the DCR was 81.8%, and the median PFS was 5.1 months, with an OS of 7.1 months.
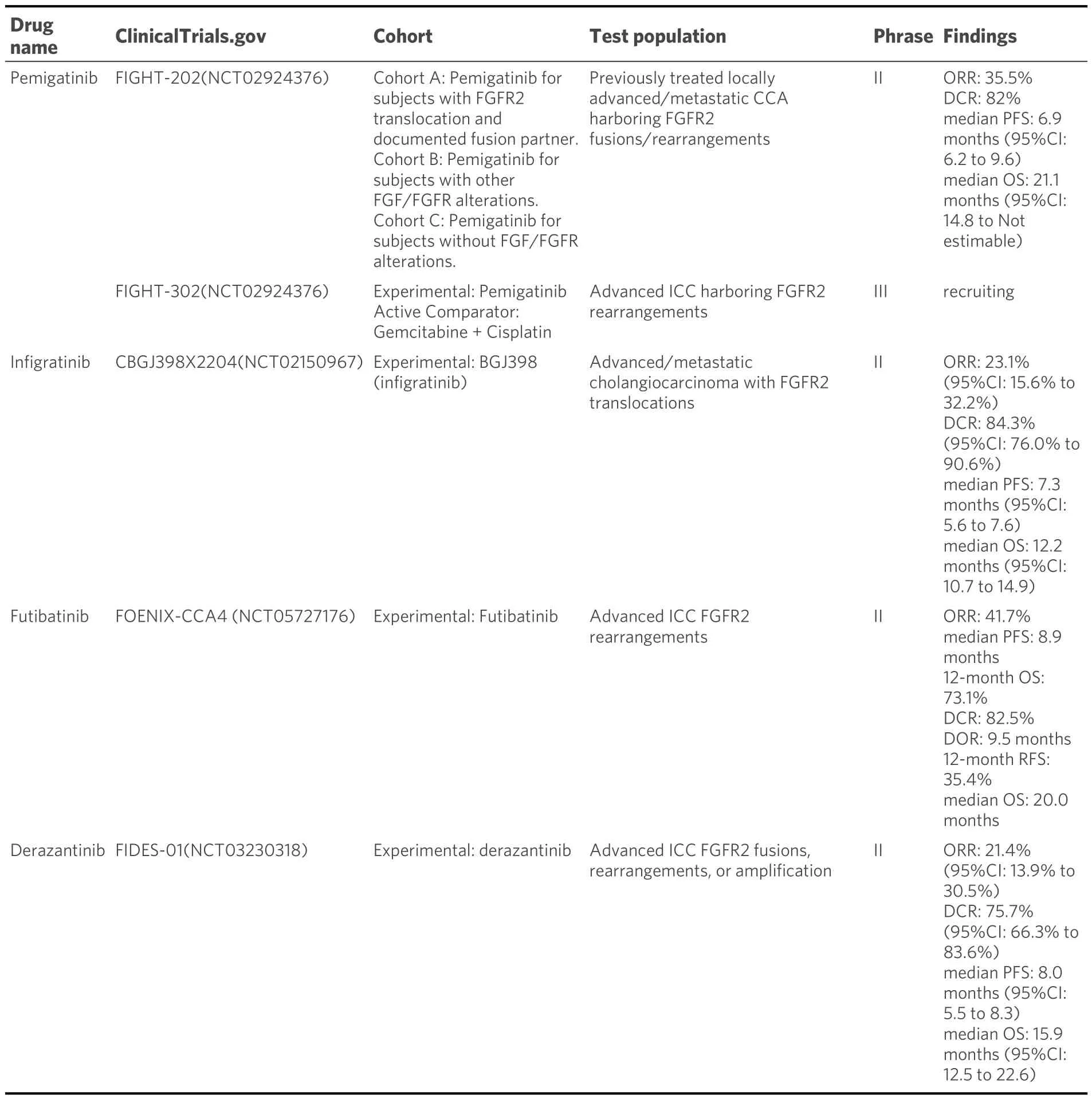
Table 2.FGFR inhibitor trials in patients with intrahepatic cholangiocarcinoma
The combination of conventional chemotherapy and trastuzumab has also exhibited effectiveness.In a phase II trial carried out in Korea, assessing the use of FOLFOX alongside trastuzumab in individuals diagnosed with HER2-positive conditions, the therapeutic regimen yielded a 29.4% ORR[48].
