Catalytic Effect of Transition Metal Complexes of Triaminoguanidine on the Thermolysis of Energetic NC/DEGDN Composite
Mohammed Dourari, Ahmed Fouzi Tarchoun, Djalal Trache, Amir Abdelaziz, Roufaida Tiliouine, Tessnim Barkat, Weiqiang Pang
(1.Energetic Materials Laboratory (EMLab), Teaching and Research Unit of Energetic Processes, Ecole Militaire Polytechnique, BP 17, Bordj El-Bahri, 16046, Algiers, Algeria;2.Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
Abstract:The transition metal complexes of triaminoguanidine (TAG-M, where M=Cobalt (Co) or Iron (Fe)) have been prepared. The catalytic effect of these complexes on the thermolysis of energetic composite based on nitrocellulose and diethylene glycol dinitrate, has been investigated. Extensive characterization of the resulting energetic composites was carried out using scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and differential scanning calorimetry (DSC). Isoconversional kinetic analysis was performed to determine the Arrhenius parameters associated with the thermolysis of the elaborated energetic formulations. It is found that TAG-M complexes have strong catalytic effect on the thermo-kinetic decomposition of NC/DEGDN by decreasing the apparent activation energy and significantly increased the total heat release. The models that govern the decomposition processes are also studied, and it is revealed that different reaction processes are accomplished by introduction metal complexes of triaminoguanidine. Overall, this study serves as a valuable reference for future research focused on the investigation of catalytic combustion features of solid propellants.
Keywords:triaminoguanidine; transition metal complexes; nitrocellulose; diethylene glycol dinitrate; catalytic effect
Introduction
In recent years, the exploration of energetic materials (EMs) has emerged as a focal point of scientific investigations, owing to their widespread applications in both civilian and defense sectors[1-3]. These materials are strategically designed to rapidly release substantial amounts of stored chemical energy upon ignition through various means, including thermal forces, external mechanical forces, and laser ignition[4-6]. Consequently, study of the thermolysis and combustion processes of these EMs has garnered significant global attention[7-9].To enhance the reactivity and combustion performance of existing energetic composites, researchers have embarked on specialized strategies, encompassing surface modifications such as coating and functionalization of EMs, incorporation of highly energetic molecules, introduction of nanometer additives, and innovative motor designs[10,11]. Notably, among these strategies, the utilization of combustion catalysts has emerged as a cost-effective avenue for improving the performance of energetic formulations. This approach becomes particularly promising when catalysts are deposited on energetic materials, forming energetic complexes[12-14].
Triaminoguanidine (TAG), characterized by its nitrogen-rich building blocks, emerges as a promising candidate for catalytic enhancement[15,16]. The catalytic effect of TAG, coupled with its high energy characteristics, makes it an attractive choice for improving the performance of conventional high-energy materials, including double base propellant[17-19]. This innovative category of materials incorporates a substantial fraction of nitrogen functional groups, contributing to elevated energy levels due to their remarkably positive heat of formation. This phenomenon is primarily attributed to the abundance of inherently energetic N—N and C—N bonds within the TAG structure[20]. In this context, our study focuses on investigating the catalytic effect of transition metal complexes of triaminoguanidine on the thermolysis of an energetic composite containing nitrocellulose (NC), as a binder, and diethylene glycol dinitrate (DEGDN), as a plasticizer. Through this exploration, we aim to uncover new insights into the potential synergistic interactions between transition metal complexes and triaminoguanidine, thereby advancing our understanding of catalytic strategies to enhance the performance of energetic material.
1 Experimental section
1.1 Materials and methods
Triaminoguanidine, cobalt nitrate, and iron nitrate were used as received from the supplier Sigma-Aldrich. Nitrocellulose with nitration degrees of 12.61%, and diethyleneglycol dinitrate were synthesized in our laboratory following the procedures indicated in our recent works[21,22].
1.2 Preparation of TAG-M
The methodology for synthesizing transition metal complexes of TAG, involving cobalt (Co) and iron (Fe), was adopted from the research conducted by He et al.[18]. The preparation involves dissolving 2.4mmol of TAG-HNO3in 40 mL of distilled water into two beakers, respectively, followed by the addition of 3mmol of metal nitrates (cobalt nitrate or iron nitrate) under agitation. The mixtures were stirred for 2hours at a temperature of 75℃. The obtained products were then neutralized by sodium bicarbonate (NaHCO3) until there is no bubble releases. Finally, the precipitates were filtrated and washed several times with ethanol. A schematic presentation of the preparation procedure is represented in Fig.1. The use of protective gloves and safety glasses or other suitable full-body personal protective equipment (PPE) is strongly recommended when manipulating triaminoguanidine due to the potential dangers associated with its handling, including the risk of unexpected explosions under certain conditions.
