Diabetes and high-glucose could upregulate the expression of receptor for activated C kinase 1 in retina
Jian Tan,Ang Xiao,Lin Yang,Yu-Lin Tao,Yi Shao,Qiong Zhou
Abstract BACKGROUND Diabetic retinopathy (DR) is a major ocular complication of diabetes mellitus,leading to visual impairment.Retinal pigment epithelium (RPE) injury is a key component of the outer blood retinal barrier,and its damage is an important indicator of DR.Receptor for activated C kinase 1 (RACK1) activates protein kinase C-ε (PKC-ε) to promote the generation of reactive oxygen species (ROS) in RPE cells,leading to apoptosis.Therefore,we hypothesize that the activation of RACK1 under hypoxic/high-glucose conditions may promote RPE cell apoptosis by modulating PKC-ε/ROS,thereby disrupting the barrier effect of the outer blood retinal barrier and contributing to the progression of DR.AIM To investigate the role and associated underlying mechanisms of RACK1 in the development of early DR.METHODS In this study,Sprague-Dawley rats and adult RPE cell line-19 (ARPE-19) cells were used as in vivo and in vitro models,respectively,to explore the role of RACK1 in mediating PKC-ε in early DR.Furthermore,the impact of RACK1 on apoptosis and barrier function of RPE cells was also investigated in the former model.RESULTS Streptozotocin-induced diabetic rats showed increased apoptosis and upregulated expression of RACK1 and PKC-ε proteins in RPE cells following a prolonged modeling.Similarly,ARPE-19 cells exposed to high glucose and hypoxia displayed elevated mRNA and protein levels of RACK1 and PKC-ε,accompanied by an increases in ROS production,apoptosis rate,and monolayer permeability.However,silencing RACK1 significantly downregulated the expression of PKC-ε and ROS,reduced cell apoptosis and permeability,and protected barrier function.CONCLUSION RACK1 plays a significant role in the development of early DR and might serve as a potential therapeutic target for DR by regulating RPE apoptosis and barrier function.
Key Words: Diabetic retinopathy;Receptor for activated C kinase 1;Protein kinase C-ε;Adult retinal pigment epithelium cell line-19
lNTRODUCTlON
Diabetic retinopathy (DR) is the main ocular complication of diabetes mellitus (DM) and a common cause of visual impairment and blindness.The International Diabetes Federation predicts that the number of DM patients worldwide will increase from 460 million in 2019 to 700 million in 2045[1],and approximately 30% of DM patients will eventually develop DR[2].Without effective treatment,DR patients will experience visual impairment or even blindness;hence,precise treatment in the early stage of DR is particularly important[3].Retinal pigment epithelium (RPE) is the main component of the outer blood-retinal barrier (oBRB),and leakage caused by blood-retinal barrier injury is a sign of DR[4].However,to date,research on DR has mostly focused on the internal retinal barrier,while research on the damage mechanism of the oBRB in diabetes is limited.
In the early stage,through bioinformatics analysis of retinal tissue samples of DR patients and normal people without diabetes,our research team obtained four hub genes and found that only the receptor for activated C kinase 1 (RACK1) was the most highly and differentially expressed (P=0.003) hub gene (P=0.003)[5].RACK1 is a multifunctional signal transduction protein,also known as the anchoring protein of protein kinase C (PKC)[6].PKC is a serine/threonine kinase involved in signal transduction that can respond to the stimulation of specific hormones,neurons,and growth factors[7].The PKC family consists of 12 subtypes,among which PKC-α,-β,-δ,and-ε are activated and play an important role in the occurrence and development of DR[7,8].Among them,PKC-ε can enhance the activity of nicotinamide adenine dinucleotide phosphate oxidase,thus promoting the production of reactive oxygen species (ROS) in RPE cells.The overaccumulation of ROS induces mitochondrial damage,apoptosis,inflammation,lipid peroxidation,and structural and functional changes in the retina[9].
Based on the above-mentioned research,we hypothesized that the activation of RACK1 under hypoxic/high-glucose (HG) conditions might promote apoptosis and migration of RPE cells by modulating PKC-ε/ROS,thereby disrupting the oBRB and leading to the progression of early DR.Therefore,this study aims to investigate the impact of knockdown RACK1 on alleviating PKC-ε/ROS induced damage to the oBRB,thus delaying the progression of early DR.RPE cells are highly polarized monolayer cells that are usually induced by HG to simulate the DR environment[10].Considering that HG concentration and hypoxia are the two main components in the diabetic environment,we used both hypoxia and HG concentration to simulate this environment[9].
