Ferritinophagy: A new idea for liver diseases regulated by ferroptosis
Zi-Bing Qin ,Jun-Feng Li ,b ,Wn-Yun Xiong ,Xio-Rong Mo ,c,*
a The First Clinical Medical College of Lanzhou University, Lanzhou 730000, China
b Institute of Infectious Diseases, The First Hospital of Lanzhou University, Lanzhou 730000, China
c Department of Infectious Disease, The First Hospital of Lanzhou University, Lanzhou 730000, China
Keywords: Ferritinophagy Ferroptosis Liver disease
ABSTRACT Background: The discovery of regulatory cell death has led to a breakthrough in the therapeutic field.Various forms of cell death,such as necrosis,apoptosis,pyroptosis,autophagy,and ferroptosis,play an important role in the development of liver diseases.In general,more than one form of cell death pathways is responsible for the disease state.Therefore,it is particularly important to study the regulation and interaction of various cell death forms in liver diseases.Data sources: We performed a PubMed search up to November 2022 with the following keywords: ferritinophagy,ferroptosis,and liver disease.We also used terms such as signal path,inducer,and inhibitor to supplement the query results.Results: This review summarized the basic characteristics of ferritinophagy and ferroptosis and the regulation of ferroptosis by ferritinophagy and reviewed the key targets and treatment strategies of ferroptosis in different liver diseases.Conclusions: Ferritinophagy is a potential therapeutic target in ferroptosis-related liver diseases.
Introduction
The rapid development of the global economy in the last decade has made tremendous changes in public health habits due to the increase in per capita income.These health habits are observed mainly in the form of expanded vaccination coverage,availability of a high-fat diet,and an increase in global per capita alcohol consumption.As a result,an increasing number of studies are involved in updating and analyzing the epidemiology of various diseases,including diseases related to the liver.In the last decade,statistically significant changes have been observed in the epidemiology of hepatocellular carcinoma (HCC),which is the third leading cause of death from tumors worldwide.Furthermore,viral hepatitis remains the leading cause of liver cancer deaths globally.Notably,an increasing trend has also been observed in the number of nonalcoholic fatty liver disease (NAFLD)-associated HCC,with the fastest growth rate of mortality [1].Therefore,many studies have been conducted to understand the cell death pathways in liver diseases,and ferritinophagy and ferroptosis are the two hot spots areas of research in recent years.Mechanisms of these cell death have become a new hot spot.
Ferritinophagy
Autophagy is a normal metabolic process that maintains the intracellular environment by degrading damaged organelles and associated proteins and releasing the products of decomposition for reuse.The process of autophagy starts with the endoplasmic reticulum and Golgi vesicles enveloping mitochondria,endosomes,peroxisomes,and other organelles in the cytoplasm,and eventually forming the initial autolysosomes.After the fusion of endosomes and the initial autolysosomes,the intermediate autolysosomes are formed.Subsequently,the lysosomes are fused with the initial autolysosomes to form the degrading autolysosomes.The contents and inner membrane of the initial autophagic vesicles are degraded by various enzymes in lysosomes to finally complete cell autophagy (Fig.1) [2].Moderate autophagy can increase the survival rate of cells under stress,but high autophagy levels and impaired lysosomal activity can promote ferroptosis,which mainly includes the following events.(1) Nuclear receptor co-activator 4 (NCOA4)-related autophagy (ferritinophagy) and ferroptosis [3].NCOA4 is a selective transport receptor that mediates ferritinophagy [4].(2) Sequestosome 1 (SQSTM1/p62)-associated autophagy (clockophagy),and ferroptosis [5].Liu et al.[5]identified a selective type of autophagy in which the biological clock regulator aryl hydrocarbon receptor nuclear translocator-like (ARNTL)is involved in the degradation process.Here,SQSTM1,which is a carrier receptor protein,carries ARNTL for autophagic degradation.In this type of autophagy,ARNTL degradation can block hypoxia-inducible factor-1α(HIF-1α)-mediated cellular uptake of fatty acids and lipids,causing ferroptosis by lipid peroxidation.(3)RAB7A-associated autophagy (lipoautophagy) and ferroptosis [6].The small GTPase Ras-related protein Rab-7a serves as a key organizer of the endosomal-lysosomal system.During cell death,lipid droplets can act as antioxidants,and the autophagic degradation of these intracellular lipid droplets promotes RAS-selective lethal 3 (RSL3)-induced ferroptosis in hepatocytes.And (4) the endoplasmic reticulum stress-mediated autophagy (ER-phagy) and ferroptosis [7].Mitochondrial reactive oxygen species (MitoROS) mediates ferroptosis induced by ER stress-autophagy pathway during the renal tubular injury [8](Fig.2).The bioavailability of intracellular iron ions depends on ferritinophagy,which is a special type of ferritin-selective autophagy [9].Excessive ferritinophagy activation increases intracellular iron levels and induces glutathione (GSH)depletion and a decrease in GSH peroxidase 4 (GPX4),eventually causing cellular ferroptosis [10].Furthermore,NCOA4 downregulation decreases the ferritin autophagic flow,reducing susceptibility to ferroptosis,whereas overexpression of NCOA4 increases susceptibility to ferroptosis due to an increase in intracellular bioavailable iron levels [11].Nuclear factor erythroid 2-related factor 2 (Nrf2) is closely associated with the regulation of both ferroptosis and autophagy [12].A previous study concluded that tumor cell death caused by the toxic accumulation of free iron leading to ferroptosis is the most effective way to kill drug-resistant cancer cells [13].Nrf2 maintains iron homeostasis by controlling HERC2(E3 ubiquitin ligase binding NCOA4 and FBXL5) and VAMP8 (mediates autophagosome-lysosome fusion).AfterNrf2knockdown,the levels of both ferritin and NCOA4 were increased in cells,and apoferritin was recruited to autophagosomes,eventually blocking autophagosome-lysosome fusion,inhibiting ferritinophagy,and increasing the labile iron pool (LIP) [13].Therefore,Nrf2 plays a role in reducing the "toxic" iron load and alleviating ferroptosis.