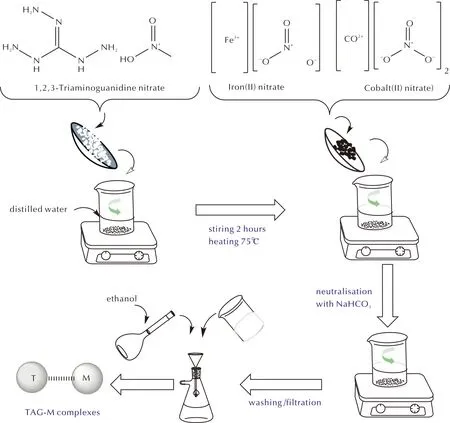
Fig.1 Preparation of transition metal complexes of triaminoguanidine (TAG-M)
1.3 Preparation of NC/DEGDN composites doped with TAG-M
The preparation process of the desired composites is detailed in Fig.2. Initially, 1g of nitrocellulose was dissolved in 60mL of acetone and stirred for 60min at 20℃ to obtain a homogeneous solution. Subsequently, diethylene glycol dinitrate was introduced to the previous mixture using a mixing mass ratio of 1∶1 for NC/DEGDN. Following this step, a specified amount of TAG-M (mass fraction of 5%) was dissolved in 40mL of acetone and the resulting TAG-M solution was then combined with the NC/DEGDN mixture and stirred for over 1hour. Upon air-drying, thin films of NC/DEGDN/TAG-Co and NC/DEGDN/TAG-Fe were successfully obtained.
1.4 Experimental techniques
1.4.1 Scanning electron microscope (SEM) analyses
Morphological characteristics of the studied samples were analyzed using a FEI Quanta 600 scanning electron microscope. The microscope was set with a 10mm distance from the samples and operated at an accelerating voltage of 10kV. Before the experiment, the samples were dried, placed on a sticky carbon grid, and coated with a thin conductive carbon films.
1.4.2 Infrared spectroscopy characterization
FTIR analysis of the composite materials was conducted using a Perkin-Elmer 1600 Fourier-transform infrared spectrometer in transmission mode. Prior to analysis, the samples were dried to eliminate moisture. The FTIR measurements took place at room temperature, covering a frequency range from 4000 to 400cm-1with a spectral resolution of 4cm-1.
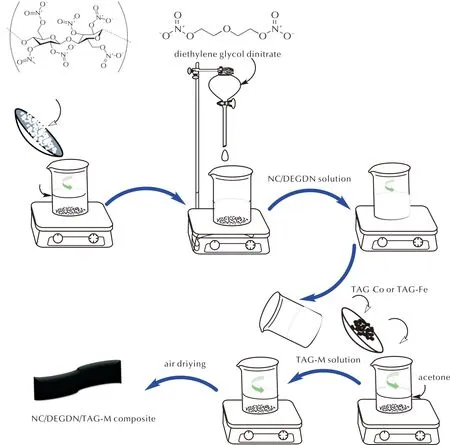
Fig.2 Preparation process of NC/DEGDN/TAG-M composites
1.4.3 X-ray diffraction characterization
Sample crystal structures were investigated through an X-Ray diffractometer (PANalytical X′pert PRO, UK) with the use of Cu Kα radiation at 45kV and 40mA. The data were scanned within an angular range of 2θ=5°-80° with a step size of 0.02°.
1.4.4 Thermal analysis by TGA
Thermogravimetric measurements were carried out using TGA (Perkin-Elmer 8000 analyzer, USA) within the temperature range of 50—350℃ using the same heating rate (β=10℃/min) under nitrogen atmosphere. The analyzed samples were prepared by introducing 2mg of dried samples in a ceramic crucible.
1.4.5 Thermal analysis by DSC
Thermal behavior of the elaborated compositeswas assessed using a differential scanning calorimeter (Perkin-Elmer 4000 analyzer), calibrated with highly pure indium before operating. A sample mass of 1—2mg was deposited into a sealed aluminum crucible, while another identical pan was used as a reference. The measurements were carried out within the temperature range of 50—350℃ at different heating rates (i.e., 10, 15, 20, and 25℃/min) under an inert nitrogen atmosphere.