MATERlALS AND METHODS
Animal model
All male Sprague-Dawley rats (8 wk old,weighing 180-220 g) were purchased from the Animal Center of Nanchang University.All rats were housed in standard rat cages under standardized environmental conditions with controlled temperature (23 ± 2 °C),humidity (50%),and a 12-h light/dark cycle.The diabetic group received intraperitoneal injection of streptozotocin (STZ) (60 mg/kg body weight,Sigma-Aldrich;Merck Millipore,Darmstadt,Germany) dissolved in citrate buffer (pH 4.5),while the control group received an equivalent volume of citrate buffer.Rats were considered diabetic if their blood glucose levels exceeded 16.7 mmol/L 72 h after STZ injection and remained elevated for 1 wk.A total of 24 rats were included in the study,with 12 rats in each group.The rats were raised for 8 or 10 wk (n=6 per group).All experiments were conducted according to the guidelines for the care and use of laboratory animals and approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University.
Hematoxylin and eosin staining of the retina
Six rats from each group were sacrificed,and their eyeballs were harvested at 8 and 10 wk after successful modeling.The eyeballs were then fixed in a 20% paraformaldehyde solution at 4 ℃ for 2 h.Subsequently,the samples were sectioned into 5 μm slices,stained with hematoxylin and eosin,and examined under a light microscope (magnification,400 ×;Zeiss,OberCoring,Germany),to determine the number of RPE cells in the samples.
Cells and culture
Adult RPE cell line-19 (ARPE-19) cells were purchased from Procell (Wuhan,China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (containing 5.5 mM glucose) (Procell,Wuhan,China) supplemented with 10% fetal bovine serum (HyClone,Logan,UT,United States) and 1% penicillin/streptomycin (Thermo Fisher) in 5% CO2at 37 ℃.The HG and hypoxia cell model was chemically induced by the adjustment of the glucose concentration of the culture medium to 25 mM,while 400 μM cobalt chloride (CoCl2) (Merck,Germany) was added to the cell culture medium for 24 h before the experiment.
Small interfering RNA transfection
The RACK1-specific small interfering RNA (siRNA) (5′-3′GTCTCTGGATCTCGAGATA) used in this study was obtained from Ruibo RIBOBIO (China).Transfection was performed in ARPE-19 cells when they reached 50%-70% confluency.The transfection was carried out using Lipofectamine 2000 (Invitrogen) with 100 nmol of RACK1 siRNA,and the medium was replaced with fresh medium 4-6 h after transfection.Transfected ARPE-19 cells were subsequently cultured for 48 h for mRNA experiments or 72 h for cell function and protein expression experiments.
Flow cytometry
ARPE-19 cells were grown in DMEM supplemented with 10% FBS for 12 h in a six-well plate,followed by treatment with HG and hypoxia for 24 h.The treated cells were collected (1 × 105cells/mL) after digestion with pancreatic enzyme (Solarbio,Beijing,China) without ethylenediaminetetraacetic acid and then washed twice with pre-cooled phosphatebuffered saline (PBS).The cells were resuspended in 100 μL of binding buffer and stained with 5 μL Annexin V-FITC and 10 μL propidium iodide (Yeasen,Shanghai,China) for 15 min while protected from light for 15 min.Subsequently,400 μL of binding buffer was added to resuspend the cells.The percentage of apoptotic cells was analyzed by flow cytometry (BD,FACSCalibur,United States).
Real-time quantitative polymerase chain reaction
Total RNA was extracted at room temperature using TRIzol reagent (Invitrogen;Thermo Fisher Scientific) and immediately reverse transcribed or stored at -80 ℃ as needed.mRNA was reverse transcribed into cDNA using the Strand cDNA Synthesis SuperMix for quantitative polymerase chain reaction (qPCR) kit (11141ES60,Yeasen,China) and subsequently quantified using specific primers from the SANGON primer group (Shanghai,China) for each mRNA.Realtime qPCR (RT-qPCR) was performed using the ABI PRISM 145 real-time polymerase chain reaction system (Applied Biosystems;Thermo Fisher Scientific) that used the Quick Start General SYBR Green (Roche,Basel,Switzerland).The cycle threshold (Ct) values were obtained,and relative mRNA expression levels were calculated based on the 2-ΔΔCtmethod.The oligonucleotide sequences of RT-qPCR primers are listed in Table 1.
