Methanolic extract of Ephedra alata inhibits breast cancer cells in vitro and in vivo
Fairouz Sioud, Aida Lahmer, Mouna Selmi, Fadwa Chaabane, Leila Chekir-Ghedira
1Research Unit Bioactive Natural Products and Biotechnology UR17ES49, Faculty of Dental Medicine of Monastir, University of Monastir, Avicenne Street, 5000 Monastir, Tunisia
2Laboratory of Pharmacognosy, Faculty of Pharmacy, University of Monastir, Avicenne Street, 5000 Monastir, Tunisia
ABSTRACT Objective: To determine the anticancer potential of the methanolic extract from Ephedra alata against breast cancer both in vitro and in vivo.Methods: The effects of the methanolic extract of Ephedra alata on the viability, migration as well as apoptosis of breast cancer 4T1 cells were measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, Transwell assay, and annexin V-FITC staining assay, respectively.Histological examination was also carried out.Moreover, a murine breast cancer model was established to evaluate the inhibitory effect of the extract.Biochemical parameters including hepatic and non-hepatic enzymes,malondialdehyde, and glutathione were investigated.Results: The methanolic extract of Ephedra alata showed a strong anti-proliferative and anti-migratory activity against 4T1 cells in a dose-dependent manner.It also induced apoptosis in 4T1 cells.In an in vivo mouse model, the extract markedly inhibited tumor growth,reduced malondialdehyde, and hepatic and non-hepatic enzymes as well as increased glutathione level.Conclusions: The methanolic extract of Ephedra alata inhibits breast cancer in vitro and in vivo, which may be a promising anticancer agent.
KEYWORDS: Ephedra; Breast cancer; Apoptosis; Antioxidant;Migration
1.Introduction
Malignancies and cancer have recently become a primary focus for health-active communities all around the world.Following lung tumor, breast cancer has been recorded as the second prevalent cause of death among women[1].In 2020, 2.3 million cases and 685 000 deaths were recorded[2], and 4.4 million cases are anticipated to be reported in 2070[3].
Cancer is the uncontrolled growth of aberrant cells that can be distinguished from benign and malignant tumors.Breast and colon cancer are two of the most prevalent malignancies discovered.Furthermore, malignant cells frequently exhibit irregular metabolism.Increased formation of reactive oxygen species (ROS)has been detected when cancer progresses, contributing to excessive cell proliferation and invasion[4].
ROS plays an essential role in tumor promotion through direct chemical reactions or perturbation of cellular metabolic processes.Superoxide dismutase, catalase, and glutathione (GSH) are ROS scavengers that serve as inhibitors at various phases of carcinogenesis[5].
Finding a drug that can modify the redox system could be helpful and useful for tumor eradication.The metabolic imbalance produced by free radicals can potentially be reduced by bioactive substances derived from plants.Recently, researchers have become interested in discovering powerful anti-cancer drugs inside natural items.Several plant extracts and products have been found to protect against free radicals by inducing the expression of antioxidant genes[6,7].
Recently, we have shown the abundance of flavonoids, phenolic acids, and proanthocyanidins in the methanolic extract ofEphedra alata(E.alata).The LC-MS/MS analysis showed a predominant presence of quercetin 3-O-[6”-(3-hydroxy-3-methylglutaryl)]-Dgalactoside[8].We have also demonstrated that this extract plays a significant role in preventing and neutralizing free radicals, actively preventing cisplatin-induced oxidative damagein vivo[9].Thus, the objective of this work was to assess the antitumor and antioxidant effects of the methanolic extract ofE.alatain vivo.
2.Materials and methods
2.1.Chemicals and reagents
DMEM medium, trypsin, streptomycin, penicillin, and fetal bovine serum were purchased from Sigma Cell Culture (Courtaboeuf,France).3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Euromedex (Mundolsheim,France).Triton X-100, ribonuclease A (RNase), phosphate-buffered saline (PBS), sodium hydroxide and propidium iodide (PI) were purchased from Sigma-Aldrich (Steinheim, Germany).
2.2.Preparation of methanolic extracts of E.alata
E.alatawas sourced from the Sahara region of Tataouine, located in the southeast of Tunisia, in February 2017.The plant material was shade dried, powdered and stored at room temperature.The plant was taxonomically identified by Pr.Fethia Skhiri (Higher Institute of Biotechnology of Monastir, Tunisia) according to the Tunisian Guideon Flora.A voucher specimen (EA001) was deposited in our laboratory.
