miR-24-3p promotes proliferation and inhibits apoptosis of porcine granulosa cells by targeting P27
Shengjie Shi ,Lutong Zhang ,Liguang Wang ,Huan Yuan,Haowei Sun,Mielie Madaniyati,Chuanjiang Cai,Weijun Pang,Lei Gao#,Guiyan Chu#
1 College of Animal Science and Technology,Northwest A&F University,Yangling 712100,China
2 Key Laboratory of Animal Genetics,Breeding and Reproduction of Shaanxi Province,Yangling 712100,China
Abstract Ovarian follicle development is associated with the physiological functions of granulosa cells (GCs),including proliferation and apoptosis.The level of miR-24-3p in ovarian tissue of high-yielding Yorkshire×Landrace sows was significantly higher than that of low-yielding sows.However,the functions of miR-24-3p on GCs are unclear.In this study,using flow cytometry,5-ethynyl-2′-de-oxyuridine (EdU) staining,and cell count,we showed that miR-24-3p promoted the proliferation of GCs increasing the proportion of cells in the S phase and upregulating the expression of cell cycle genes,moreover,miR-24-3p inhibited GC apoptosis.Mechanistically,on-line prediction,bioinformatics analysis,a luciferase reporter assay,RT-qPCR,and Western blot results showed that the target gene of miR-24-3p in proliferation and apoptosis is cyclin-dependent kinase inhibitor 1B (P27/CDKN1B).Furthermore,the effect of miR-24-3p on GC proliferation and apoptosis was attenuated by P27 overexpression.These findings suggest that miR-24-3p regulates the physiological functions of GCs.
Keywords: miR-24-3p,granulosa cells,proliferation,apoptosis
1.lntroduction
Granulosa cells (GCs) are the largest cell population in ovarian follicles,and their physiological functions are critical for follicle development (Kossowska-Tomaszczuket al.2009).GC proliferation is essential for the continuous development of primordial,primary,secondary,and antral follicles.In most cases,cell-cycle proteins (Cyclins) and cell-cycle dependent protein kinase (CDKs) control cell proliferation (Biet al.2021).In mammalian ovaries,only a few follicles grow to the preovulatory stage,while most undergo atresia during development (Matsudaet al.2012;Tiwariet al.2015).Follicular atresia is characterized by decreased numbers of follicles caused by GC apoptosis.The B-cell lymphoma-2 (BCL-2) family performs vital roles in the processes of GC apoptosis (Qiuet al.2014).Abnormal follicular atresia is associated with polycystic ovary syndrome,primary ovarian insufficiency,and other diseases (Matsuda-Minehataet al.2006;Sunet al.2019).MicroRNAs (miRNAs) might participate in the regulatory mechanisms of GCs.
miRNAs are small non-coding RNA molecules about 22 nucleotides in length,they participate in many critical physiological processes.MiRNAs target mRNA by binding to specific sequences in the 3′-untranslated region transcribed from target genes to degrade them or inhibit translation,thereby suppressing gene expression at the post-transcriptional or translational level (Huang 2017;Vishnoi and Rani 2017).Several lines of evidence suggest that miRNA regulates proliferation and apoptosis in GCs and their targets (Salilew-Wondimet al.2020;Zhuet al.2022).For example,miR-130b promotes oocyte maturation by targeting SMAD Family Member 5 (SMAD5) and ribosomal protein S6 kinase (MSK1) to regulate the survival and proliferation of bovine GCs (Sinhaet al.2017).miR-27a-3p targeting Vangl1 and Vangl2 inhibits proliferation in GCs (Taoet al.2023).In terms of GC apoptosis,miR-93-5p promotes GC apoptosis and ferroptosis by the NF-κB signaling pathway in polycystic ovary syndrome (Tanet al.2022).miR-484 contributes to diminished ovarian reserve by regulating GC functionviaYAP1-mediated mitochondrial function and apoptosis (Liet al.2022).Nevertheless,the regulatory mechanisms of miRNAs on porcine GC proliferation and apoptosis need further exploration.
miR-24-3p is a highly conserved non-coding RNA in animals.Its mature sequence contains 22 nucleotides and exhibits specific expression profiles at various developmental stages in tissues and cells of different species (Lianet al.2012;Dinget al.2020).Studies targeting miR-24-3p focused on human cancer (Zhanget al.2021),cardiac fibrosis (Zhanget al.2022),myogenesis (Huet al.2019) and other processes.Solexa sequencing technology compared ovarian miRNAs of Yorkshire pigs with large and small litter sizes and found that the level of miR-24-3p in the ovaries of highyielding sows (>16.4 piglets/litter) was significantly higher than that in low-yielding sows (<7.4 piglets/litter) (Huanget al.2016).In addition,we have verified through experiments that the miR-24-3p expression level was higher in the ovarian tissues of high-yielding sows of Yorkshire×Landrace compared with low-yielding sows.More importantly,the level of miR-24-3p in the follicular fluid of women with polycystic ovary syndrome was lower than that of ordinary women,suggesting that miR-24-3p is critical for follicle development (Sorensenet al.2016).The proliferation and apoptosis of GCs affect follicle development.It has been reported that the expression of miR-24-3p in GCs isolated from dominant follicles of cows on day 7 of estrus was higher than that in subordinate follicles (Salilew-Wondimet al.2014).Moreover,we predicted the target genes of miR-24-3p.And we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analyses on the target genes,which indicated that miR-24-3p target genes may be related to the cell proliferation and apoptosis and may also be involved in signaling pathways,such as Wnt and mTOR,that have apparent effects on follicular growth processes.