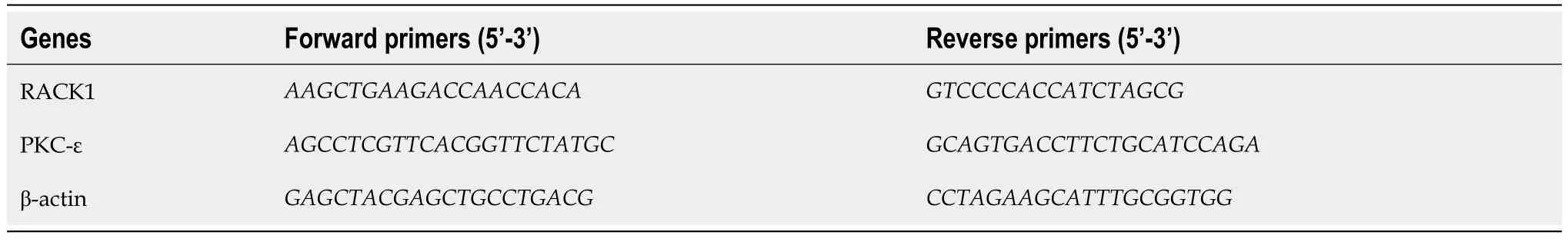
Table 1 Primer sequences for quantitative reverse transcription-polymerase chain reaction
Western blotting analysis
Crushed tissue or cells were lysed using a buffer (Thermo Fisher Scientific,Waltham,United States) to extract proteins.The protein extracts were subsequently separated using 10% SDS-PAGE,and the separated proteins were transferred onto a PVDF membrane (Amersham,Cytiva,Germany).The membrane was incubated with 10% skim milk powder for 2 h to ensure its proper sealing.The membrane was then incubated overnight at 4 °C with a primary antibody for GAPDH (1:1000,Abcam),along with RACK1 and PKC-ε,to detect the target protein.The following day,a secondary antibody (1:5000,Abcam) conjugated with horseradish peroxidase was incubated with the membrane at room temperature for 1.5 h.Finally,the membrane was treated with an ECL reagent (Amersham Pharmacia Biotech,Inc.,United States),and the protein bands were visualized using ImageJ software.
Determination of intracellular ROS
ROS were measured by assessing the intracellular peroxide-dependent oxidation of 2',7'-Dichlorodihydrofluorescein diacetate (DCFH-DA) that produces the fluorescent compound 2′,7′-dichlorofluorescein.Cells were seeded in 24-well plates at a density of 2 × 104cells per well and cultured for 24 h.After two washes with PBS,the cells were treated with fresh medium containing 25 mM glucose and 400 μM CoCl2and incubated for an additional 24 h.Subsequently,20 μM DCFH-DA was added,and the cells were incubated for 30 min at 37 °C.After two more washes with PBS,400 μL of PBS was added to each well,and the fluorescence intensity was measured using a TECAN SPARK 450M (Tecan Group,Ltd.,Manedoff,Switzerland).
Monolayer permeability assay
A 0.4-μm pore polycarbonate membrane insert with a 6.5-mm Transwell assay format (3413,Corning,NY,United States) was used to assess the vascular permeability of ARPE-19 cells.In each well,105 cells in 200 μL of complete medium were transferred to the top chamber,while the bottom chamber was filled with 500 μL of the same medium.To conduct the permeability assays,the top chamber was loaded with 100 μL of a 1 mg/mL solution of fluorescein isothiocyanate dextran (FITC-dextran) (40 kDa,FD40,Sigma-Aldrich,St.Louis,MO,United States),while the bottom chamber was filled with 500 μL of PBS.Following a 30-min incubation in the darkroom,100 μL samples were collected from the bottom chamber and plated onto 96-well plates.The leakage of FITC-dextran was subsequently analyzed using a TECAN SPARK 450M (Tecan Group,Ltd.,Manedoff,Switzerland),with an excitation wavelength of 490 nm and an emission wavelength of 520 nm.
Statistical analysis
A minimum of three repetitions were conducted for each experiment.All data were presented as mean ± SD.Statistical analyses were performed using Prism 9.0 software (GraphPad Software,San Diego,CA,United States) with Student’sttest and one-way ANOVA.P< 0.05 indicated statistical significance.
RESULTS
STZ-induced rats exhibited a typical diabetic phenotype
In our study,an animal model of diabetes was established through STZ injection.Both rat body weight and blood glucose levels were measured at weeks 0,2,4,6,8,and 10 to assess the progression of diabetes (Figure 1).The results showed a significant increase in blood glucose levels in diabetic rats,which was > 4 times higher than those in the normal control group throughout the study.Additionally,diabetic rats experienced significant weight loss compared to the normal control group.