To prepare the methanolic extract, 100 g sample of dried aerial parts of the plant was extracted by maceration in 1 L of methanol for 7 d at room temperature.Following this, the liquid was filtered, and the solvent was removed using a rotary evaporator under reduced pressure at 40 ℃, resulting in a methanol extract[8].
2.3.MTT assay
Using the MTT assays, cell proliferation was evaluated.In a 96-well plate, we seeded the 4T1 cells [a murine mammary carcinoma cell line that was obtained from American Tissue Culture Collection(ATCC, Molsheim, France)] at a density of 1 × 104cells per well.These cells were subsequently cultured in 200 μL of DMEM media including various doses of the methanolic extract ofE.alata(0, 20,40, 60, 80, and 100 μg/mL).The MTT solution was applied to each well and incubated for 24 and 48 h[10].A Thermo Scientific (Vantaa,Finland) plate reader was used to measure the solution’s absorbance at a wavelength of 492 nm.
2.4.Annexin V-FITC staining assay for apoptosis analysis
PI and annexin V-FITC staining were used to measure the apoptosis of 4T1 cells.First, different doses of the methanolic extract ofE.alatawere applied to 4T1 cells over 48 h.After incubation, the cultivated cells were completely cleaned with PBS three times,stained for 5 min in the dark with annexin V-FITC and PI, and then analyzed on a flow cytometer (FACS system, Switzerland)[11].
2.5.Transwell chamber assay
To assess the migrative capacity of 4T1 cells, we employed a Transwell chamber test.Cells were treated with various concentrations (0, 30, and 60 μg/mL) of the methanolic extract ofE.alatafor 48 h.The cells were then seeded to the upper chamber with serum-free DMEM medium and serum-containing DMEM medium was added to the lower chamber as a chemoattractant for 24 h.After incubation, the cells under the membrane were stained with 0.1%crystal violet and photographed[12].
2.6.Experimental animals
A total of 24 female BALB/c mice (8 weeks old and weighing ≈25 g) were received from the Pasteur Institute in Tunisia.Mice were housed in an approved pathogen-free facility with conventional settings of 24 ℃, 50% humidity, and 12 hours of light/dark.All animals had free access to normal rodent food and filtered water.
2.7.Experimental design and tumorigenesis assessment
To generate a breast tumor, 1 × 1064T1 cells were subcutaneously injected into the mammary fat pad area.All mice were checked by palpation every week to monitor tumorigenesis.Treatment started on the seventh day.All mice were treated for two weeks (three times per week) by intraperitoneal injection.Animals were randomly divided into four groups: the untreated group without a breast tumor(as a negative control), the untreated group with a breast tumor (as a positive control), the tumor group treated group with 50 or 150 mg/kg of the methanolic extract ofE.alata.For all groups, dimethyl sulfoxide(DMSO) was used at the final concentration of 0.1% and the two control groups received a saline solution with 0.1% of DMSO.
2.8.Determination of tumor inhibition rate
Throughout the investigation, the tumor volumes of mice were calculated after 18 days using the following formula:
where d and D are the shortest and the longest diameters, respectively.24 h after the last administration, mice were sacrificed by cervical dislocation under anesthesia with 2% isoflurane inhalation, and tumors were peeled off and weighed.
The tumor inhibition rate was calculated according to the following formula:
Tumor inhibition rate = (average tumor weight of the control group- average tumor weight of the experimental group)/average tumor weight of the control group × 100
2.9.Histological study
Collected tumors were fixed with 10% formaldehyde.They were then placed in various baths with alcohol to eliminate the water.This will then allow the impregnation of the formalin-containing paraffin in the tissue to dehydrate and dissolve the fats in the tissue.The samples are finely cut into 5 μm thick sections with a microtome(KD-2268 Manual Microtome).The sections are then stained with hematoxylin and eosin (H&E) for observation using an optical microscope (Leica Microsystems, Germany)[13].
2.10.Biochemical analysis
Aspartate transaminase (AST), alanine transaminase (ALT),creatinine, and alkaline phosphatase (ALP) were analyzed by the Biochemistry Department Laboratory of the University Hospital Center using an automated analysis with a commercial Cobas Integra kit (Roche, Boulogne-Billancourt, France).
2.11.Non-enzymatic antioxidant assay
The Moron technique was used to determine the glutathione (GSH)content present in liver and kidney homogenates[9].Briefly, to precipitate the tissue proteins, 100 μL of homogenate was mixed with 10% sulfosalicylic acid and vortexed.The mixture was centrifuged at 5 000 rpm for 20 min.Subsequently, supernatant, sodium phosphate dibasic (Na2HPO4), and 5,5′-dithiobis-(2-nitrobenzoic acid) were added successively.The absorbance was measured within 10 min at 412 nm.Commercially available GSH was used as a standard.The level of GSH was expressed as μmol GSH/mg of protein.