Based on this analysis,we hypothesized that miR-24-3p might regulate the physiological function of GCs.We clarified the role of miR-24-3p in the proliferation,and apoptosis of ovarian GCs and its regulatory mechanism,suggesting that miR-24-3p is a critical regulatory factor in GC physiology.
2.Materials and methods
2.1.ldentification and collection of ovarian tissue from high-and low-yielding sows
We collected and collated litter size records (a total of 8,657 parity) from Hanshiwei Food Ltd.,Co.(Dahua,Guangxi,China) from 2016 to 2018 and used SPSS25.0 to perform normal distribution processing on the data.After normal transformation and testing,the total litter size (12.9±2.17) was found to be approximately normally distributed,with a critical value of 15% right tail probability (14.7 head/litter) and a critical value of 15% left tail probability of (9.3 head/litter).Therefore,we defined the lower yield groups below 9.3 head/litter as smaller litter sizes and the higher yield groups above 14.7 head/litter as larger litter sizes (Shiet al.2020).The left ovarian tissues of three sows in each group were selected to detect the expression of miR-24-3p.
2.2.Porcine granulosa cell culture and treatment
Fresh Landrace ovaries were isolated from local slaughterhouses,placed in 0.9% saline at 37°C supplemented with 100 IU mL-1penicillin and 100 mg mL-1streptomycin,and returned to the laboratory within 2 h.Fluid was extracted from follicles with diameters of 3-6 mm using needles,centrifuged at 1,000 r min-1for 10 min at room temperature,and then the supernatants were discarded.The pellets were resuspended in DMEM/F12 (Cytiva,Shanghai,China) containing 10% fetal bovine serum (Gibco,ThermoFisher Scientific,Shanghai,China) and seeded in cell culture plates at 37°C in humidified 5% CO2(Huet al.2022).After 24 h of culture,the cells were gently washed with phosphate-buffered saline (PBS) to remove other tissue and cell fragments.In each experiment,ovaries obtained from ten sows were used,and 20 ovaries were collected to obtain pooled GCs.These GCs were equally divided into control and experimental groups with an inoculation density of 105cells cm-2.
2.3.Transfection of miR-24-3p mimics and inhibitor
GCs were seeded in 12-or 6-well plates and transfected with miR-24-3p mimics or negative control (NC) and miR-24-3p inhibitor or NC (GenePharma,Shanghai,China) at 100 nmol L-1using X-tremeGENE HP DNA transfection reagent (Rochelll,Mannheim,Germany).The culture medium was changed to a fresh medium after 12 h.
2.4.RNA isolation and quantitative real-time PCR
Total RNAs from GCs were extracted using TRIzol reagent (TaKaRa,Otsu,Japan),the OD260,OD280and concentrations were measured using a NanoDrop 2000 spectrophotometer (ThermoFisher Scientific,Waltham,MA,USA),and OD260/OD280ratio was between 1.8 and 2.0.Total RNA (500 ng) was reverse transcribed to cDNA using a Reverse Transcription Kit (TaKaRa,Otsu,Japan).qPCR was performed in Bio-Rad PCR instrument (Foster City,CA,USA) using 500 ng RNA,0.5 μL gDNA Eraser,1.0 μL 5× gDNA Eraser Buffer,the rest with RNase-free dH2O,42°C,2 min.The PCR product of the first step was added 2 μL 5× PrimeScript Buffer 2,2 μL RNase-free dH2O,0.5 μL PrimeScript RT Enzyme Mix I,and 0.5 μL RT Primer Mix at 37°C for 15 min and 85°C for 5 s,then diluted appropriately and stored at -20°C.RT-qPCR was performed on a StepOne real-time PCR machine (Applied Biosystems,Carlsbad,CA,USA) using SYBR Premix Ex TaqTM(2×) 5 μL,upstream primer (10 μmol L-1) 0.3 μL,downstream primer (10 μmol L-1) 0.3 μL,cDNA template 1 μL,ddH2O 3.4 μL.A two-step PCR reaction procedure was used: predenaturation at 95°C for 30 s,deformation at 95°C for 5 s,and annealing at 60°C for 30 s,a total of 45 cycles.The specificity of each PCR amplification was verified by melting curve analysis,and the data were normalized to the level of theβ-actingene expressed as a control.Primer sequences used for RT-qPCR are displayed in Table 1.