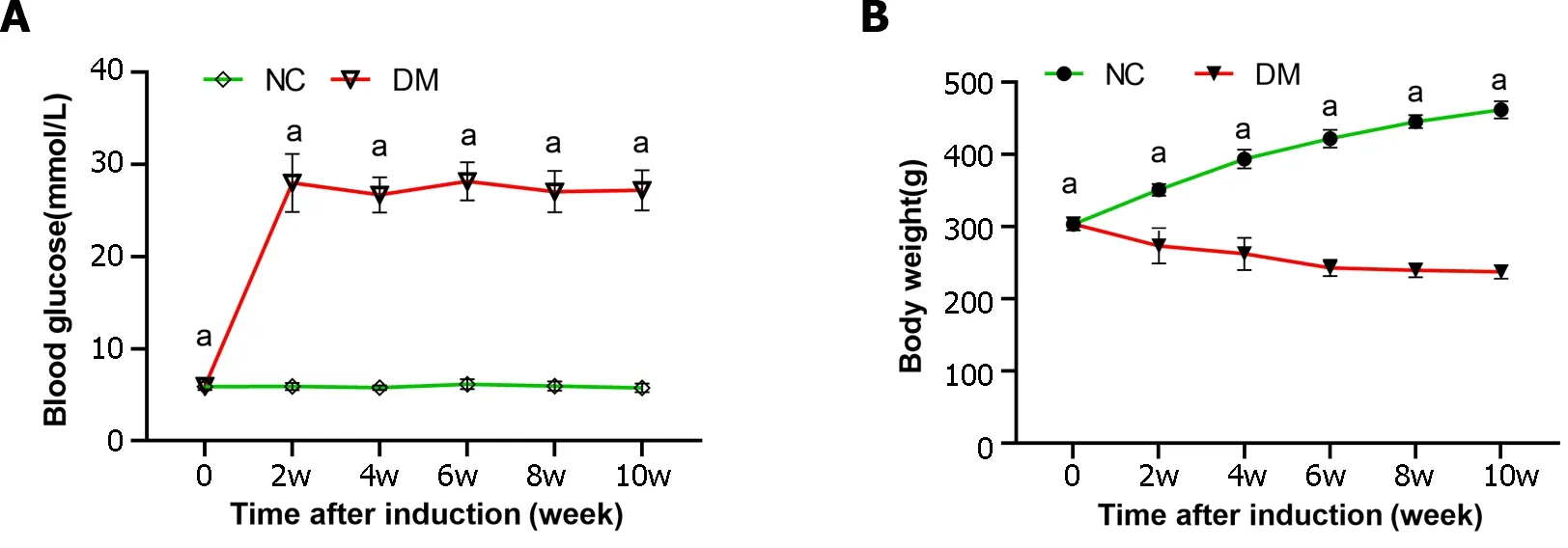
Figure 1 Blood glucose levels and body weight of rats following intraperitoneal injection of streptozotocin (65 mg/kg) (pH 4.5) in citrate buffer. A: Blood glucose level;B: Body weight.The results are presented as the mean ± SD.aP < 0.05 vs control group (n=6 in each group).NC: Normal control;DM: Diabetes mellitus.
In this study,we examined the number of cells in the RPE layer in each group at various time points following STZ injection.Cell counting was performed at 0,2,4,6,8,and 10 wk post-injection (Figure 2).There was no significant difference in the number of RPE cells between the control and diabetic groups at 0,2,4,and 6 wk after STZ injection (P> 0.01).However,at weeks 8 and 10,a notable decrease in the number of RPE cells was observed in the diabetic group.Furthermore,a significant decrease in the number of RPE cells was observed in the diabetic group compared to the normal control group at week 8 and 10(P< 0.01).These findings suggested a progressive loss of RPE cells in the diabetic group over time.
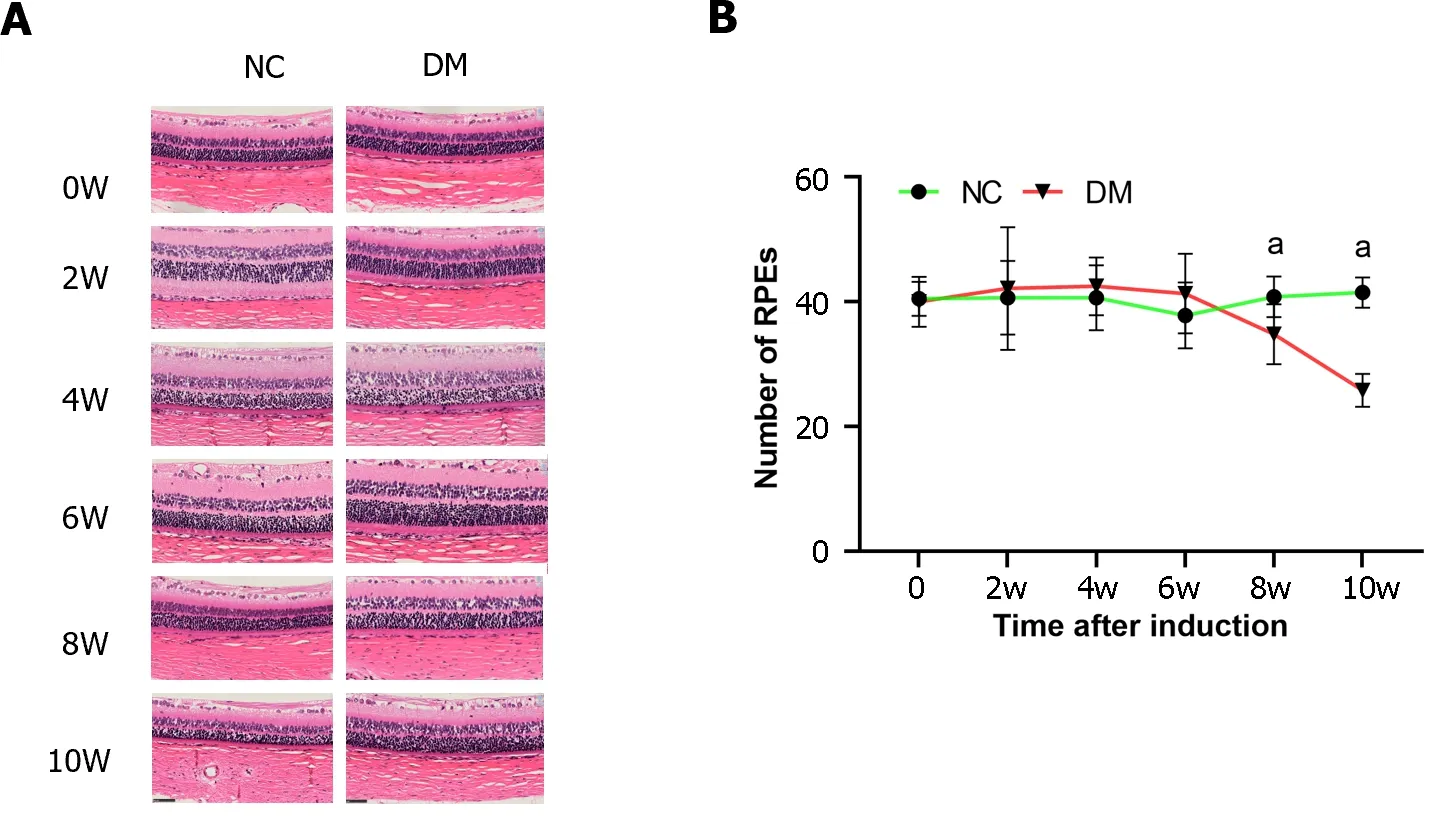
Figure 2 Retinal hematoxylin and eosin staining (magnification,400) and retinal pigment epithelium cell counts were performed in normal and diabetic rats. A: The hematoxylin and eosin staining of rat retina;B: The quantification of retinal pigment epithelium cells.The results are expressed as the mean ± SD.aP < 0.05 vs control group.NC: Normal control;DM: Diabetes mellitus.
Aberrant expression of RACK1 and PKC- ε in the retina of diabetic rats
The protein levels of RACK1 and PKC-ε in retinal tissues of normal and diabetic rats were assessed using western blot analysis at weeks 8 and 10 following STZ injection (Figure 3).The findings revealed that the protein levels of RACK1 and PKC-ε were significantly elevated in the retinal tissues of diabetic rats as compared to the normal group (P< 0.05).