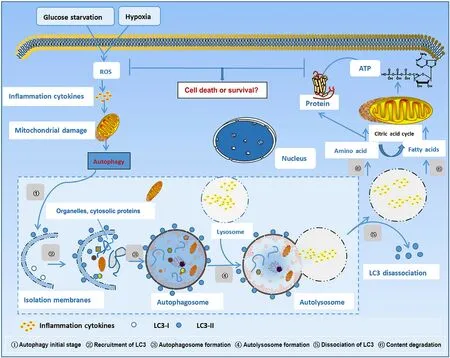
Fig.1. Autophagy process.ROS: reactive oxygen species;LC3: light chain 3;ATP: adenosine triphosphate.
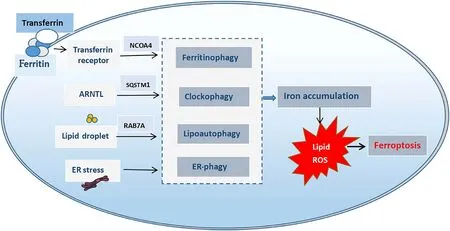
Fig.2. Autophagy-driven ferroptosis.NCOA4: nuclear receptor co-activator 4;ARNTL: aryl hydrocarbon receptor nuclear translocator-like;SQSTM1: sequestosome1;RAB7A:Ras-related protein Rab-7a.ER: endoplasmic reticulum;ROS: reactive oxygen species.
Ferroptosis
Ferroptosis is a type of cell death that is morphologically,biochemically,and genetically distinct from apoptosis,necrosis,and autophagy [14].In terms of morphological changes,ferroptosis is characterized by mitochondrial atrophy,reduction or disappearance of mitochondrial cristae,increase of mitochondrial membrane density,and rupture of the outer mitochondrial membrane.Further,during ferroptosis,the following biochemical changes are characterized: (1) increased lipid peroxidation;(2) aggregation of iron and ROS;(3) activation of mitogen-activated protein kinase (MAPK);and (4) depletion of intracellular GSH,release of arachidonic acid (ARA) and other mediators [15].Genetic level changes during ferroptosis are mainly influenced by ribosomal protein L8 (RPL8),iron response element binding protein 2 (IREB2),ATP synthase F0 complex subunit C3 (ATP5G3),tetratricopeptide repeat domain 35 (TTC35),citrate synthase (CS),acyl-CoA synthetase family member 2 (ACSF2),and regulation by metabolic and storage genes.Ferroptosis is triggered by several inducers,including erastin,RSL3,buthionine sulfoximine,sulfasalazine,sorafenib,and artesunate.Further,molecules such as vitamin E,GSH,ferrostatin-1,liproxstatin-1,and iron chelators inhibit ferroptosis[16-20](Table 1).
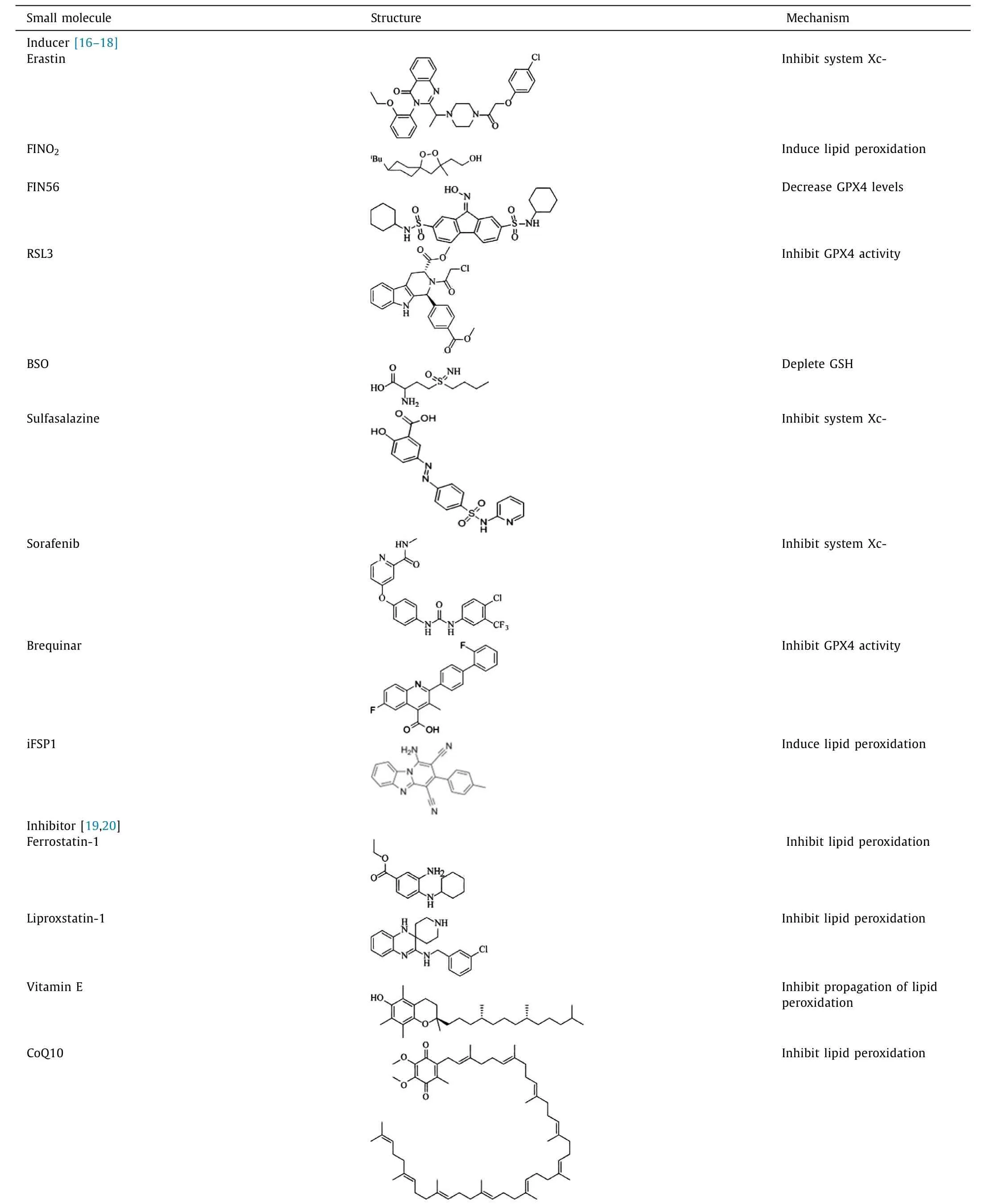
Table 1Inducers and inhibitors of ferroptosis.