2 Theoretical background of kinetic analysis
Kinetic analysis plays a crucial role in evaluating the key decomposition parameters such as the activation energy (Ea), the pre-exponential coefficient (Aa), and the kinetic model (f(α)).The following kinetic equations are typically used by the International Committee of Thermal Analysis and Calorimetry (ICTAC) to explain the single-step thermal decomposition process[23].
(1)
(2)
Where ,A,Ea,f(α) andg(α) are the preexponential factor, activation energy, differential and integral forms of the model, respectively, andαis the extent of conversion (0<α<1). The collection of these parameters is termed the kinetics triplet (Ea, lg (A),g(α)). It is interesting to point out that forty-one types of common kinetic models have previously been presented based onf(α) org(α)[24,25]. DSC technique is the commonly employed tool for studying the kinetic processes, as it allows the determination of the kinetic factors by linking the heat flow to the reaction rate. The value ofαcan be determined from the temperature integral of the thermograms by using Equation (3), in which ΔHis the measured heat change and ΔHtotalis the total reaction heat[26,27].
(3)
Isoconversional methods are considered the most dependable approaches for determining the kinetic triplet of thermally activated reactions[28-30]. In this study, the kinetic triplet was achieved using isoconversional models, namely, Trache-Abdelaziz-Siwani (TAS)[24], iterative Kissinger-Akahira-Sunose (it-KAS)[31], and Vyazovkin′s method (VYA) coupled with the compensation effect approach (CE)[32]. Additional information and detailed explanations about these kinetic approaches can be found in our previously published papers[33,34].
3 Results and discussion
3.1 Morphological features of TAG-M complexes and NC/DEGDN based composites
SEM was employed for the morphological analysis of both transition metal complexes of triaminoguanidine and composites based on NC/DEGDN. Examining the images of TAG-M, depicted in Fig.3(a) and 3(b), it is evident that TAG-Co displays an irregular shape with a rough surface, while TAG-Fe exhibits a spherical aspect. Furthermore, Fig.3(c)illustrates that the NC/DEGDN composite has a smooth and uniform morphology; while Fig.3(d)-(e)highlight the uniform distribution of transition metal complexes of triaminoguanidine (TAG-Fe and TAG-Co) within the NC/DEGDN matrix, without any apparent aggregation or agglomeration. This effective dispersion is crucial for maintaining a consistent and stable material structure, thereby positively influencing factors such as high reaction rates and heat release rates, as elaborated in subsequent sections.
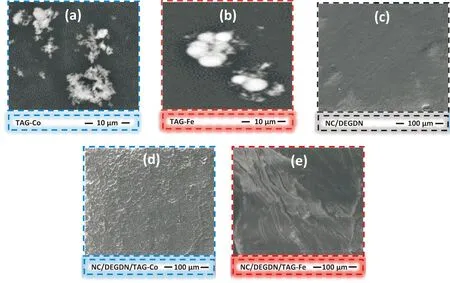
Fig.3 Scanning electron microscopy images of transition metal complexes of triaminoguanidine and composites based on NC/DEGDN
3.2 Crystalline and chemical structures
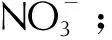
3.3 TGA results
The thermal behavior of the prepared NC/DEGDN/TAG-M composites were investigated using TGA/DTG, and the resulting curves are depicted in Fig.5. Analysis of the recorded TGA/DTG curves reveals that the overall mass loss trends of the composites are consistent with those of the NC/DEGDN baseline, wherein two decomposition processes were observed. The initial step is corresponded to the moisture evaporation and the volatilization of a small amount of DEGDN plasticizer[42]. The subsequent decomposition, observed between 160 and 240℃, is attributed to the thermolytic cleavage of the nitric ester groups[4, 21].
In order to achieve a quantitative comparison, the detailed parameters of the TGA/DTG profiles are summarized in Table 1. It is found that NC/DEGDN/TAG-Co and NC/DEGDN/TAG-Fe composites have the same total mass of 80% under the same heating rate. Another interesting finding is that the addition of both TAG-Co and TAG-Fe decrease the onset and peak temperatures of the decomposition steps of NC/DEGDN composite, which suggests that the addition of triaminoguanidine metal complexes could improve the thermal reactivity of the NC/DEGDN composite.