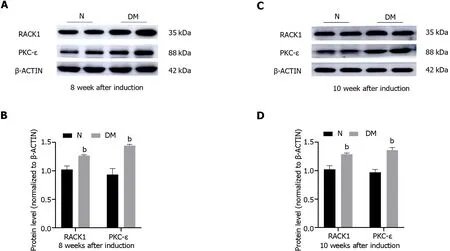
Figure 3 Aberrant expression of receptor for activated C kinase 1 and protein kinase C-ε in the retina of diabetic rats. A and B: The protein levels of receptor for activated C kinase 1 and protein kinase C-ε in the retinas of N and DM rats were measured at 8 wk following streptozotocin injection;C and D: The protein levels of receptor for activated C kinase 1 and protein kinase C-ε in the retinas of N and DM rats were measured at 10 wk following streptozotocin injection.The results are expressed as the mean ± SD.bP < 0.01 vs control group.N: Normal control;DM: Diabetes mellitus;8 W: 8 wk;10W: 10 wk;RACK1: Receptor for activated C kinase 1;PKC-ε: Protein kinase C-ε.
HG combined with hypoxia up-regulated transcription and increased protein levels of RACK1 and PKC-ε in ARPE-19 cells
ARPE-19 cells were subjected to HG hypoxic conditions to mimic diabetesin vitro.The results indicated that the transcription and protein levels of RACK1 and PKC-ε were significantly elevated in the HG hypoxia group (Hg+Hypo) compared to the control group (P< 0.05) (Figure 4).

Figure 4 High glucose combined with hypoxia up-regulated transcription and increased protein levels of receptor for activated C kinase 1 and protein kinase C-ε in adult retinal pigment epithelium cell line-19 cells. A and B: The protein levels of receptor for activated C kinase 1 (RACK1) and protein kinase C-ε (PKC-ε) in the adult retinal pigment epithelium cell line-19 (ARPE-19) cells of normal control and high glucose combined with hypoxia;C: The ratio of mRNA of RACK1 and PKC-ε in ARPE-19 cells.The results are expressed as the mean ± SD.aP < 0.05 vs control group.bP < 0.01 vs control group.NC: Normal control;Hg+Hypo: High glucose combined with hypoxia;RACK1: Receptor for activated C kinase 1;PKC-ε: Protein kinase C-ε.