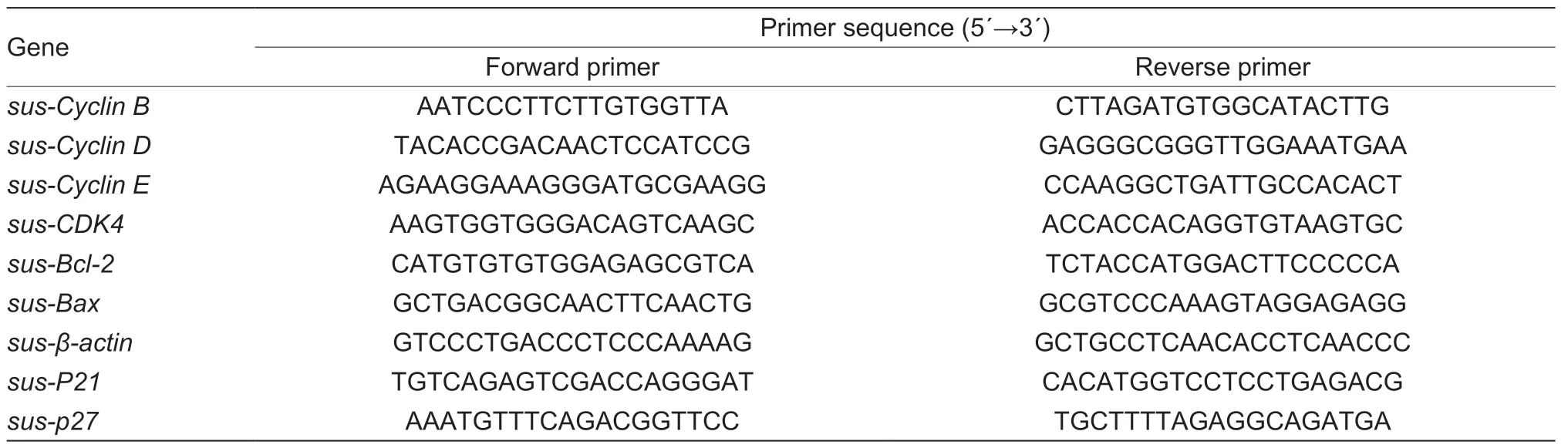
Table 1 Primers used in real-time quantitative PCR (porcine)
2.5.Western blot
After washing the cells with PBS,the total protein was extracted from GCs using RIPA buffer (Beyotime,Shanghai,China) and protease inhibitor mix (Cwbio,China).All extraction processes were according to standard protocols.The protein concentration was measured using a BCA Assay Kit (Cwbio,China).A total of 20 μg of protein were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gels,followed by transfer to polyvinylidene fluoride membranes (Cell Signaling Technology,Boston).The membranes were blocked with 5% bovine serum albumin at 4°C for 2 h,incubated with antibodies (1:1,000) against Cyclin B (CY5378,60 kDa),Cyclin D (CY5404,34 kDa),Cyclin E (CY1028,49 kDa),CDK4 (CY5836,34 kDa),P27 (CY5354,27 kDa,Abways,Shanghai,China),BCL-2 (sc-7382,26 kDa,Santa Cruz,Dallas,TX,USA),BAX (sc-20067,23 kDa,Santa Cruz,Dallas,TX,USA).Secondary antibodies (1:3,000) were horseradish peroxidase-labeled goat anti-rabbit IgG antibodies and goat anti-mouse IgGs (BOSTER,Wuhan,China).Bands were visualized using a gel imaging system (Bio-Rad,Foster City,CA,USA) and analyzed using Image J software (http://imagej.nih.gov/ij/).
2.6.Flow cytometry
Porcine GCs were cultured in six-well culture plates at the appropriate density.The cells were treated with miR-24-3p mimics and miR-24-3p inhibitors at 50% confluence.Cell samples were collected at 24 h after treatment and fixed in cold 70% ethanol overnight at 4°C.The methods for detecting the cell cycle were performed as described (Shiet al.2022).Cell samples were collected after 48-h treatment to measure apoptosis.The cell samples weredigested with trypsin,centrifuged,resuspended in a medium,and sent to Zimu Biotechnology Company (Zimu,Shaanxi,China) for apoptosis assays.
2.7.Target prediction and luciferase activity assay
The target genes of miR-24-3p were predicted using miRDB,miRWalk,miRBase,and TargetScan 6.2.Luciferase reporter plasmids (psi-CHECK2) containing the wild-type 3′UTRs ofP27(WT-P27) and mutant 3′UTRs ofP27(Mut-P27) were obtained from General Biosystems (Anhui,China).HEK293T cells were seeded in 48-well plates and cotransfected with miR-24-3p mimics or the NC with psiCHECK-2-P27-reporter or mutant vector.The cells were harvested 24 h after transfection.Luciferase activities were measured on a Dual-Glo Luciferase Assay System (Promega,Madison,WI,USA) following the manufacturer’s instructions.
2.8.EdU staining and cell counting kit-8 (CCK-8)
GC proliferation was measured using 5-ethynyl-20-deoxyuridine (EdU) staining.GCs were seeded in 100 mL in 96-well plates (2×103cells/well) with three repetitions and harvested after treating the cells with miR-24-3p mimics or inhibitors for 24 h.Cells were stained with 50 mmol L-1EdU (RiboBio,Guangzhou,China) for 2 h at a final concentration of 50 mmol L-1and with Hoechst dye at 25°C for 15 min after washing three times with PBS.The cells were observed using a Nikon TE2000 Microscope (Nikon,Tokyo,Japan),and the data were analyzed using Image J software (http://imagej.nih.gov/ij/).
GCs were seeded in 96-well plates at 2×103cells per well.After treating the cells with miR-24-3p mimics and inhibitor for 24 h,10 μL CCK8 reagent was added to each well and incubated for 3 h at 37°C.Finally,the absorbance was measured at 450 nm.