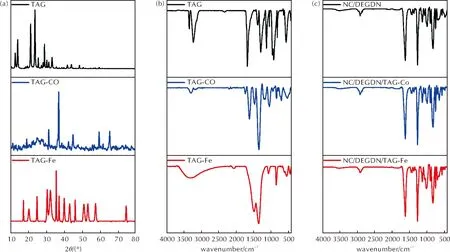
Fig.4 (a) XRD patterns of triaminoguanidine and its complexes; (b) FTIR spectra of triaminoguanidine and its complexes; (c) FTIR spectra of the energetic composites

Fig.5 Thermogravimetry-differential thermal analysis of NC/DEGDN with or without TAG-M complexes at the heating rate of 10℃/min
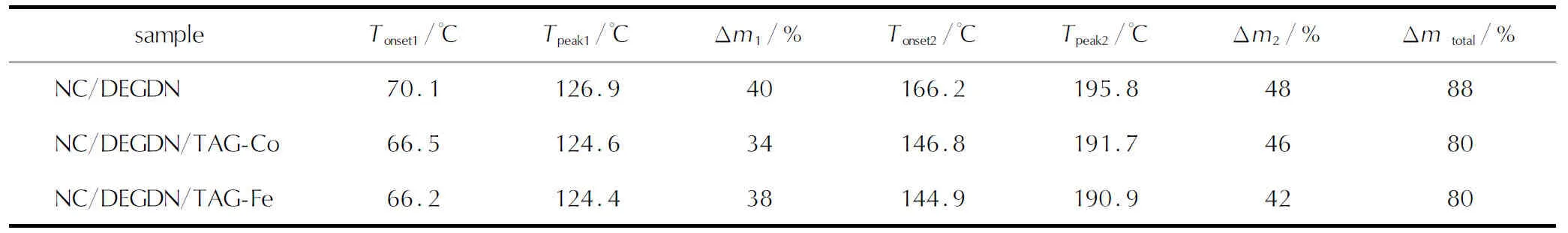
Table1 Thermal parameters determined via TGA at the heating rate of 10℃/min
3.4 DSC findings
The thermal decomposition mass loss parameters of the energetic composites under investigation can be further supported by DSC data. The recorded thermograms at different heating rate(10, 15, 20, and 25℃/min) are presented in Fig.6, and the detailed parameters are listed in Table 2. A notable observation is the consistent profile exhibited by all thermograms across varying heating rates, revealing a singular exothermic peak, which is attributed to the decomposition of explosophoric nitrate esters[42,43].
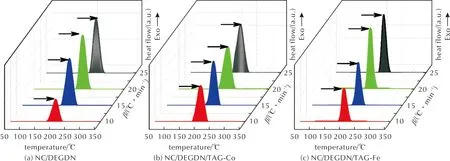
Fig.6 Differential scanning calorimetry of NC/DEGDN with or without TAG-M complexes at different heating rate
It is essential to highlight that DSC analyses did not clearly reveal the first peak observed in TGA curves, which signifies the evaporation of DEGDN. This difference might be explained by the lower sample weight used in DSC, leading to reduced sensitivity in detecting enthalpy changes associated with DEGDN evaporation[21, 44]. It can be also deduced from Fig.5 and Fig.6 that the temperature of peak decomposition from DSC is consistently higher than that obtained from DTG curves, indicating that the major mass loss peaks are responsible for the heat releases[19]. Moreover, Fig.6 highlights that the exothermic decomposition event of the studied energetic composites is significantly influenced by the heating rate. Specifically, with increasing heating rate, the peak representing decomposition shifts towards higher temperatures. This behavior is attributed to the accelerated rate of thermal energy input at higherβ, reducing the time available for the reaction to occur[45,46]. These findings align with observations, reported in the literature, for non-isothermal DSC experiments[47-49]. Based on the results presented in Fig.6 and Table 2, it is evident that the incorporation of TAG-Co and TAG-Fe into the NC/DEGDN matrix leads to a decrease in the maximum peak temperature to almost the same values of 194.5℃ for NC/DEGDN/ TAG-Co and 194.6℃ for NC/DEGDN/ TAG-Fe, as confirmed by TGA analyses. These results provide evidence for the comparable catalytic impact of the two investigated TAG-M complexes on the thermolysis of NC/DEGDN. This outcome can be further sustained by the work of Hanafi et al.