Silencing of RACK1 in ARPE-19 cells under HG hypoxia down-regulated PKC-ε
We examined the mRNA and protein levels of PKC-ε by siRNA after siRNA-silencing of RACK1 expression in ARPE-19 cells exposed to HG hypoxic conditions (Figure 5).The results showed that the inhibition of RACK1 expression in ARPE-19 cells reduced PKC-ε transcription and protein levels under HG hypoxia (P< 0.05).

Figure 5 Silencing of receptor for activated C kinase 1 in adult retinal pigment epithelium cell line-19 cells under high glucose combined with hypoxia down-regulated protein kinase C-ε. A and B: The mRNA levels of receptor for activated C kinase 1 (RACK1) and protein kinase C-ε (PKC-ε) in the normal control,high glucose combined with hypoxia,non-silent siRNA,and silenced RACK1 siRNA groups;C-E: The protein levels of RACK1 and PKC-ε in adult retinal pigment epithelium cell line-19 cells of each group.The results are expressed as the mean ± SD.aP < 0.05 vs normal control.bP < 0.05 vs non-silent siRNAnormal control.NC: Normal control;Hg+Hypo: High glucose combined with hypoxia;siRNA-NC: Non-silent siRNA;siRNA-RACK1: Silenced RACK1 siRNA;RACK1: Receptor for activated C kinase 1;PKC-ε: Protein kinase C-ε.
Silencing RACK1 inhibits ROS elevation, apoptosis, and cell leakage in ARPE-19 cell monolayers under HG hypoxia
We subsequently examined ROS levels,apoptosis,and permeability between monolayers after silencing RACK1 expression by siRNA in ARPE-19 cells under HG hypoxia (Figure 6).The results showed that the inhibition of RACK1 expression in ARPE-19 cells down-regulated ROS levels,apoptosis,and cell permeability in monolayers under HG hypoxia (P< 0.05).

Figure 6 Silencing receptor for activated C kinase 1 inhibits reactive oxygen species elevation,apoptosis,and cell leakage in adult retinal pigment epithelium cell line-19 cell monolayers under high-glucose hypoxia. A and B: The apoptosis rate of adult retinal pigment epithelium cell line-19 cells was measured in the normal control (NC),high glucose combined with hypoxia,non-silent siRNA-NC,and silenced receptor for activated protein kinase C1 siRNA groups under high glucose hypoxia;C: The FITC-dextran leakage level in the cells of each group,used to analyze the permeability between cell levels in monolayers;D: The reactive oxygen species (ROS) production level of ROS detection kit in the cells of each group.The results are expressed as the mean ± SD.aP
DlSCUSSlON
DR is a major ocular complication of diabetes that significantly impacts global health[11].The mechanisms underlying its occurrence and development are complex and poorly understood[12].Further elucidation of these mechanisms might aid in mitigating DR progression.RPE cells treated with HG are commonly used as an ideal model for investigating DR[13],and RPE cells are often exposed to HG and hypoxic conditions during DR development[14].Studies suggested that the disruption of RPE barrier function in DR might result from apoptosis of RPE cells under HG and hypoxic conditions[15],although the specific mechanisms remain unclear.In this study,we aimed to explore the effect of RACK1 on RPE barrier function through bothin vitroandin vivoexperimental models to verify its role in the occurrence and development of early DR.
The RPE consists of highly specialized single-layer chromatophores,which are located between microvessels of the villus and outer segments of photoreceptors[16,17].The RPE and photoreceptors in the outer retinal layer usually act as units to maintain normal visual function.Similarly,mutations in photoreceptor cells or RPE can lead to retinal degeneration[18].In this study,we observed that apoptosis of RPE cells occurred in the retina of STZ-induced diabetic rats at week 8,highlighting apoptosis as an important factor in RPE and oBRB damage in early DR.Furthermore,we investigated the expression of RACK1 and PKC-ε in the retina of diabetic rats at weeks 8 and 10,showing that both mRNA and protein levels of RACK1 and PKC-ε were significantly higher in the retina of diabetic rats compared to the control group.Prior studies similarly demonstrated that elevated levels of RACK1 can promote cell apoptosis induced by polyglutamine[19],while the inhibition of PKC-ε can protect RPE cells from lipopolysaccharide-induced injury.However,whether PKC-ε can be suppressed by regulating RACK1 remains unclear[20].In contrast,inhibition of RACK1 might potentially reduce damage and cell apoptosis in RPE cells in diabetes.Further investigations are needed to fully understand the mechanisms by which RACK1 and PKC-ε contribute to RPE cell damage and apoptosis in diabetes.