2.9.Bioinformatics analysis
MiRNA target genes were predicted using online software,including miRBase (https://www.mirbase.org/),RNAhybrid (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid/),miRDB (https://mirdb.org/),miRWalk (http://mirwalk.umm.uni-heidelberg.de/),and TargetScan 6.2 (https://www.targetscan.org/).We also used KOBAS 3.0 (http://kobas.cbi.pku.edu.cn/index.php) to complete GO and KEGG.
2.10.Statistical analysis
All graphs were created using GraphPad Prism 8.0,and the data represent the mean±SEM.The significance of differences between the groups was assessed using the Student’st-test or one-way ANOVA (*,P<0.05;**,P<0.01).
3.Results
3.1.Analysis of miR-24-3p expression characteristics
To investigate the expression characteristics of miR-24-3p,we detected the expression level of miR-24-3p in the ovarian tissues of both high-and low-litter Yorkshire×Landrace sows.In the high-yielding group,the ovaries were collected from three Yorkshire×Landrace sows with average litter sizes of 16.75±1.11,16.25±1.75 and 16.00±1.22,respectively.In the low-yielding group,the ovaries were sampled from three sows with average litter sizes of 7.75±1.80,8.50±1.66,and 6.50±2.40,respectively.The miR-24-3p expression level was higher in the ovarian tissues of high-yielding sows compared with low-yielding sows (Fig.1-A).The mature sequence of miR-24-3p is highly conserved across multiple species (e.g.,mouse,pig,human,rat) (Fig.1-B).Additionally,KEGG and GO analyses of the target genes of miR-24-3p revealed that miR-24-3p might also be involved in MAPK,Wnt and mTOR signaling pathways that influence follicular growth (Fig.1-C),and it may also be related to cell proliferation and apoptosis (Fig.1-D).These data suggest that miR-24-3p may be involved in the regulation of GC physiological functions.

Fig.1 Biological characteristics of miR-24-3p.A,expression levels of miR-24-3p in ovarian tissues of high-litter (H;16.75±1.11,16.25±1.75 and 16.00±1.22) and low-litter sows (L;7.75±1.80,8.50±1.66,and 6.50±2.40).B,sequence of the mature miR-24-3p is highly conserved across species.C,KEGG pathway analysis of miR-24-3p.D,GO analysis of miR-24-3p target genes.Results are mean±SEM of three independent experiments.*,P<0.05,**,P<0.01.
3.2.miR-24-3p overexpression promotes GC proliferation
The proliferation of GCs is crucial for follicular development (Li Xet al.2021).Significantly,miRNA is involved in this process.To explore the regulatory effects of miR-24-3p on GC proliferation,miR-24-3p mimics and inhibitors were evaluated.The expression level of miR-24-3p in GCs was significantly increased or decreased after transfection with miR-24-3p mimics and inhibitors (Figs.2-A and 3-A).Flow cytometry results showed that overexpression of miR-24-3p significantly increased the proportion of GCs in the G2 phase,and the proportion of G1 cells tended to decrease (Fig.2-B and C),while inhibition of miR-24-3p significantly reduced the proportion of cells in S phase (Fig.3-B and C).Moreover,treatment with miR-24-3p mimics increased the number of EdU-positive cells (Fig.2-D-E) and cell viability (Fig.2-F).Treatment with miR-24-3p inhibitor produced opposite results (Fig.3-DF).The mRNA (Figs.2-G and 3-G) and protein levels (Figs.2 and 3-H and I) of cell cycle-related genes (Cyclins B,D,and E) were also changed significantly.

Fig.2 Overexpression of miR-24-3p promotes porcine granulosa cell (GC) proliferation.A,transfection efficiency of overexpressed miR-24-3p compared with control group.B,cell cycle analysis of GCs detected by flow cytometry after transfection with miR-24-3p mimics and NC for 24 h.C,statistical analysis of cell cycle.D,the number of positive cells detected by EdU 24 h after transfection,the DNA replicated cells were stained with EdU (red),and the nucleus was stained with Hoechst (blue).E,the proportion of positive cells stained with EdU.F,cell viability detected by CCK-8 24 h after transfection.G,RT-qPCR detection of mRNA expression of proliferation-related genes CDK4,CyclinB,CyclinD and CyclinE after transfection of miR-24-3p mimics.H,Western blot to detect the protein levels of proliferation-related genes CDK4,CyclinB,CyclinD and CyclinE after treatment.I,quantitative analysis of CDK4,CyclinB,CyclinD and CyclinE proteins.NC represents the negative control.Results are mean±SEM of three independent experiments.*,P<0.05;**,P<0.01;ns,not significant.