Ferritinophagy induces ferroptosis
After the initiation of ferritinophagy,free Fe2+ions are released by the degradation of intracellular ferritin,and the excess Fe2+ions cause a large ROS accumulation through the Fenton reaction,which is important for the development of ferroptosis [21].Gao et al.[10]demonstrated that NCOA4 or ATG5/ATG7 deletion using shRNA inhibited the autophagic degradation of ferritin in murine embryonic fibroblasts and reduced intracellular iron levels.In contrast,NCOA4 overexpression promoted iron levels.Proteomic analysis of purified autophagosomes identified NCOA4 as a ferritin transporter during ferritinophagy,and that the C-terminal domain of NCOA4 can bind to the arginine residue (R23) on the FTH1 surface to translocate ferritin into the autophagic lysosomes,regulating ferroptosis [9].It was demonstrated that artesunate and the RNA-binding proteins ELAVL1/HuR promote hepatic stellate cell (HSC) ferroptosis by activating ferritinophagy,eventually exacerbating liver fibrosis [22].Yoshida et al.showed the effects of smoking on iron availability [23].Smoke exposure upregulates ferritinophagy,activates ferroptosis,which plays a role in the pathological process of chronic obstructive pulmonary disease.In summary,ferroptosis is closely associated with the pathophysiological processes of many diseases,and understanding the regulation of ferritinophagy by which ferroptosis can be reduced is the new hotspot research topic in this field.
Regulation of ferritinophagy
Regulation of ferritinophagy by the iron content
The ferritinophagy flux determines the NCOA4 levels,which are tightly regulated by intracellular iron levels post-translationally.Since NCOA4 can increase the cytoplasmic Fe2+ions content,a relatively simple negative feedback process mediated by iron content inhibits NCOA4 [24].NCOA4 has multiple ubiquitination sites with high affinity to E3 ubiquitin-protein ligase 2 (HECT and RLD domain containing E3 ubiquitin-protein ligase 2,HERC2) [25].HERC2 is associated with the degradation of many proteins and may have multiple ubiquitination targets.When iron is abundant in cells,HERC2 uses its CUL7-homologous structural domain to recognize NCOA4 and mediates NCOA4 degradation through the ubiquitin-proteasome system,thereby decreasing NCOA4 levels,increasing ferritin content,promoting iron storage,and decreasing free iron concentration.In contrast,under iron-deficient conditions,the binding of HERC2 to NCOA4 decreases,while NCOA4 becomes more stable and its content increases in autophagic vesicles,thereby inducing ferritin degradation by lysosomes via the autophagic pathway and increasing free iron in cells [11,26].Since the HERC2-binding site on NCOA4 overlaps with the FTH1-binding site,there is a possibility of mutual exclusion and competition between HERC2 and FTH1 binding.Further studies are required to understand the gaps such as clarity on the interaction between NCOA4 and ferritin,the nature of binding between HERC2 and NCOA4,ferritin and NCOA4,and methods used by NCOA4 to recognize iron and trigger autophagy.
Regulation of ferritinophagy by autophagy
It has been found that autophagy inhibition also leads to the inhibition of NCOA4-mediated ferritinophagy [27].In addition to HERC2,autophagy can also mediate NCOA4 degradation [9].In mouse embryonic fibroblasts (MEFs) and HT1080 cells,decreased NCOA4 expression was detected,which was reversed by autophagy inhibitors [10,27].Another study found that HepG2 cells expressed higher levels of NCOA4 and lower levels of FTH1 than those of Hep3B cells.More interestingly,the ferroptosis inducer formosanin C induces significant ferroptosis and autophagic flux in HepG2 cells [28].These studies demonstrated that ferritinophagy itself is a type of autophagy,and that autophagy inhibition also inhibits ferritinophagy.Although most recent studies focused on understanding the core role of NCOA4,future studies are required to focus on novel target or other interfering regulatory mechanisms of NCOA4.