Therefore,in this study,we conducted anin vitroexperiment to simulate the HG hypoxic environment of ARPE-19 cells in diabetes.We observed that mRNA and protein levels of RACK1 and PKC-ε in ARPE-19 cells were significantly higher under those conditions compared to the control group.RACK1 serves as a scaffold protein that mediates PKC activation[21].PKC is a member of the family of serine/threonine protein kinases that are crucial in regulating many biological processes,such as cell division,growth,apoptosis,and cellular responses to environmental stressors.Meanwhile,the PKC pathway is an important pathway involved in DR.
However,mRNA and protein levels of PKC-ε were significantly reduced in the control group after inhibiting RACK1 expression,suggesting the possibility of down-regulating PKC-ε by inhibiting RACK1 expression.Hyperglycemia and tissue hypoxia in diabetes patients both increase the production of ROS,leading to retinal and tissue damage[22].Our findings showed that the production of ROS by ARPE-19 cells was significantly increased under hyperglycemic and hypoxic conditions but could be significantly reduced by inhibiting RACK1.PKC is known to be involved in ROS production,and the increase in PKC stimulates ROS production in the mammalian target of rapamycin complex 1 pathway,which is related to autophagy[23].PKC-ε plays a tissue-specific role in redox biology,with specific isoforms being both a target of ROS and an upstream regulator of ROS production[24].Therefore,this effect might result in the accumulation of unfolded proteins and dysfunctional organelles in cells,contributing to DR pathophysiology[25].Furthermore,our study also revealed that under HG and hypoxic conditions,ARPE-19 cell viability decreased,and apoptosis increased.This could potentially be attributed to the activation of PKC-ε under HG and hypoxic conditions,leading to increased ROS production and subsequent cell autophagy.Therefore,reduction of PKC-ε activation by inhibiting RACK1 expression might be possible,thereby decreasing ROS production and alleviating cell autophagy and cellular damage.
In diabetes,the activation of PKC is mediated by the formation of diacylglycerol (DAG),a physiological activator of PKC[26].Blocking DAG,the activator of PKC,can interrupt the metabolic signaling cascade and inhibit ROS production.Therefore,inhibiting DAG formation is a potential method to control this signaling pathway[27].During the onset and progression of diabetes,the inhibition of phospholipase D and phospholipase C can lead to an increase in the level of DAG through de novo synthesis.This increase in DAG levels can contribute to the induction of more severe oxidative stress in diabetes[28-30].Phosphate hydrolase 1 and 2 can catalyze the conversion of phosphatidic acid into DAG through a process called de novo synthesis[31,32].However,because of its biochemical complexity and multiple sources,direct DAG inhibition is not the best treatment option for diabetes.RACK1,as a scaffold protein involved in multiple signal transduction cascades,can promote the expression of PKC and enhance its activity in cells in a manner highly dependent on PKC-ε[33,34].This makes it a promising therapeutic target to replace DAG inhibition.By inhibiting RACK1,the expression and activity of PKC-ε can be reduced,leading to a decrease in ROS production,potentially mitigating oxidative stress in diabetes.Furthermore,HG and hypoxia can induce the expression of apoptosis-promoting transcription factor C/EBP homologous protein in ARPE-19 cells and disrupt the integrity of tight junctions[35].In our study,we observed that silencing RACK1 reduced FITC leakage in ARPE-19 cells under HG and hypoxia conditions.However,the specific downstream mechanisms related to the changes in tight junction proteins are not yet fully understood and require further research to elucidate the underlying mechanisms by which RACK1 disrupts the oBRB.
However,this study has certain limitations.First,all mechanistic experiments were conducted in ARPE-19 cells.Future studies need to confirm the effect of RACK1 on the oBRB in diabetic rats.Studies conducted solely in cell lines,such as ARPE-19 cells,might not fully reflect the in vivo effects of RACK1 on the oBRB in diabetic rats or other animal models.Therefore,animal models are essential for studying complex physiological processes and evaluating potential therapeutic < 0.05vsnormal control.bP< 0.05vssiRNA-normal control.NC: Normal control;Hg+Hypo: High glucose combined with hypoxia;siRNA-NC: Non-silent siRNA;siRNA-RACK1: Silenced RACK1 siRNA;ROS: Reactive oxygen species;RACK1: Receptor for activated C kinase 1.
interventions because they provide a more comprehensive understanding of in vivo effects,including systemic factors and interactions between different cell types within the tissues of interest.Therefore,future studies should aim to confirm the effect of RACK1 on the oBRB in animal models of diabetes,such as diabetic rats.These studies might assess the expression and localization of RACK1,PKC isoforms,and DAG signaling components in the retina of diabetic animals.Additionally,functional assays can be performed to evaluate the integrity and permeability of the oBRB.