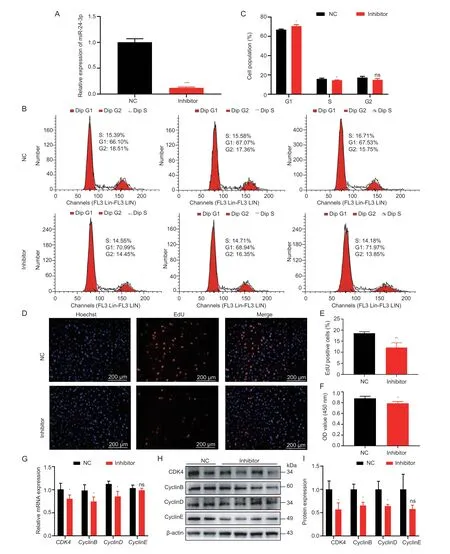
Fig.3 miR-24-3p inhibitor inhibits porcine granulosa cell (GC) proliferation.A,interfering with the transfection efficiency of miR-24-3p compared to the control group.B,cell cycle analysis of GCs detected by flow cytometry after transfection with miR-24-3p inhibitor and NC for 24 h.C,statistical analysis of cell cycle.D,the number of positive cells detected by EdU 24 h after transfection,the DNA replicated cells were stained with EdU (red),and the nucleus was stained with Hoechst (blue).E,the proportion of positive cells stained with EdU.F,CCK-8 detection of cell viability 24 h after transfection.G,RT-qPCR detection of mRNA expression of GC proliferation-related genes CDK4,CyclinB,CyclinD and CyclinE after transfection of miR-24-3p inhibitor.H,Western blot to detect the protein levels of proliferation-related genes CDK4,CyclinB,CyclinD and CyclinE after treatment.I,quantitative analysis of CDK4,CyclinB,CyclinD and CyclinE proteins.NC represents the negative control.Results are mean±SEM of three independent experiments.*,P<0.05;**,P<0.01;ns,not significant.
3.3.miR-24-3p inhibits GC apoptosis
The primary cause of follicle atresia is GC apoptosis,therefore,we tested the effect of miR-24-3p on GC apoptosis.miR-24-3p mimics significantly upregulated miR-24-3p levels in GCs (Fig.4-A) but inhibited the proportion of cells in early and late apoptosis (Fig.4-B and C).Moreover,overexpression of miR-24-3p significantly increased mRNA levels of the anti-apoptotic geneBCL-2and significantly downregulated mRNA and protein levels of the pro-apoptotic gene BAX (Fig.4-DF).However,interfering with miR-24-3p significantly reduced the level of miR-24-3p in GCs (Fig.5-A) and markedly increased the proportion of early-stage apoptotic cells (Fig.5-B and C),and it upregulated the expression of BAX and downregulated the expression of BCL-2 at the transcription and translation levels (Fig.5-D-F).These results suggest that miR-24-3p regulates the expression of apoptosis-related genes and inhibites GC apoptosis.
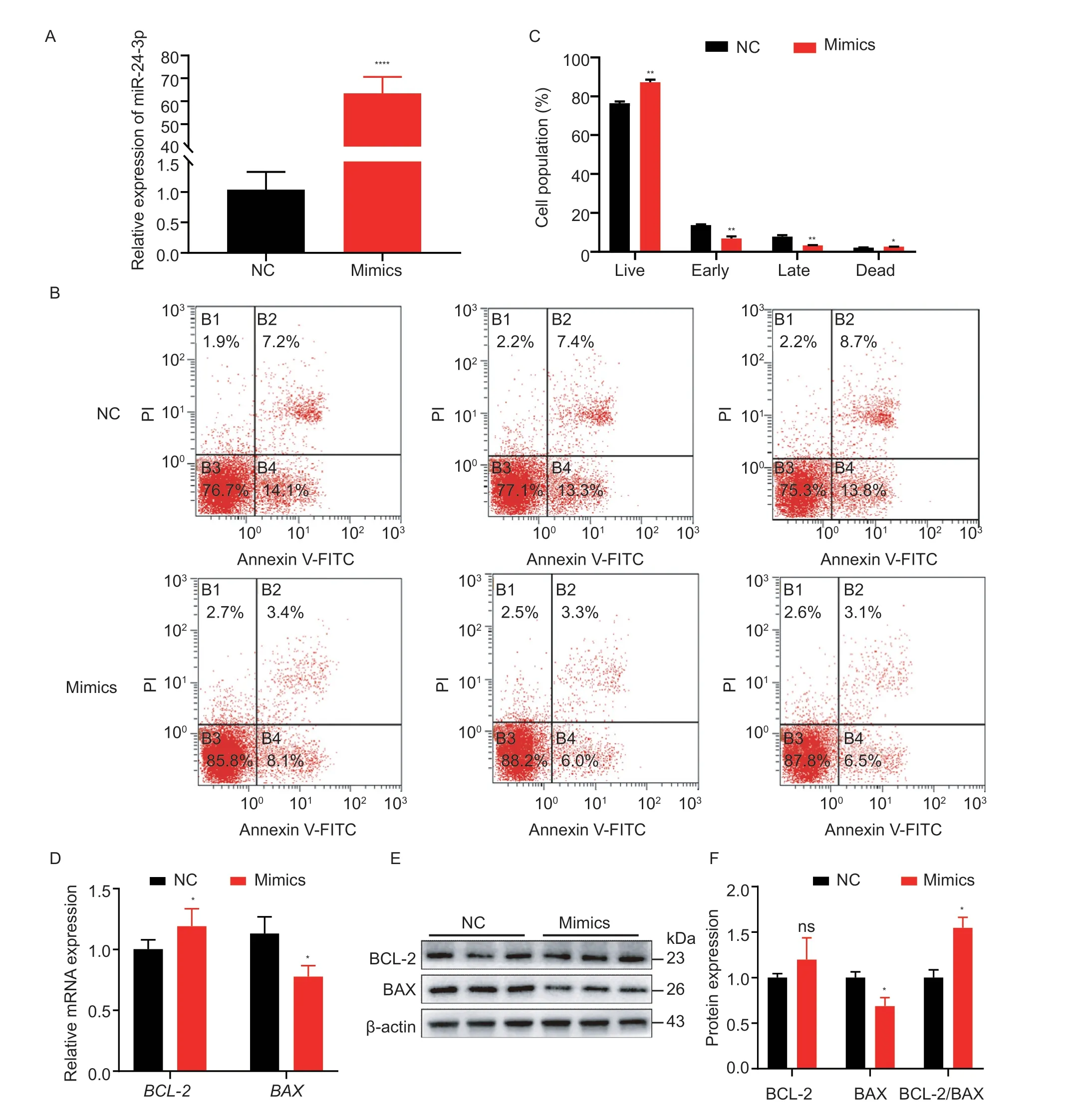
Fig.4 miR-24-3p inhibits porcine granulosa cell (GC) apoptosis.A,compared with the control group,the overexpression efficiency of miR-24-3p mimics transfected.B,the apoptosis state of GCs was detected by flow cytometry.C,the proportion of apoptosis in each period was counted.D,RT-qPCR was used to detect the expression levels of apoptosis-related genes BAX and BCL-2 mRNA after transfection of miR-24-3p mimics.E,the levels of apoptosis-related proteins BAX and BCL-2 were detected by Western blot.F,quantitative analysis of BAX and BCL-2 proteins.NC represents the negative control.Results are mean±SEM of three independent experiments.*,P<0.05;**,P<0.01;ns,not significant.