Lysosomal targeting regulates ferritinophagy
The mechanism by which the NCOA4-FTH1 complex reaches the lysosome remains unclear.In addition to the classical LC3Bdependent delivery pathway,a novel,non-classical,and distinct endosomal sorting complex required for transport (ESCRT)-mediated delivery pathway is also described where ferritin transport involves a complex of NCOA4 binding partner tax1 binding protein 1 (TAX1BP1),VPS34 (class III PI3K vacuolar protein sorting 34),ATG9A,and ULK1/2-FIP200 [29].In this non-classical pathway,direct binding of TAX1BP1 to NCOA4 is required for ferritin lysosomal transport under basal and iron-deficient conditions.shRNA-based screening analysis of 390 cancer cell lines revealed the strongest regulation of TAX1BP1 with the down-regulation of NCOA4 levels [30].Interestingly,when TAX1BP1 was knocked down,NCOA4 bound to ferritin and accumulated at the periphery,suggesting that NCOA4 interaction with ferritin alone is not suffi-cient to promote ferritin degradation [30].However,specific regulatory events in this mechanism require further investigation.
Modulation of ferritinophagy by hypoxia
NCOA4 is also regulated by HIF.Increased NCOA4 mRNA levels were detected in cells with increased HIF-1αor HIF-2αactivity [31].In macrophages,hypoxia decreases NCOA4 expression.Hypoxic macrophages store iron by reducing intracellular free iron and increasing the expression of ferritin,including mitochondrial ferritin (FTMT).Further,NCOA4 is also shown to directly regulate the expression of FTMT [32].However,in contrast,it was also shown that ferritinophagy processes initiated by the degradation of FTH-NCOA4 complex autophagosomes were activated under normoxic conditions,and interestingly,hypoxia prevented these processes [33].Further research is required to understand the regulation of ferritinophagy under different oxygen concentrations and for exploring other factors involved in the regulation of ferritinophagy under hypoxic conditions.
Ferroptosis regulates the signaling pathway
Since its discovery,ferroptosis is believed to be the result of multiple regulatory pathways acting simultaneously.
Cystine/glutamate antiporter (system Xc-)
The system Xc-is located on the cell membrane and consists mainly of solute carrier 3A2 (SLC3A2) and solute carrier 7A11(SLC7A11) [34].This system transports amino acids in a 1:1 ratio;it pumps extracellular cystine inside and pumps intracellular glutamate outside the cell.Once inside,cystine is enzymatically converted into cysteine.Subsequently,cysteine is converted intoγ-glutamylcysteine by ATP-dependent glutamate-cysteine ligase,andγ-glutamylcysteine is converted into GSH by GSH synthetase (GSHS).As a key factor in the regulation of ferroptosis,GSH can scavenge free radicals and is also an important antioxidant in the human body.Thus,cellular uptake of cysteine is a key step in GSH synthesis,and GSH production and maintenance are critical for protecting cells from damage caused by oxidative stress.When the system Xc-is selectively inhibited,intracellular GSH synthesis is reduced,and ROS accumulation causes a series of reactions that eventually lead to ferroptosis [15].The system Xc-acts as a "carrier" in the cell,and p53 and Nrf2-Keap1 exert their "competence"through the system Xc-.
p53 mediates ferroptosis by inhibiting the system Xc-
It is well established that activation of p53 alone is not suffi-cient to directly induce ferroptosis,but p53 can regulate ferroptosis in the presence of ferroptosis inducers.Ferroptosis is induced by downregulating the expression of SLC7A11,which is a key component of the system Xc-and inhibits the uptake of cysteine,thereby increasing the cellular sensitivity to ferroptosis.A further study [35]found that erastin upregulated p53 expression,which subsequently downregulated SCL7A11 expression and thus inhibited the system Xc-,leading to ferroptosis.p53 down-regulates SLC7A11 and inhibits the system Xc-uptake of cystine and interferes with GSH synthesis to induce ferroptosis [36].
Nrf2-Keap1 induces ferroptosis via the system Xc-
Although the protective effect of Nrf2 on cell death has been considered for a long time,the Nrf2-mediated regulation of ferroptosis has only been studied recently.The kelch-like ECH-associated protein 1 (Keap1)-Nrf2 system is the main response mechanism of the body against oxidative stress injury.Under normal cellular conditions,intracellular Nrf2 binds specifically to Keap1,which leads to Nrf2 activity inhibition.But during cellular injury,Keap1 dissociation activates Nrf2,which in turn activates the downstream GPX4 levels and enhances the antioxidant capacity of tissues,protecting them from oxidative stress injury [37].Fan et al.[38]found that cancer cells with inhibited Nrf2 expression were susceptible to ferroptosis inducers,whereas those with increased Nrf2 expression resisted the onset and execution of ferroptosis by upregulating the system Xc-.Meanwhile,Gai et al.[39]found that erastin and acetaminophen could synergistically inhibit the expression of Nrf2 in non-small cell lung cancer,thereby inhibiting the system Xc-and inducing ferroptosis.Thus,Nrf2 regulates ferroptosis through the system Xc-.
Inhibition of GPX4 induces ferroptosis
GPX4 is a membrane lipid repair enzyme that reduces phospholipid hydrogen peroxide and H2O2in membranes to the corresponding alcohol or water [40].A few compounds,such as Rasselective lethal small molecule 3 (RSL3),can inhibit the GPX4 function,leading to ferroptosis [40].GPX4 inhibition promotes the accumulation of lipid peroxides and induces ferroptosis.Park et al.[41]found that GPX4 inhibition during myocardial infarction results in the accumulation of lipid peroxides,which leads to ferroptosis in cardiomyocytes.Jin et al.[42]found that solanine inhibits GPX4 activity and increases lipid ROS levels to induce ferroptosis.Hu et al.[43]demonstrated that GPX4 protects hematopoietic stem cells and inhibits lipid peroxidation and ferroptosis.In addition,selenocysteine,which is one of the amino acids at the center of the GPX4 activity and is embedded in GPX4 via the transporter-selenocysteine tRNA,maintains the GPX4 activity and exerts a scavenging effect on lipid ROS,thereby inhibiting ferroptosis [44].