2.12.Evaluation of lipid peroxidation status
Kidney and liver homogenates were mixed with trichloroacetic acid and thiobarbituric acid and then incubated at 95 ℃ for 2 h.The reaction was stopped by immediate cooling in ice, and then the samples were centrifuged at 2 500 rpm for 10 min at 4 ℃.The reaction between thiobarbituric acid and the lipids produced by oxidative degradation generates red complexes measured using a microplate reader (Vantaa, Finland) at 530 nm[9].The concentration of malondialdehyde (MDA) (μmol per mg proteins) was obtained by extrapolating absorbance values to concentrations of the standard curve of MDA.
2.13.Statistical analysis
The data were presented as mean ± standard deviation (SD).Statistical comparisons between groups were conducted using oneway and two-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test.AP-value < 0.05 was considered statistically significant.
2.14.Ethical statement
Directives regulating the welfare of experimental animals, and experiments were approved by the Life Sciences and Health Research Ethics Committee of the Institute of Biotechnology(University of Monastir, Tunisia; Reference ethical approval: CERSVS/ISBM 009/2020) in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no.85-23, revised 1996).
3.Results
3.1.Methanolic extract of E.alata inhibits the proliferation of 4T1 cells
The methanolic extract ofE.alatainhibited the proliferation of 4T1 cells in a dose-dependent manner.The IC50value was 65 μg/mL after 48 h of incubation (Figure 1).
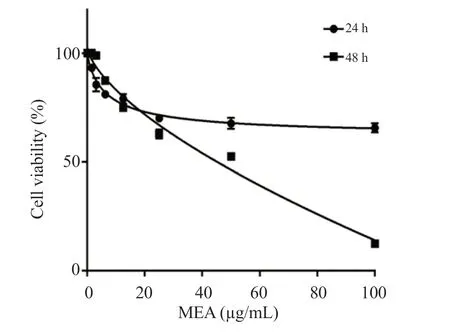
Figure 1.Antiproliferative effect of the methanolic extract of Ephedra alata on 4T1 cells.Cells were treated with different concentrations of Ephedra alata for 24 and 48 h and cell proliferation was measured by MTT assay.MEA: Methanolic extract of Ephedra alata.
3.2.Methanolic extract of E.alata induces apoptosis in 4T1 cells
The flow cytometry analysis showed that the methanolic extract ofE.alatamarkedly increased the early and late stages of apoptosis in 4T1 cells (Figure 2).Specifically, apoptotic cells significantly increased at 60 μg/mL and 120 μg/mL.More than 70% apoptotic cells were observed in cells treated with 120 μg/mL of the methanolic extract ofE.alata.
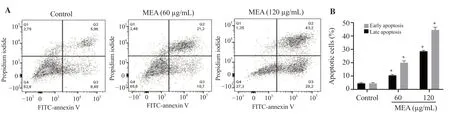
Figure 2.Flow cytometric analysis of early and late apoptosis in 4T1 cells treated with the methanolic extract of Ephedra alata.After 48 hours of treatment,4T1 cells were stained with FITC-annexin V/propidium iodide.Apoptosis was assessed using flow cytometry.(A) Apoptosis profile plots.(B) The percentages of early and late apoptosis in 4T1 cells treated with the methanolic extract of Ephedra alata.The data are presented as mean ± standard deviation (SD) of three independent experiments and analyzed by one way ANOVA followed by Tukey’s multiple comparison test.*P < 0.01 compared to the control group.
3.3.Methanolic extract of E.alata inhibits the migration of 4T1 cells
The methanolic extract ofE.alataefficiently inhibited 4T1 migration in a dose-dependent manner (Figure 3A) and the inhibition rates were 76% and 85% after treatment with 30 and 60 μg/mL of the methanolic extract, respectively (Figure 3B).

Figure 3.Anti-migratory effect of the methanolic extract of Ephedra alata in 4T1 cells.A Transwell chamber was used for the migration assay and images were captured.(A) Representative photomicrographs of stained cells (Magnification, ×40).(B) The percentage of migrative cells.The results are expressed as mean ± SD of three independent experiments and analyzed by one way ANOVA followed by Tukey’s multiple comparison test.*P < 0.01 compared to the control group.