BRAF proto-oncogene inhibitors
BRAF, a proto-oncogene, exerts a pivotal role in the regulation of cell processes[49].While BRAF mutations are rare in advanced BTC, accounting for only 1%-5% of actionable findings, they are more prevalent in ICC[27].Specifically, the BRAF V600E mutation in ICC accounts for 27% of the identified BRAF variants,which leads to activation of the MAPK/ERK signaling pathway, causing uncontrolled cell proliferation and contributing to tumor progression[49].
The phrase II, single-arm study, ROAR, examined 43 previously treated individuals with advanced BTC carrying documented mutations[50].These individuals underwent combination inhibition of the MEK pathway and the BRAF pathway.The ORR was 47%, with a median PFS of 9 months and a median OS of 14 months.However, around 40% of patients experienced serious adverse events, including elevated γ-glutamyl transferase, fever, reduced white blood cell count, and increased blood pressure.
To evaluate the potential survival advantage of this treatment compared to chemotherapy as an earlier line of systemic therapy, larger studies would be required.
Other mutation-based therapy
One analyzed study included 54 adults with advanced or metastatic solid tumors positive for NTRK fusion from 10 different tumor types and 19 histology types[51,52].The ORR was notable, with 57% of patients demonstrating a response.Among the responders, 7% achieved complete response (CR) and 50% had PR.The median DOR was estimated to be 10 months, indicating a sustained and meaningful clinical benefit.However, it is essential to acknowledge some limitations of the studies analyzed.The data presented in this summary were based on a specific patient population and may not be generalizable to all patients with NTRK fusion-positive ICC.Further investigations are needed to evaluate the efficacy of these targeted therapies in larger cohorts and diverse patient populations.
Previous studies involving immune checkpoint blockade showed modest response rates of 10%-20% in refractory ICC, but these results did not result in regulatory approval for the treatment[53,54].However, the TOPAZ-1 trial has reignited enthusiasm in this area[14].This study compared GEMCIS plus durvalumab versus GEMCIS plus placebo.The trial results revealed that the addition of durvalumab resulted in improved outcomes compared to the placebo group.The addition of durvalumab to GEMCIS resulted in an OS of 12.8 months, which was longer than the OS of 11.5 months for GEMCIS alone (P= 0.021).The median PFS was 7.2 months in the durvalumab arm compared to 5.7 months in the GEMCIS arm (P=0.001).The ORR was 26.7% in the durvalumab arm and 18.7% in the GEMCIS arm.Although the absolute differences in these survival endpoints are relatively small, the tail of the survival curves suggests that a significant proportion of patients (up to 25%) were alive at 2 years and experienced durable responses,which is unprecedented with traditional cytotoxic therapy.
KRAS has recently gained significant attention for its therapeutic development in multiple kinds of tumors[55-57].In a cohort study conducted by our team, which involves a collective of 1024 individuals diagnosed with ICC, 14 different subcategories of KRAS mutations were identified, impacting 127 individuals (12.4%)[58].Among the identified KRAS variants, the most common was G12D, accounting for 55 cases (43.3%).This was subsequently G12V with 25 cases (19.7%), G12C with 9 cases (7.1%), and G13D with 8 cases (6.3%).Being one of the most prevalent driver mutations in cancer, KRAS holds significant importance in the development and advancement of tumors[59,60].In the past, KRAS has long been considered an "undruggable" target due to its essential role in normal cell functioning and the toxicity associated with inhibiting its activity[59].Nevertheless, recent advancements have rekindled hope, as the FDA approval of sotorasib for KRAS G12C mutations in non-small cell lung cancer (NSCLC) signifies a breakthrough in KRAS-targeted therapy[61,62].These achievements have sparked renewed interest in exploring KRAS as a therapeutic target in ICC and offer potential avenues for novel treatment strategies.