Iron overload induces ferroptosis
Iron overload is one of the main factors for the induction of ferroptosis.Excess iron is deposited in tissues and organs,which may cause a variety of organ dysfunction.Hereditary hemochromatosis,ferroportin disease,ceruloplasmin deficiency,and others are common causes of iron overload.Further,the common acquired factors include excessive iron intake,repeated blood transfusion,and chronic liver disease [45].Serum iron overload can saturate transferrin,which leads to Fe3+ions binding to toxic non-transferrin and is more likely to be absorbed by organs such as liver and heart.Excess Fe2+ions in cells increase the ROS production and oxidation of macromolecules,especially lipid molecules [e.g.,polyunsaturated fatty acids (PUFA)],which result in the Fenton reaction,thereby promoting the development of molecules such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HN),which can cause damage to mitochondria and other cellular organelles [46].Excess iron also promotes hepatocytic apoptosis by down-regulating nuclear factor kappa B (NF-κB) and releasing cytochrome C.Ferritin is the main iron storage protein complex in cells and includes ferritin light polypeptide 1 (FTL1) and ferritin heavy polypeptide 1(FTH1) subunits [47].Excess Fe2+is stored in ferritin to form an unstable iron pool.FTL1 and FTH1 can be degraded by autophagy,releasing large amounts of free Fe2+ions and increasing intracellular iron levels.This increase in intracellular iron leads to disturbed iron homeostasis,triggering ferroptosis.
Promotion of ACSL4/LPCTA3/ALOX15 lipid metabolism pathway induces ferroptosis
Ferroptosis is characterized by the iron-dependent accumulation of lipid peroxides.As per the recent study,the accumulation of PUFA is a marker of ferroptosis,and the intracellular PUFA content determines the extent of ferroptosis in cells [48].Free PUFA is a substrate for the synthesis of lipid signaling transmitters,but it must be esterified to membrane phospholipids and oxidized to cause ferroptosis.Lipidomics studies have shown that phosphatidylethanolamine (PE) containing arachidonic acid (AA) or adrenic acid (ADA) is the key phospholipid that undergoes oxidation and drives cells toward ferroptosis [49].Therefore,decreasing the monounsaturated fatty acid (MUFA) content and increasing the PUFA content could promote the progression of lipid peroxidation process-induced ferroptosis.ACSL4 and LPCAT3 are key enzymes that regulate ferroptosis.ACSL4 is a member of the long-chain lipoyl CoA synthase [acyl-coenzymeA (CoA) synthetase;ACSL]family and is involved in fatty acid metabolism.In the first step,ACSL4 converts AA and ADA into arachidonic CoA (AA-CoA) and adrenoyl CoA (AdACoA),respectively.AA-CoA and AdACoA are then used in the synthesis of phosphatidyl CoA via lysophosphatidylcholine acyltransferase3 (LPCAT3),which is involved in the synthesis of membrane phospholipids such as PE.The membrane long-chain polyunsaturated fatty acids can often be oxidized by ferrous ions and acted upon by lipoxygenase to generate PE-AA-OOH and PEAdA-OOH,which induce ferroptosis in cells.The absence of ACSL4 and LPCAT3 affects cells positively by inhibiting cellular ferroptosis [50].Therefore,the ACSL4/LPCTA3/ALOX15 pathway is an important signaling pathway for lipid peroxidation-induced ferroptosis.
Inhibition of NAD(P)H/FSP1/CoQ10 pathway induces ferroptosis
The NAD(P)H/FSP1/CoQ10 pathway is another antioxidant pathway parallel to GPX4.Ferroptosis inhibitor protein 1 (FSP1) is a key protein to resist ferroptosis [51].FSP1 reduces ubiquinone (CoQ)to ubiquinol (CoQH2),a powerful antioxidant that prevents lipid peroxidation in the plasma membrane [52].Therefore,inhibition of NAD(P)H/FSP1/CoQ10 pathway expression could regulate ferroptosis.However,the NAD(P)H/FSP1/CoQ10 system as another central system for ferroptosis regulation needs to be further studied.
The DHODH-CoQH2 system inhibits ferroptosis
DHODH is a flavin-dependent enzyme in the inner mitochondrial membrane that catalyzes the fourth step of pyrimidine nucleotide synthesis [53,54].CoQH2 is a radical-trapping antioxidant that inhibits lipid peroxidation in mitochondria [55].DHODH has been shown to inhibit ferroptosis by regulating CoQH2 production in the inner mitochondrial membrane [17].DHODH knockdown leads to RSL3-induced ferroptosis by increasing the CoQ/CoQH2 ratio while DHODH inactivation induces extensive mitochondrial lipid peroxidation and ferroptosis in cancer cells with reduced GPX4 expression,thus identifying a defense mechanism for DHODH-mediated mitochondrial ferroptosis [17].Therefore,the DHODH-CoQH2 pathway has an important antioxidant function in inhibiting ferroptosis,and reducing the expression of DHODH may be a new strategy to induce ferroptosis in cancer treatment.