3.4.Methanolic extract of E.alata inhibits tumor growth in mice
After 6 days of treatment, the growth of tumors was significantly decreased compared to the control group (Figure 4).After 12 days,this difference was sharply accentuated.The inhibition rates were 46.16% and 68.8%, for the group treated with 50 and 150 mg/kg of the methanolic extract ofE.alata, respectively.
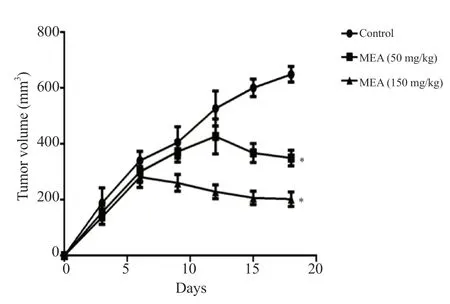
Figure 4.Tumor volume after treatment with the methanolic extract of Ephedra alata.The results are expressed as mean tumor volume ± SD of three independent experiments and analyzed by one-way ANOVA followed by Tukey’s multiple comparison test.*P < 0.01 compared to the control group.
3.5.Histopathological results
The sections of the positive control group showed infiltration of tumor cells.In neoplastic cells, large hyperchromatic nuclei and a relatively small amount of cytoplasm were noted.These neoplastic cells showed special characteristics, including pleomorphic cells and nuclei, and giant cells.The results indicate cells under mitotic division in some areas (Figure 5A).In most areas of the group treated with the extract, a significantly increased degree of cell necrosis could be observed.The tumor section of mice treated with 150 mg/kg of the extract showed larger areas of necrosis which exceeded half of the total area of the tumor (Figure 5B).
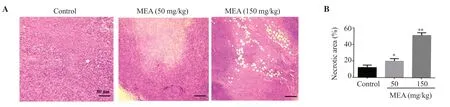
Figure 5.Effect of the methanolic extract of Ephedra alata on histology in mice with breast tumor.(A) Representative sections of tumors from untreated mice and mice treated with different doses of the methanolic extract of Ephedra alata (Magnification: 200×).(B) The percentage of necrotic cells.The results are expressed as mean ± SD of three independent experiments and analyzed by one-way ANOVA followed by Tukey’s multiple comparison test.*P < 0.05, **P <0.01 compared to the control group.
3.6.Effect of the methanolic extract of E.alata on liver function markers
An increase in ALT, AST, and ALP activities was observed in the positive control group, confirming liver damage, whereas treatment with 150 mg/kg of the extract decreased ALT and ALP activities, and 50 mg/kg of the extract only reduced ALP activity (Figure 6A-C).
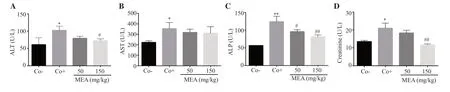
Figure 6.Effect of the methanolic extract of Ephedra alata on (A) alanine transaminase (ALT), (B) aspartate transaminase (AST), (C) alkaline phosphatase(ALP), and (D) creatinine.The results are expressed as mean ± SD of three independent experiments and analyzed by one-way ANOVA followed by Tukey’s multiple comparison test .*P < 0.05, **P < 0.01 compared with the negative control group; #P < 0.05, ##P < 0.01 compared with the positive control group.Co-:the negative control group; Co+: the positive control group.
3.7.Effect of the methanolic extract of E.alata on creatinine level
Creatinine level was significantly increased in the positive control group.However, treatment with the methanolic extract ofE.alatareduced the creatinine level in a dose-dependent manner (Figure 6D).
3.8.Effect of the methanolic extract of Ephedra alata on lipid peroxidation
An increase in hepatic and renal MDA levels was observed in the positive control group.Administration of 150 mg/kg of the methanolic extract ofE.alataattenuated lipid peroxidation in the kidney and liver,thus preventing further damage caused by tumor progression (Figure 7).
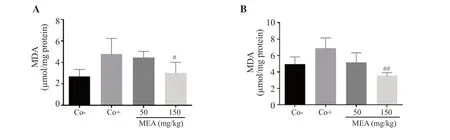
Figure 7.Lipid peroxidation level in the (A) kidney and (B) liver of treated mice with 4T1 breast cancer cell-induced tumor.The data are expressed as mean ±SD of three independent experiments and analyzed by one way ANOVA followed by Tukey’s multiple comparison test.#P < 0.05, ##P < 0.01 compared with the positive control group.MDA: malondialdehyde.
3.9.Effect of the methanolic extract of E.alata on GSH
Mice in the positive control group showed a reduction in the level of GSH.Treatment with 150 mg/kg of the methanolic extract ofE.alatasignificantly increased the level of GSH (Figure 8).