Fig.5 Interfering with miR-24-3p promotes the apoptosis of porcine granulosa cells (GCs).A,compared with the control group,the inhibition efficiency of miR-24-3p inhibitor transfected.B,the apoptosis state of GCs was detected by flow cytometry.C,the proportion of each period of apoptosis was counted by flow cytometry.D,RT-qPCR was used to detect the expression levels of apoptosis-related genes BAX and BCL-2 mRNA after transfection of miR-24-3p inhibitor.E,the levels of apoptosis-related proteins BAX and BCL-2 were detected by Western blot.F,quantitative analysis of BAX and BCL-2 proteins.NC represents the negative control.Results are mean±SEM of three independent experiments.*,P<0.05;**,P<0.01;ns,not significant.
3.4.miR-24-3p regulates GC proliferation and apoptosis by targeting P27
To elucidate the regulatory mechanisms of miR-24-3p in GCs,target genes of miR-24-3p were predicted using miRDB,miRWalk,miRTarBase,and TargetScan.We identified 17 common genes (Appendix A).miR-24-3p mimics inhibited three genes,and miR-24-3p inhibitor increased their levels (Appendices B and C).P27inhibits the effects of CDK2 and CDK4 and arrests the cell cycle in the G1 phase (Bertoliet al.2013).Apoptosis occurs in the G1 phase and is accelerated by late G1 or S phase arrest (King and Cidlowski 1995).We found that the seed sequence of miR-24-3p bound to the 3′UTR region ofP27(Fig.6-A).To determine whether miR-24-3p directly bindsP27,we constructed wild-type and mutantP273′UTR dual-luciferase reporter vectors (Fig.6-B).The dualluciferase reporter assay revealed that miR-24-3p mimics significantly inhibited the luciferase activity of the wild-type psiCHECK-2-p27-3′UTR reporter.In contrast,the activity of the mutant reporter vector did not change (Fig.6-C).Moreover,overexpression of miR-24-3p significantly inhibited P27 mRNA and protein in GCs (Fig.6-D-F),while interference with miR-24-3p significantly upregulated P27 mRNA and protein expression (Fig.6-G-I).

Fig.6 p27 is the target gene of miR-24-3p.A,BiBiServ software predicts the binding energy of miR-24-3p to P27 mRNA 3′UTR sequence.B,the 3′UTR sequence of the partial mRNA of the constructed P27 wild-type and mutant dual-luciferase reporter vectors.C,dual-luciferase activity after co-transfection.D,the mRNA level of P27 after 24 h transfection with miR-24-3p mimics.E,the protein level of P27 after 24 h transfection with miR-24-3p mimics.F,quantitative analysis of P27 protein.G,the mRNA level of P27 after 24 h transfection of miR-24-3p inhibitor.H,the protein level of P27 after 24 h of miR-24-3p inhibitor transfection.I,quantitative analysis of P27 protein.NC represents the negative control.Results are mean±SEM of three independent experiments.*,P<0.05;**,P<0.01;ns,not significant.