Ferritinophagy and ferroptosis-related assays
Iron metabolism involves complex regulatory mechanisms and multiple signaling and metabolic pathways.At present,there are various methods and strategies to study ferroptosis.Table 2 summarizes the commonly used assays for studying ferroptosis [23,56-67].Each method has advantages and disadvantages,and hence depending on the requirement and aim of a particular study,an appropriate method can be selected to study ferroptosis accurately.It is expected that in the future,the most appropriate method will be developed to better understand the regulatory mechanism of ferroptosis and its role in the treatment of diseases.
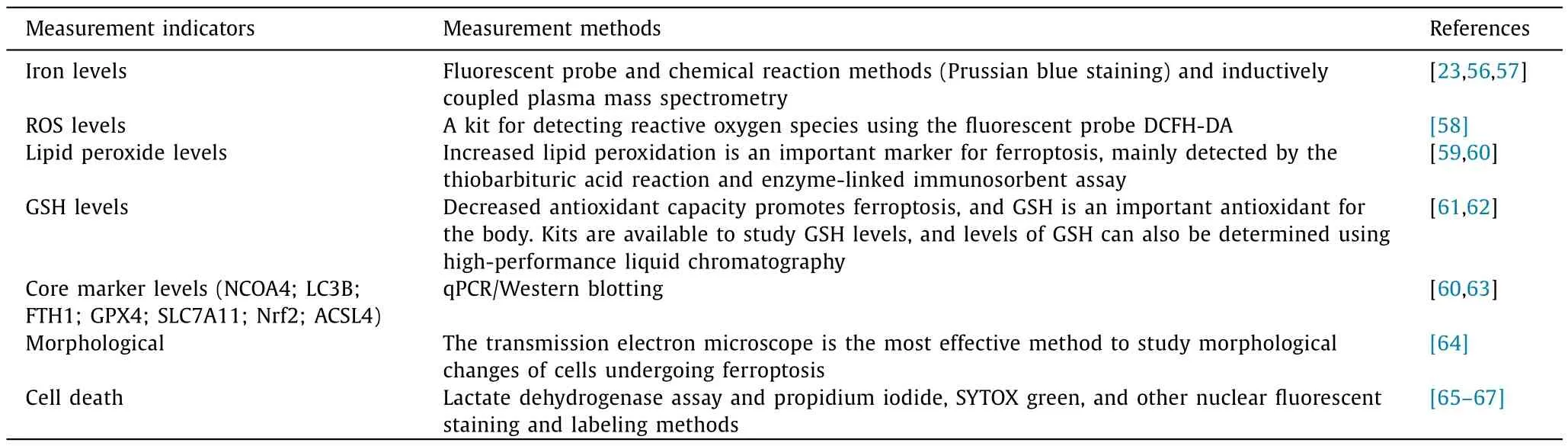
Table 2Main methods of ferroptosis detection.
Liver diseases and ferroptosis
Liver cells are the main site of iron storage in the body.A clinical threshold of ≥15 mg/g of iron in liver tissue is associated with an increased risk of cirrhosis due to increased iron content in liver tissue.It has been found that iron metabolism disorders and lipid peroxide accumulation are characteristics of ferroptosis at different stages of liver disease development,suggesting that iron metabolism plays an important regulatory role in the development of liver disease (Fig.3).The vast majority of studies on liver diseases and iron metabolism are currently focused on ferroptosis,with less research on ferritinophagy and liver diseases.The study of ferritinophagy heralds a new era in the regulation of ferroptosis,and a comprehensive study of the regulatory mechanisms of ferroptosis and ferritinophagy is a new orientation for the study of liver diseases.
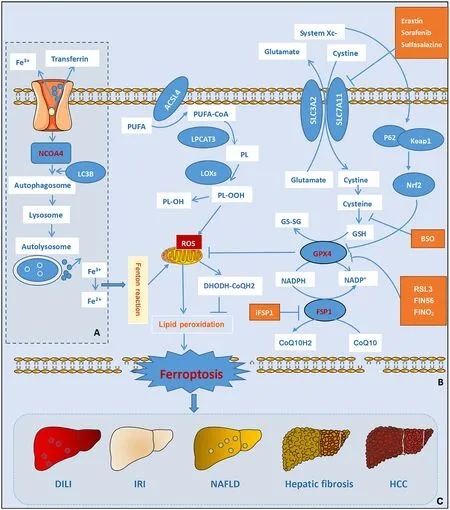
Fig.3. Schematic diagram of the signaling pathway of ferritinophagy and ferroptosis.A:Ferritinophagy;B:ferroptosis;C:liver diseases regulated by ferroptosis.NCOA4:nuclear receptor co-activator 4;LC3B: light chain 3B;PUFA: polyunsaturated fatty acid;PL: phospholipid;PL-OOH: phospholipid hydroperoxides;PL-OH: phospholipid alcohol;ACSL4: acyl-CoA synthetase long-chain family member 4;LPCAT3: lysophosphatidylcholine acyltransferase 3;LOXs: lipoxygenases;Vit E: vitamin E;ROS: reactive oxygen species;DHODH: dihydroorotate dehydrogenase;CoQH2: ubiquinol;GSH: glutathione;GS-SG: glutathione disulfide;GPX4: glutathione peroxidase 4;Keap 1: kelchlike ECH-associated protein 1;Nrf2: nuclear factor (erythroid-derived)-like 2;NADPH: nicotinamide adenine dinucleotide 2' -phosphate reduced tetrasodium salt;NADP:nicotinamide adenine dinucleotide phosphate;FSP1: ferroptosis suppressor protein 1;CoQ10: coenzyme Q10;AA: arachidonicacid;AA-CoA: acetoacetyl-CoA;PE: phosphatidylethanolamine;RSL3: RAS-selective lethal 3;FIN56: ferroptosis-inducing compound 56;SLC3A2: solute carrier 3A2;SLC7A11: solute carrier 7A11;HCC: hepatocellular carcinoma;NAFLD: nonalcoholic fatty liver disease;DILI: drug-induced liver injury;IRI: ischemia-reperfusion injury.