A comprehensive overview of the trials, medications, and effectiveness of other inhibitors is provided in Table 3.
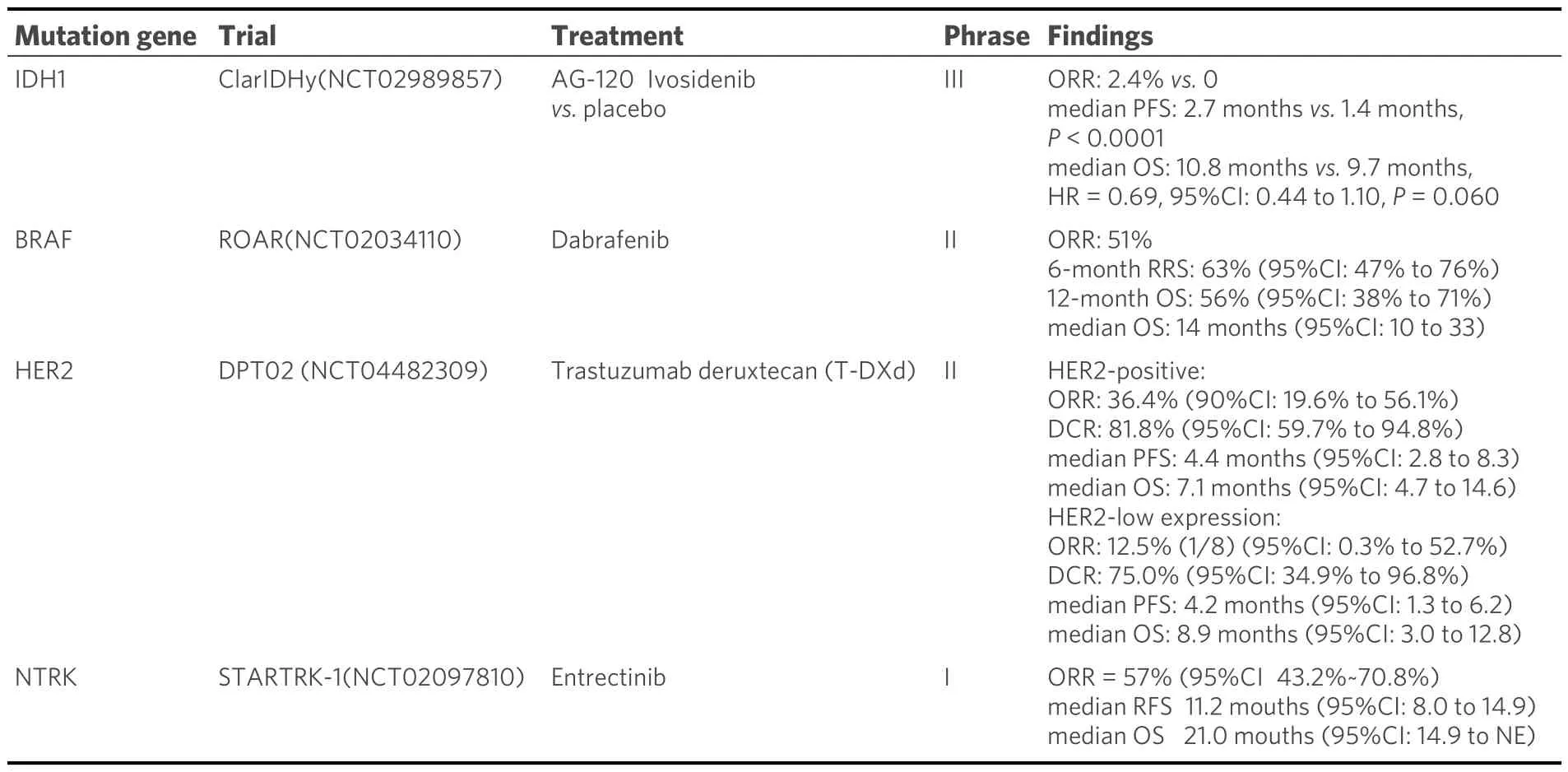
Table 3.Research on other actionable targets harboring in ICC
DISCUSSION
Writing this review, we addressed certain key targets, target-related medications, and drug-related clinical studies in ICC, and provided an outlook in the field along with a critical analysis of its limitations.In the contemporary era, there have been significant changes in the therapy landscape for advanced-stage ICC with the emergence of mutation-based therapy.Drugs such as pemigatinib and futibatinib have shown enhanced OS in comparison to conventional chemotherapeutic treatments in ICC patients who have received prior treatment, with a median OS of approximately 20 months[30].Additionally, there is a promising outlook for the combined utilization of mutation-targeted therapy and immunotherapy[39].Immunotherapy, particularly immune checkpoint inhibitors, has shown remarkable success in ICC by exploiting the immune system's ability to recognize and attack cancer cells[63].Despite this, further investigation and clinical trials are needed to explore the potential benefits and safety considerations of this combined strategy, which might offer new hope for patients.
Mutation-based therapy in ICC holds promising treatment prospects, but it also presents numerous challenges and limitations.In the first place, clinical trials in the field of mutation-based therapy may encounter various challenges.The rarity of these alterations poses difficulty in identifying eligible patients for recruitment[9,42,43].Moreover, there is competition among trials for recruiting patients with FGFR2 alterations, further complicating the enrollment process.Additionally, the outdated practice of using chemotherapy alone as a comparative arm in trials may limit the ability to accurately assess the efficacy of FGFR inhibitors.Furthermore, detecting genomic alterations, especially in cases where tissue samples are limited, can be challenging.