[50], who demonstrated that the type of metal ion in TAG-M complexes did not affect the thermolysis of HNTO/AN co-crystal. Besides that, a deeper examination of the obtained DSC results has permitted to highlight another interesting outcome, which is related to the increased heat release of NC/DEGDN/TAG-Co and NC/DEGDN/TAG-Fe compared to the NC/DEGDN baseline. Specifically, theΔHincreases from 1662.7J/g to 2003.8J/g in the presence of TAG-Fe, and to 2018.3J/g with TAG-Co. This finding can be attributed to the heightened reactivity of triaminoguanidine metal complexes, which contain a substantial fraction of nitrogen functional groups, promoting intimate contact among various components. This close interaction significantly improves heat and mass transfer leading to increased energy levels and accelerating the thermal decomposition process[20, 51]. This behavior can be supported by the outcomes reported by Ting et al.[52], who studied the thermal characteristics and thermolysis mechanisms of ammonium perchlorate (AP) in the presence of complexes of TAG doped with graphene oxide (GO). They revealed that the heat released during the decomposition of AP has significantly increased from 1088J/g to 1398J/g in the presence of triaminoguanidine-nickel. To provide a more comprehensive understanding on the effect of TAG-M complexes on the characteristics of NC/DEGDN propellant, experimental density of each of the developed energetic formulation was measured and outlined in Table 2. The primary finding is that the introduction of triaminoguanidine metal complexes (TAG-Co and TAG-Fe) into the NC/DEGDN matrix results in a notable increase in the density. This validates the effective dispersion of TAG-M within the NC/DEGDN matrix, as observed in SEM analyses[22, 53].
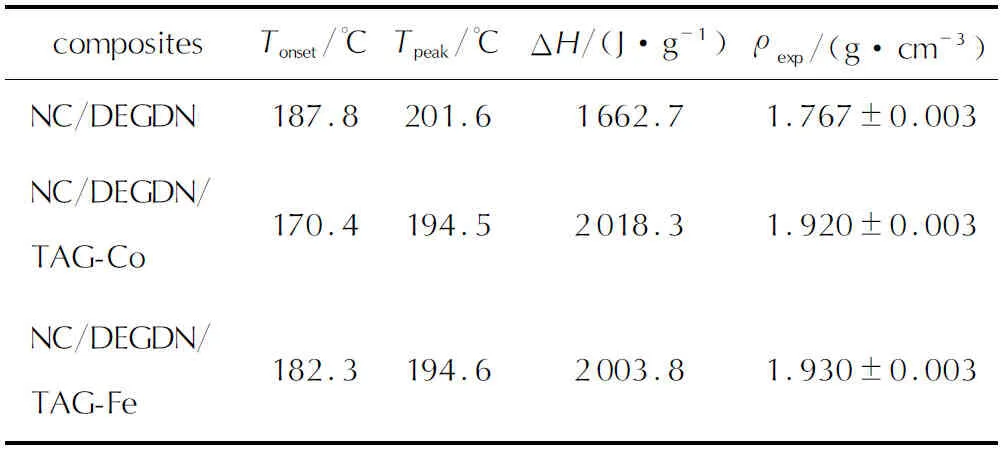
Table2 Thermal parameters determined via DSC at the heating rate of 10℃/min
3.5 Determination of the thermo-kinetic parameters
In order to obtain a more thorough understanding sand present a comprehensive overview of how the investigated triaminoguanidine metal complexes (TAG-Co and TAG-Fe) influence the thermal properties of NC/DEGDN propellant, we conducted a thermo-kinetic investigation of the formulated energetic composites utilizing isoconversional kinetic approaches, specifically TAS, it-KAS, and VYA/CE. The computations were executed using non-isothermal DSC data obtained across various heating rates, and MATLAB software was employed for calculations following the determination of extent of conversion values at each temperature[24]. This involved integrating the peak area of the DSC curves recorded at different heating rates. To mitigate errors inherent at the beginning and the end of the decomposition process, we set the conversion range from 0.02 to 0.98. Fig.7 illustrates the evolving trends ofEaand lgAwith conversion rate for all prepared composites. Conversely, Table 3 provides the average values ofEaand lgAwith their associated confidence intervals, along with the most probable decomposition modelg(α).