Drug-induced liver injury (DILI) and ferroptosis
In Europe and USA,DILI is the leading cause of the acute liver disease (ALD),and drug development failure and withdrawal from the market.At present,we are exposed to more than 30000 kinds of drugs and healthcare products in our daily life.Among them,more than 1000 kinds of drugs cause DILI.Among DILI,acetaminophen-induced liver injury is more typical [68].Yamada et al.[69]found that ALD induced by acetaminophen was associated with ferroptosis driven byω-6 PUFA.Acetaminophen acts in the liver and is converted to hepatotoxic product through its reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI),which ultimately leads to hepatocyte death [70].A previous study of cell membrane damage by ferroptosis has shown that cytochrome P450 oxidoreductase POR detoxifies xenobiotics by upregulating the peroxidation of PUFAs,and NAPQI binds to GSH and severely depletes the GSH in hepatocytes [71].A recent study shows that Maresin1 inhibits ferroptosis-induced liver injury by suppressing ROS production and Nrf2/HO-1/GPX4 biological pathway activity in a mouse model of galactosamine/lipopolysaccharide-induced acute liver injury [63].Thus,it is clear that ferroptosis is justified as a new way to treat DILI.
Ischemia-reperfusion injury (IRI) and ferroptosis
IRI occurs when the blood returns to the original supply tissue(reperfusion) after a period of tissue hypoxia and ischemia [72].Liver IRI can be caused by shock (e.g.,sepsis and hemorrhage) or liver surgery [72].Accumulating evidence suggests that complex networks involving various types of cell death,including apoptosis and necroptosis,contribute to hepatic IRI,and that ferroptosis is related to the IRI pathogenesis.Targeting ferroptosis may be a promising therapeutic approach [73].In 2014,Friedmann Angeli et al.[74]found that ferroptosis inhibitors protect against liver injury in a mouse model of IRI-induced liver injury,and experimental data demonstrate that ferroptosis is a potential regulatory mechanism in hepatic IRI.IRI-induced liver injury is largely caused by ferroptosis induced by GPX4 inactivation,and ferroptosis inhibitors can reverse liver injury and improve liver function [74].This implies that IRI injury induces ferroptosis in the liver through enhanced lipid peroxidation by iron overload [75].
NAFLD and ferroptosis
The incidence of NAFLD is on the rise [76].In early 2020,an international expert consensus on a new definition of metabolic dysfunction-associated fatty liver disease (MAFLD) was published by an international panel of 30 experts from 22 countries,recommending the use of "MAFLD" instead of "NAFLD".Based on the use of NAFLD in a large body of literature,this term is still used in this review.Lipid peroxide accumulation contributes to the pathogenesis of NAFLD,and metabolic disorders cause iron deposition [77].Patients with NAFLD usually have excessive iron levels,and ironinduced lipid peroxidation contributes to the onset of the disease [78].More than 90% of patients with NAFLD have high levels of lipid peroxidation markers,such as MDA and 4-HNE.Among patients with nonalcoholic steatohepatitis (NASH),MDA and 4-HNE are much higher than those in patients with NAFLD [79].A study has elucidated the mechanism of ferritinophagy inhibition of hepatocyte senescence through NCOA4 regulation in NAFLD,providing a promising option for cellular senescence treatment of NAFLD [80].Ferroptosis is the first striking event that triggers steatohepatitis and precedes other regulatory cell deaths [81].In animal models fed with choline-deficient,ethionine-supplemented (CDE) diet,ferroptosis inhibitors such as vitamin E inhibit cell death and inflammation during NASH.Recent advances in research have provided new insights into the molecular mechanisms of ferroptosis,particularly the relationship between ferroptosis and cellular metabolism,but many key questions remain to be addressed,for example,what are the molecules that ultimately induce ferroptosis? How does lipid peroxide aggregation trigger ferroptosis? How do the major metabolic processes that regulate ferroptosis such as iron,amino acid,and lipid metabolism interconnect and ultimately determine whether the cell survives or dies of iron?
Liver fibrosis and ferroptosis
Hepatic stellate cells and excessive extracellular matrix (ECM)deposition are the major pathological features of liver fibrosis.It damages the normal structure and function of the liver by excessive proliferation of fibrous connective tissue around the portal vein [82].A key component of liver fibrosis treatment is inhibiting HSC activation and proliferation.Ferroptosis caused by disorders of iron metabolism is closely related to HSC activation.A recent study showed that hepatic transferrin (TRF) could inhibit liver injury,fibrosis,and cirrhosis by regulating ferroptosis [56].Researchers constructed a hepatocyteTRFknockout mouse (TRFLKO) model and a hepatocyteTRFgene andSLC39A14gene double knockout (DKO) mouse model and found that TRF-LKO mice exhibited hepatic non-TRF-bound iron (NTBI) accumulation and liver fibrosis.In contrast,DKO mice significantly reduced the accumulation of hepatic NTBI,thereby reducing ferroptosis and liver fibrosis induced by a high iron diet or CCl4injection.Clinical data support the role of TRF in preventing liver fibrosis by blocking ferroptosis,which provides a possible therapeutic target for the treatment of liver fibrosis [56].It was found that the upregulation of p53 inhibits SLC7A11,which indirectly reduces the antioxidant effect of GPX4,promoting ferroptosis in HSC and improving liver fibrosis [83].In addition to the ferroptosis mechanism associated with HSC regulation,TRF and heme oxygenase-1 (HO-1),a serum-rich metal-binding protein,are also involved in the liver fibrosis process,and the accumulation of NTBI in the liver caused by knocking downTRFexpression in mouse hepatocytes further exacerbates the iron-rich diet-mediated liver fibrosis [84].Similarly,magnesium isoglycyrrhizinate-induced HO-1 upregulation leads to HSC ferroptosis by promoting intracellular iron and lipid peroxide accumulation in hepatic fibrosis rats,whereas the antifibrotic effect of magnesium isoglycyrrhizinate was abolished when Ferrostatin-1 was used or HO-1 was knocked down [85].These studies suggest that the induction of ferroptosis in HSC may be a viable option for the treatment and prevention of liver fibrosis development.However,the question remains as to how HSC can maintain a benign relationship between the induction of ferroptosis and the prognostic level of the disease so that this strategy can minimize the impact on healthy cells.