To address these challenges, it is essential to implement strategies that enhance patient recruitment and improve the accessibility of sequencing methods.This can be achieved by integrating increased dynamism and decentralization into recruitment and examination.One promising approach is the utilization of circulating cell-free tumor DNA (ctDNA) analysis[64,65].This method offers an alternative means of identifying and detecting genetic modifications, especially in cases where tissue samples are scarce.Ongoing studies are actively investigating the use of ctDNA as a diagnostic tool, although certain platforms still have limitations in detecting specific fusion events[65].
On another front, the emergence of drug resistance poses a significant challenge in the mutation-based therapy of ICC[66-68].For FGFR2 inhibitors, several mechanisms of drug resistance have been recognized,with gatekeeper mutations as a prominent example[67].These mutations sustain FGFR pathway signaling and confer resistance to currently available inhibitors.On the other hand, studies primarily focused on acute myeloid leukemia (AML) have revealed receptor tyrosine kinase (RTK) pathway genes as a cause of resistance against IDH1 inhibitors[69].However, the mechanism of resistance in IDH mutations in ICC requires further investigation.
By gaining a comprehensive understanding of these mechanisms, novel strategies can be developed to overcome resistance and improve patient outcomes.This may involve the development of new drugs,combination therapies, or alternative treatment approaches tailored to the specific resistance mechanisms identified.The ultimate goal is to maximize the benefits of targeted therapies and optimize their long-term effectiveness in the management of ICC.