CONCLUSlON
Knockdown of RACK1 can reduce PKC-ε activity and ROS production,thereby alleviating cellular oxidative stress and inflammatory responses.By reducing the excessive activation of PKC-ε/ROS,the occurrence and progression of early DR can be reduced.This may be achieved through the reduction of cellular oxidative stress and inflammatory response,improvement of retinal cell survival and function,and the reduction of vascular lesions and inflammatory infiltration.Therefore,inhibiting RACK1 might be a potential therapeutic strategy to slow down the progression of early DR by regulating PKC-ε/ROS.However,further research is needed to determine the safety and efficacy of this strategy and definitively explore its potential clinical applications.
ARTlCLE HlGHLlGHTS
Research background
Diabetic retinopathy (DR) is a major ocular complication of diabetes mellitus,leading to visual impairment.Retinal pigment epithelium (RPE) injury is a key component of the outer blood retinal barrier,and its damage is an important indicator of DR.
Research motivation
Therefore,inhibiting receptor for activated C kinase 1 (RACK1) may be a potential therapeutic strategy to slow down the progression of early DR by regulating protein kinase C-ε/ reactive oxygen species (PKC-ε/ROS).
Research objectives
Knockdown of RACK1 can reduce the activity of PKC-ε and the production of ROS,thereby alleviating cellular oxidative stress and inflammatory responses.By reducing the excessive activation of PKC-ε/ROS,the occurrence and progression of early DR can be reduced.
Research methods
In this study,Sprague-Dawley rats and adult RPE cell line-19 (ARPE-19) cells were used asin vivoandin vitromodels,respectively,to explore the role of RACK1 in mediating PKC-ε in early DR.Furthermore,the effect on the apoptosis and barrier function of RPE cells was also investigated in the former model.
Research results
Knockdown of RACK1 can reduce the activity of PKC-ε and the production of ROS,thereby alleviating cellular oxidative stress and inflammatory responses.By reducing the excessive activation of PKC-ε/ROS,the occurrence and progression of early DR can be reduced.
Research conclusions
this study proposes that by reducing the excessive activation of PKC-ε/ROS,the occurrence and progression of early DR can be reduced.This may be achieved through the reduction of cellular oxidative stress and inflammatory response,improvement of retinal cell survival and function,and the reduction of vascular lesions and inflammatory infiltration.
Research perspectives
One of the main limitations of this study is that all the mechanistic experiments were conducted in ARPE-19 cells.Future studies need to confirm the effect of RACK1 on the oBRB in diabetic rats.
FOOTNOTES
Co-first authors:Jian Tan and Ang Xiao.
Author contributions:Tan J,Xiao A,Yang L,Tao YL,Shao Y and Zhou Q designed the research study;Tan J,Xiao A and Yang L performed the research;Tan J and Shao Y contributed new reagents and analytic tools;Tan J and Zhou Q analyzed the data and wrote the manuscript;all authors have read and approve the final manuscript.Tan J and Xiao A contributed equally to this work as co-first authors.The reasons for designating Tan J and Xiao A as co-first authors are threefold.First,Tan J and Xiao A made equal contributions to the project research.Secondly,both Tan J and Xiao A actively participated in subsequent revisions and communication related to the paper.Finally,co-authorship serves to better exemplify collaboration within the team.In summary,we believe that designating Tan J and Xiao A as co-first authors of is fitting for our manuscript as it accurately reflects our team's collaborative spirit,equal contributions,and diversity.
Supported byNational Natural Science Foundation of China,No.82 260211;Key Research and Development Project in Jiangxi Province,No.20203BBG73058;Chinese Medicine Science and Technology Project in Jiangxi Province,No.2020A0166.
lnstitutional review board statement:This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University.
lnstitutional animal care and use committee statement:All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Nanchang University.
Conflict-of-interest statement:The authors declare no conflicts of interest.
Data sharing statement:Technical appendix,statistical code,and dataset available from the corresponding author at zqndyfy@163.com.
ARRlVE guidelines statement:The authors have read the ARRIVE Guidelines,and the manuscript was prepared and revised according to the ARRIVE Guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Jian Tan 0000-0002-1225-8637;Qiong Zhou 0009-0006-3077-2425.
S-Editor:Qu XL
L-Editor:A
P-Editor:Chen YX
 World Journal of Diabetes2024年3期
World Journal of Diabetes2024年3期
- World Journal of Diabetes的其它文章
- Regulatory role of peroxynitrite in advanced glycation end products mediated diabetic cardiovascular complications
- Effects of vitamin family members on insulin resistance and diabetes complications
- Chiglitazar and Thiazolidinedione in patients with type 2 diabetes: Which is better?
- Glucagon-like-peptide-1 receptor agonists and the management of type 2 diabetes-backwards and forwards
- Acute worsening of microvascular complications of diabetes mellitus during rapid glycemic control: The pathobiology and therapeutic implications
- Unlocking new potential of clinical diagnosis with artificial intelligence: Finding new patterns of clinical and lab data