HCC and ferroptosis
HCC is characterized by high malignancy,rapid progression,and poor prognosis.Liver cancer is the second leading cause of cancerrelated death throughout the world and its incidence is steadily increasing [86].Despite treatments such as resection,radiofrequency ablation,radiation therapy,and chemotherapy,liver cancer cells can still evade death,continuing to proliferate,invade,and metastasize.This has led to recent investigations of molecular-targeted therapy for HCC.Tumor cells have higher iron demands than normal cells as iron is required for increased growth and metabolic needs.The resultant increase in iron uptake also leads to elevated levels of oxidative stress [87].To compensate for the increases in oxidative stress,tumor cells tend to activate and upregulate the transcription and expression of antioxidant factors and genes,including GPX4 and SLC7A11.Guo et al.found that SLC7A11 was highly expressed in HCC tissues and cells compared to that in normal tissues and cells,and that inhibition of SLC7A11 reduced the growth of HCC cells in vivo and in vitro [88].Transcriptomic analysis of the association between SLC7A11 and the clinical features of HCC suggested that SLC7A11 has prognostic value in HCC [89].Guerriero et al.[90]demonstrated that increased expression of GPX4 was associated with higher grades of malignancy in HCC patients.ACSL4 is also an important factor in predicting the sensitivity of cells to ferroptosis.ACSL4 expression is elevated in ferroptosis-sensitive cells,and hepatic ACSL4 expression is higher in patients with a complete or partial response to sorafenib compared with patients with a poor response to sorafenib [91].As a key licensed first-line therapy for advanced HCC,sorafenib can improve patient survival rates,and the functional relationship between drug resistance and ferroptosis has recently attracted wide attention [92].A previous study has shown that MT-1 G is both an important regulator of ferroptosis and a target of sorafenib resistance in human liver cancer cells [93].MT-1 G is reported to negatively regulate ferroptosis,which can increase the resistance of liver cancer cells to sorafenib,leading to poor prognosis in patients with liver cancer [94].Reduced expression of MT-1 G increases GSH depletion and lipid peroxidation,thereby promoting sorafenib-induced ferroptosis.The expression of MT-1 G was found to be significantly upregulated in HCC cells after treatment with sorafenib.MT-1 G inhibits ferroptosis by preventing lipid peroxidation mediated by GSH depletion,a process that can promote sorafenib resistance in liver cancer cells [93,95],and down-regulation of Nrf2 and MT-1 G expression may provide a promising strategy for mitigating sorafenib resistance.
Summary and prospect
The concept of induced ferroptosis therapy is recently gaining attention for the treatment of liver diseases,particularly in liver cancer treatment.With continuous research in this field,it is expected that more details on the process of ferroptosis will emerge.It is important to have an in-depth knowledge of the induction of ferroptosis under various pathological conditions,which will help in developing therapeutic interventions for treating liver-related diseases.The followings are the main aspects related to the process of ferroptosis requiring further studies: (1) the identification of ways for better differentiating various cell death modes and the mechanisms of interaction between different death modes;(2) the development of more specific assays and methods to study ferroptosis;(3) the role of iron and lipoxygenase in triggering or propagating lipid peroxidation during iron metabolism;(4) identifying pathways (if any) where ferroptosis imposes an inhibitory effect;and (5) the mode of ferroptosis action (active or passive).
Acknowledgments
None.
CRediT authorship contribution statement
Zi-Bing Qian:Data curation,Formal analysis,Writing -original draft.Jun-Feng Li:Funding acquisition,Supervision,Writing -review &editing.Wan-Yuan Xiong:Conceptualization.Xiao-Rong Mao:Funding acquisition,Supervision,Writing -review &editing.
Funding
This study was supported by grants from the National Natural Science Foundation of China (82360132),the Natural Science Foundation of Gansu Province (20JR5RA364),the Fund of the First Hospital of Lanzhou University (ldyyyn2020-02,ldyyyn2020-14),Gansu Clinical Medical Research Center of Infection &Liver Diseases (21JR7RA392),the Natural Science Foundation of Gansu Province (21JR1RA070),and Lanzhou Science and Technology Planning Project (2023-2-76).
Ethical approval
Not needed.
Competing interestNo benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2024年2期
Hepatobiliary & Pancreatic Diseases International2024年2期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Editors
- Information for Readers
- Meetings and Courses
- Liver transplantation and liver resection as alternative treatments for primary hepatobiliary and secondary liver tumors: Competitors or allies?
- Laparoscopic anatomical liver resection of segment 7 using a sandwich approach to the right hepatic vein (with video)
- Severe liver injury and clinical characteristics of occupational exposure to 2-amino-5-chloro-N,3-dimethylbenzamide: A case series