The integration of immunotherapy with chemotherapy has emerged as a recent focal point in ICC.In a phase II trial, the investigational combination of nivolumab, gemcitabine, and cisplatin exhibited compelling effectiveness and well-tolerated safety in 32 patients[70].The cohort included individuals resistant to gemcitabine or cisplatin and chemotherapy-naive patients.Encouragingly, the regimen achieved a noteworthy 55.6% ORR, with 18.6% CR, showcasing notable efficacy even in cases resistant to conventional chemotherapy.The DCR reached an impressive 92.6%.For cohort A, where patients were resistant to standard chemotherapy, one achieved a CR, and one achieved a PR.In the chemotherapy-naive cohort B,61.9% attained an OR.The median PFS for all patients (cohorts A+B) was a robust 6.1 months, and the median (OS) stood at 8.5 months, accompanied by a notable 33.3% 12-month OS rate.Noteworthy adverse events included thrombocytopenia (56%) and neutropenia (22%).These comprehensive findings underscore the promising potential of nivolumab combined with GEMCIS as an effective and tolerable therapeutic approach for patients with advanced BTC, especially in cases resistant to traditional chemotherapy.Despite promising outcomes in the 32-patient cohort, the relatively modest sample size and a median follow-up of 12.8 months may pose challenges in extrapolating findings to a broader patient spectrum and understanding potential long-term implications.While these findings are encouraging, careful consideration of these limitations underscores the need for future well-controlled studies to substantiate and refine these preliminary observations.
Presently, the realm of new adjuvant and adjunct therapies for ICC lacks involvement in clinical studies with mutation-based targeted treatment[71,72].This absence can be attributed to two primary factors.Firstly,there is an absence of small-molecule targeted drugs that have transitioned into the forefront of treating unresectable ICC.Unlike chemotherapy approaches like GEMCIS, targeted therapies typically come into play after the establishment of a molecular diagnosis.While clinical trials have investigated the responsiveness of unidentified ICC subtypes to HER2-targeted drugs, the limited patient enrollment and scarcity of associated research impede the widespread acceptance of these treatments as primary therapies.Secondly, the significance of postoperative adjuvant therapy in preventing recurrence introduces the consideration that genetic mutations recurring from primary ICC may have undergone substantial changes.In essence, gene mutation types initially identified through surgical specimens may no longer manifest in recurrent tumors.Instead, these tumors might rely on different gene mutations for progression.This assumption implies that targeted therapy based on prior genetic testing results would lose its specificity.Despite explorations into the molecular subtypes of ICC, a universally accepted viewpoint remains elusive,as acknowledged by the majority of scholars[73-75].Anticipation surrounds the development of a more refined molecular classification of ICC and the progress of non-invasive methods for detecting genetic mutation profiles.Looking ahead, as the molecular classification of ICC matures and non-invasive methods for detecting genetic mutation profiles advance, targeted therapies for ICC can ascend to a new echelon,potentially paving the way for frontline adjuvant and adjunct therapies.
In summary, we summarized the progress and deficiencies in mutation-based therapies for ICC in this review.Mutation-based therapies have offered innovative treatment options for particular groups of patients and acted as potential members in combination therapy, but they still face certain obstacles.In the age of individualized and precision medicine, further research and clinical practice are needed to advance the field of mutation-based therapies.
DECLARATIONS
Authors’ contributions
Manuscript conception and design: Zhou SL, Zhou J, Fan J, Zhou ZJ Manuscript draft and revision: Pan SY, Ye YH
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study was jointly supported by the National Natural Science Foundation of China (No.82173260, No.81972708, No.82072681, No.82003082, No.81773069, No.81830102, No.81772578); the National Key R&D Program of China (No.2019YFC1315800, 2019YFC1315802, 2018YFA0109400); Shanghai Technical Standard Program (21DZ2201100).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
? The Author(s) 2024.
- Hepatoma Research的其它文章
- Introduction of the Chinese expert consensus on postoperative adjuvant therapy for hepatocellular carcinoma (2023 Edition)
- Precise staging of advanced HCC promotes higher quality of personalized treatment management:Chinese experts consensus on precision diagnosis and management of advanced hepatocellular carcinoma (2023)
- Crosstalk between cancer cell plasticity and immune microenvironment in cholangiocarcinoma
- Interpretation of Chinese expert consensus on the whole-course management of hepatocellular carcinoma (2023 edition)
- Racial, ethnic, and socioeconomic differences in hepatocellular carcinoma across the United States
- Associations between physical activity and risk of liver cancer: results from a population-based cohort study in Chinese women