These results suggest that miR-24-3p inhibits the transcription and translation of P27;therefore,we determined whether P27 would alter the effect of miR-24-3p on proliferation and apoptosis.We conducted rescue experiments and found that the P27 overexpression attenuated miR-24-3p-induced effects on GC proliferation by decreasing EdU-positive cells (Fig.7-A and B)
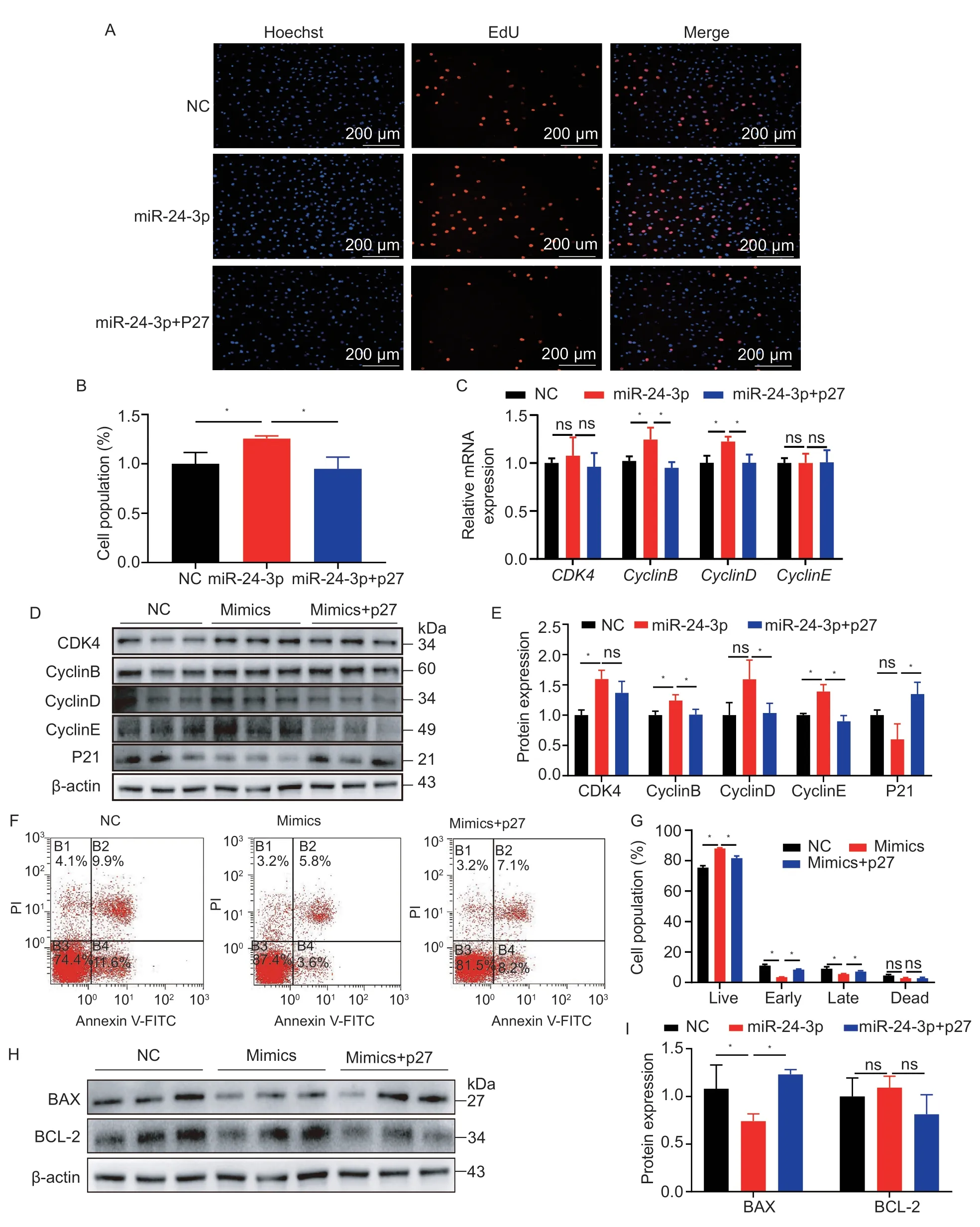
Fig.7 miR-24-3p regulates the proliferation and apoptosis of ovarian granulosa cells (GCs) by targeting P27.A,EdU staining to detect GCs after co-transfection of miR-24-3p mimics and P27 overexpression plasmid for 24 h.B,statistical results of EdUpositive cells.C,the mRNA levels of cell cycle-related genes CDK4,CyclinB,CyclinD and CyclinE after co-treatment.D,the protein levels of CDK4,CyclinB,CyclinD,CyclinE and P21 after co-treatment.E,quantitative analysis of protein results.F,flow cytometry detection of GC stage after 24 h co-transfection of miR-24-3p mimics and P27 overexpression plasmid.G,statistical results of flow cytometry.H,the levels of apoptosis-related proteins BAX and BCL-2 after co-treatment.I,quantitative analysis of protein results.NC represents the negative control.Results are mean±SEM of three independent experiments;*,P<0.05,**,P<0.01;ns,not significant.
,upregulated the proportion of early and late apoptotic cells,and downregulated the proportion of living cells (Fig.7-F and G).Co-transfection of miR-24-3p and P27 attenuated the upregulation of miR-24-3p on cell proliferation marker genes,including cyclins B and D (Fig.7-C-E),and increased levels of the pro-apoptotic protein BAX (Fig.7-H and I).