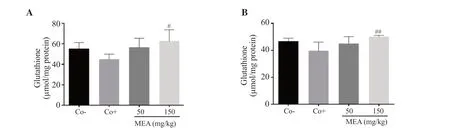
Figure 8.Glutathione level in the (A) kidney and (B) liver of treated mice with 4T1 breast cancer cell-induced tumor.The data are expressed as mean ± SD of three independent experiments and analyzed by one way ANOVA followed by Tukey’s multiple comparison test.#P < 0.05, ##P < 0.01 compared with the positive control group.
4.Discussion
Inhibiting the growth of tumor cells or eradicating them without harming healthy cells is the primary goal of the use of anti-cancer drugs.One of the most efficient cancer treatment strategies is the use of natural products, which are less harmful than chemotherapy and radiation treatment[14].
The current study was conducted to assess the anticancer and antioxidant effects of the methanolic extract ofE.alatain mammary carcinoma-bearing mice.The 4T1 mammary carcinoma is a transplantable tumor cell line that is extremely tumorigenic and invasive.Unlike most tumor models, it can spontaneously metastasize from the initial tumor in the mammary gland to various distant locations including lymph nodes, blood, liver, lung, brain,and bone[15].
Our findings showed that the methanolic extract ofE.alatahad anticancer propertiesin vitro.The methanolic extract ofE.alatagreatly inhibited the development of 4T1 cells by preventing cell migration and proliferation as well as inducing apoptosis.The IC50value of the methanolic extract ofE.alatawas found to be 65 μg/mL, substantially lower than those of other plant extracts[16,17].These findings suggest the methanolic extract ofE.alatais a potent anticancer agent.In mice with breast tumors, treatment with 50 and 150 mg/kg of the methanolic extract ofE.alatasignificantly reduced the tumor volume compared to the control group.An obvious necrosis was observed in tumor mice that received the extract treatment.Serum enzymes have been investigated for a long time as potential early markers of neoplasia[18] as well as a method for early detection of liver metastatic lesion in patient with breast cancer[19].
In the present study, the analysis of biochemical markers revealed that the untreated group with tumors showed elevated levels of liver enzymes such as ALT, AST, and ALP due to hepatocellular injury.Treatment with the extract reduced the increased markers, showing protection against tumor cell-induced hepatotoxicity.
Oxidative stress can cause macromolecule damagein vivo, such as lipid peroxidation[20].In the present study, the positive control group showed significantly higher levels of MDA in the liver and kidney, which were lowered to near-normal levels in the extracttreated groups.This reflects the decrease in free radical formation and subsequent reduction in oxidative stress, which are the primary risk factors for the disease.GSH, an effective inhibitor of neoplastic development, functions as an endogenous antioxidant system.It was detected in significant amounts in the liver and is thought to play an important role in the protective process[15].Its production increases under oxidative stress and inflammation.The present findings show that GSH levels were decreased in control mice, most likely due to a high level of free radicals.These results are consistent with previous studies that have shown that glutathione levels are significantly reduced in the blood of breast cancer patients compared to those of healthy control subjects[21].Treatment with the methanolic extract ofE.alataincreased GSH concentration in the liver and kidney.The findings of thein vitroandin vivoexperiments demonstrated that the methanolic extract ofE.alatapossesses an anti-cancer effect.However, there are some limitations in this study.The anticancer mechanism of the methanolic extract ofE.alatais not elucidated and the active compounds responsible for the anticancer activity of this plant extract are not determined.Therefore, further studies are needed to unravel these aspects.
In conclusion, the methanolic extract ofE.alatainhibits the migration and proliferation of breast cancer cells and tumor volume in mice, which suggests that it may be a promising anticancer agent for the treatment of breast cancer.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Acknowledgments
The authors acknowledge the Tunisian Ministry of Higher Education and Scientific Research for the financial support of this study.
Funding
The authors received no extramural funding for the study.
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’contributions
FS and LCG supervised and designed the study.FS and AL performedin vivostudy.MS and FC performed data collection and analytic calculation.FS contributed to the final version of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2024年4期
Asian Pacific Journal of Tropical Biomedicine2024年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
- Pachymic acid exerts antitumor activities by modulating the Wnt/β-catenin signaling pathway via targeting PTP1B
- Ellagic acid inhibits gastric cancer cells by modulating oxidative stress and inducing apoptosis
- Isoimperatorin alleviates acetic acid-induced colitis in rats
- Ethyl acetate fraction of Sargassum pallidum extract attenuates particulate matterinduced oxidative stress and inflammation in keratinocytes and zebrafish