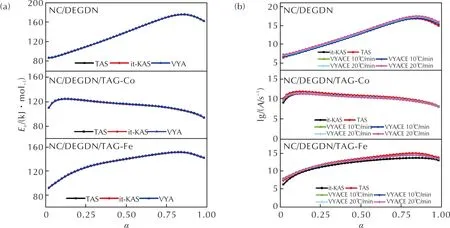
Fig.7 Evolution of Ea and lg A with conversion rate for NC/DEGDN based composites
The primary outcome depicted from Fig.7 and Table 3 is that for all the studied NC/DEGDN based composites, the employed isoconversional integral methods provide close values ofEaandLog(A), indicating the excellent consistency of the used methods and the high precision of the calculated values. Another crucial fact that can be observed from Fig.7 is the identical trend evolution of lgAandEaversus the reaction conversion, which is attributed to the well-known kinetic compensation effect (KCE) betweenEaand lgA[54-56]. It can be also deduced from Fig.7 that the incorporation of the triaminoguanidine metal complexes into the NC/DEGDN matrix effects its kinetic behavior. Specifically, it is found that the addition of TAG-Co and TAG-Fe caused a decrease in the apparent activation energy of NC/DEGDN composite. The mean value ofEadecreases from 137.4kJ/mol for NC/DEGDN to 135.4kJ/mol in the presence of TAG-Fe and even further to 114.8kJ/mol when TAG-Co is added. This finding suggests that TAG-Co has a more pronounced catalytic effect on the thermal decomposition of NC/DEGDN than TAG-Fe. These findings can be substantiated by the research conducted by Hanafi et al.[50], who examined the catalytic reactivity of a graphene oxide-stabilized iron complex of triaminoguanidine on the thermolysis of an energetic cocrystal consisting of hydrazinium 3-nitro-1,2,4-triazol-5-one and ammonium nitrate (HNTO/AN). The authors demonstrated that the introduction of the iron complex of triaminoguanidine, TAG-Fe, led to a reduction in the activation energy of the co-crystal by 31kJ/mol.
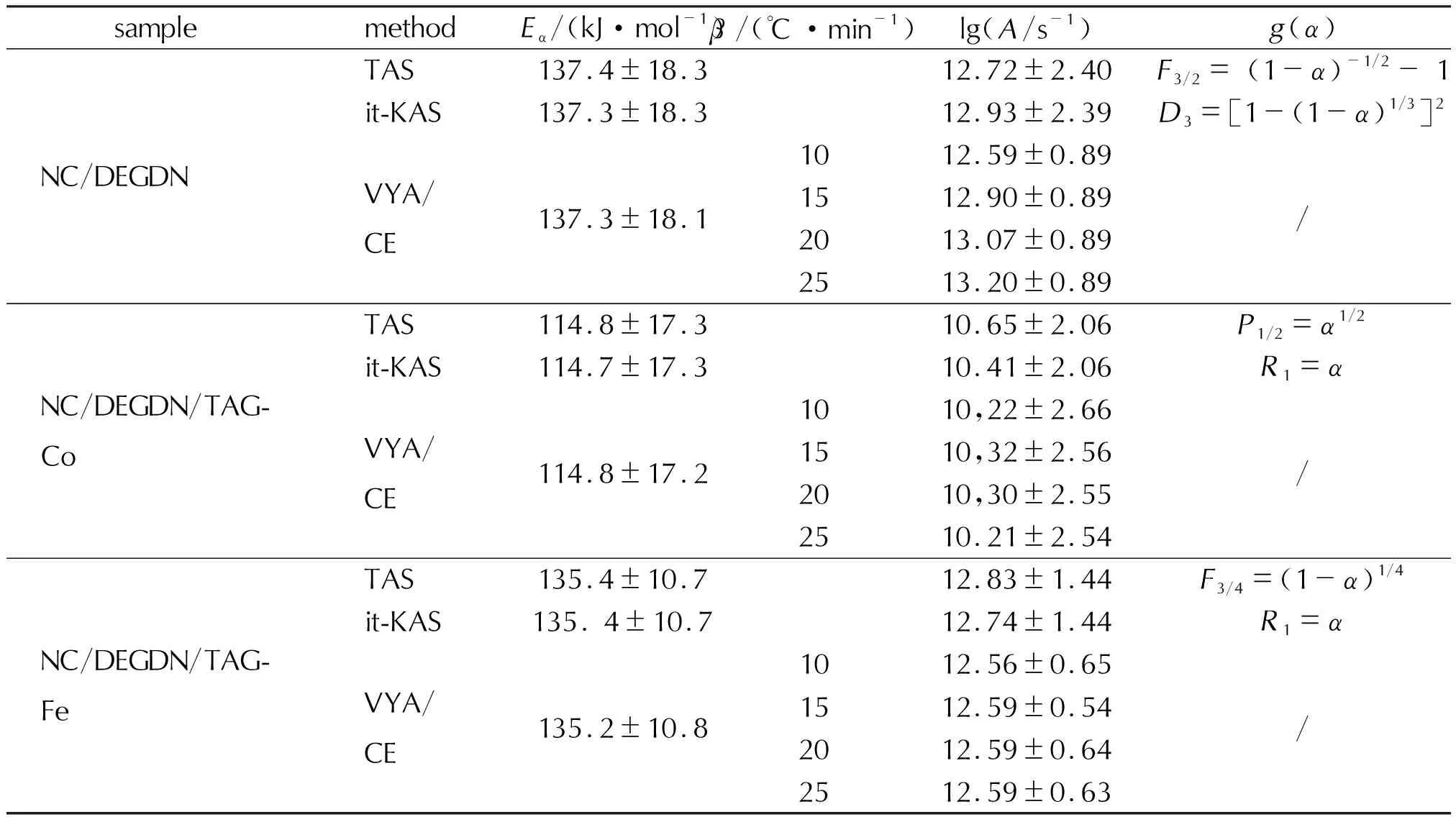
Table3 Kinetic results of the investigated energetic composites
On the other hand, the most probable models for the solid-state reactions described byg(α) functions, were identified. The expression of the determinedg(α) is presented in Table 3 and graphically plotted in Fig.8.

Fig.8 Evolution of the most probable integral reaction model as a function of conversion for the prepared composites