4.Discussion
We found that miR-24-3p plays a vital role in regulating GC physiological function.It stimulates cell proliferation and inhibits apoptosis by targetingp27.These findings highlight the crucial role of miR-24-3p in the GC physiological regulation.
miRNAs are critical for GC proliferation,apoptosis,oocyte growth and maturation,and other physiological functions (Li Qet al.2021).Based on Solexa sequencing,miR-24-3p was selected because of its significantly higher levels in high-yielding sows’ ovarian tissues than in low-yielding ones (Huanget al.2016).In addition,we also determined the miR-24-3p expression level was higher in the ovarian tissues of high-yielding sows compared with low-yielding sows.This finding captured our attention.We found that miR-24-3p was highly conserved among various species.Therefore,we speculated that miR-24-3p might be involved in regulating GC physiology.
In the present study,miR-24-3p contributed to GC proliferation by increasing the number of S phase cells and upregulating the expression of cyclins B and D at the mRNA and protein levels.Previous studies showed that miR-24-3p participates in the proliferation of various cell types.For example,in lung cancer cells,miR-24-3p promotes proliferation by directly targeting the sexdetermining region Y-box7 (SOX7) (Yanet al.2018),miR-24-3p blocks proliferation after heat damage by targeting peroxisome proliferator-activated receptor β/δ (PPAR-β) and is positively regulated by NF-κB (Cuiet al.2022),indicating that miR-24-3p is involved in regulating cell proliferation.
Follicular atresia occurs during follicular development when 10% of GCs undergo apoptosis (Zhanget al.2018),and increased apoptosis levels have been demonstrated in polycystic ovary syndrome patients and animals.Moreover,several lines of evidence support the notion that miRNAs are essential participants in follicular growth and atresia in animal ovaries.For example,miR-383-5p promotes apoptosis of human ovarian GCs by targeting the cold-induced RNA-binding protein (CIRP) through the PI3K/AKT signaling pathway (Liet al.2022),while the overexpression of insulin-like growth factor 1 (IGF1) reversed the inhibitory effect of miR-381 on GC apoptosis (Zhenet al.2021).BAX participates in mitochondrial apoptosis by continuous reverse translocation from mitochondria to cytoplasm mediated by BCL2L1/Bcl-xL to reduce the accumulation of BAX in the outer membrane (Pena-Blanco and Garcia-Saez 2018).In the present study,overexpression of miR-24-3p inhibited the pro-apoptotic geneBAXand could upregulate the expression of the anti-apoptotic geneBCL-2,while the result was the opposite after miR-24-3p inhibition.
Generally,miRNA functions by regulating the expression of target genes (Guoet al.2017).In this study,P27was another target gene identified by the dualluciferase reporter assay.Previous studies revealed thatp27suppresses proliferation and accelerates apoptosis (Reiset al.2000),miR-196a in cervical cancer (Houet al.2014),miR-24 in prostate cancer (Lynchet al.2016),and miR-152-3p in chronic myelogenous leukemia promote proliferation by targetingp27(Wanget al.2018).Besides,overexpression of P27 led to death and apoptosis of lung cancer cells,and P27 upregulated the expression ofBAXto promote the spontaneous apoptosis of oropharyngeal squamous cells (Fujiedaet al.1999;Naruseet al.2000).In the present study,we showed that miR-24-3p mimics inhibited the transcription and translation of p27,suggesting thatp27is a direct target of miR-24-3p.In addition,to further verify the role ofp27mediating miR-24-3p in regulating the proliferation and apoptosis of GCs,we constructed an overexpression plasmid of P27 and transfected it into GCs for a recovery experiment.We found that overexpression of P27 inhibited GC proliferation and promoted apoptosis.P27 weakened the promoting effect of miR-24-3p on GC proliferation and the inhibiting effect on apoptosis.
5.Conclusion
In summary,as shown in Fig.8,miR-24-3p is a regulatory molecule that promotes proliferation and inhibits apoptosis by targetingP27.These results enrich our understanding of the physiological functions of miRNAs in regulating GCs and provide candidate target genes for follicular development and the improvement of female reproductive success.

Fig.8 Schematic diagram of the miR-24-3p-associated regulatory mechanisms affecting prcine granulosa cells (GCs).miR-24-3p promotes proliferation and inhibits apoptosis of porcine ovarian GCs by targeting P27.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32272849),the National Key R&D Program of China (2021YFF1000602),and the earmarked fund for CARS-35-PIG.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of Animal Welfare and Health of Northwest A&F University,China (NWAFU-514030069).
Appendicesassociated with this paper are available on https://doi.org/10.1016/j.jia.2023.04.008
 Journal of Integrative Agriculture2024年4期
Journal of Integrative Agriculture2024年4期
- Journal of Integrative Agriculture的其它文章
- Invasion of fall armyworm led to the succession of maize pests in Southwest China
- Genome-wide and candidate gene association studies identify BnPAP17 as conferring the utilization of organic phosphorus in oilseed rape
- Impacts of agri-food e-commerce on traditional wholesale industry: Evidence from China
- Transcriptomic and metabolomic analysis provides insights into lignin biosynthesis and accumulation and differences in lodging resistance in hybrid wheat
- Responses of growth performance,antioxidant function,small intestinal morphology and mRNA expression of jejunal tight junction protein to dietary iron in yellow-feathered broilers
- Genome-wide identification of the CONSTANS-LIKE (COL) family and mechanism of fruit senescence regulation by PpCOL8 in sand pear (Pyrus pyrifolia)