It is worth noting that the non-linear VYA method does not provide the reaction model directly. However, when combined with the compensation effect approach, it can yield numerical data for the functiong(α)[56]. Based on the forty one types of mechanism functions discussed elsewhere[24], the NC/DEGDN decomposition is determined to follow a chemical reaction (F3/2) according to TAS approach and a three-dimensional diffusion (D3, Jander) according to it-KAS. It is also evident from the data in Table 3 that the use of the catalysts involved (TAG-Fe and TAG-Co) has altered the decomposition mechanism of the NC/DEGDN composite. According to it-KAS approach, both NC/DEGDN/TAG-Co and NC/DEGDN/TAG-Fe composites decompose following a contracting disk mechanism characterized byR1=α. As per TAS approach, the NC/DEGDN/TAG-Co composite decomposes through a nucleation mechanism identified byP1/2(power law), while the NC/DEGDN/TAG-Fe composite decomposes through a chemical reaction mechanism identified byF3/4.
4 Conclusions
In this study, transition metal complexes of triaminoguanidine(TAG-M, where M=Cobalt (Co) or Iron (Fe)) were synthesized and their catalytic effect on the thermolysis of double base NC/DEGDN propellant was investigated. The designed TAG-Co and TAG-Fe complexes exhibited distinctive X-ray diffraction (XRD) patterns with sharp and intense peaks, indicating their crystalline nature. The successful preparation of TAG-M complexes was further supported by FTIR findings. Moreover, SEM analysis confirmed the effective dispersion of TAG-M catalysts within the NC/DEGDN matrix. Thermal assessments through TGA and DSC revealed that the addition of TAG-M complexes reduced both the onset and maximum peak decomposition temperatures of the double base NC/DEGDN matrix while enhancing its total heat release. Isoconversional kinetic study using various methods, including Trache-Abdelaziz-Siwani, iterative Kissinger-Akahira-Sunose, and Vyazovkin′s method coupled with the compensation effect approach provided insights into the thermo-kinetic parameters of the decomposition process. It is found that the incorporation of TAG-Co or TAG-Fe complexes led to a decrease in the apparent activation energy of the NC/DEGDN composite, indicating their catalytic influence. In addition, examination of models governing the decomposition processes revealed that the introduction of metal complexes of triaminoguanidine resulted to different reaction processes. This investigation offers valuable insights into catalytic strategies for enhancing the performance of double base propellants and offer a foundation for future research in the field of solid propellants.

