Uveal melanoma: Recent advances in immunotherapy
Francesco Saverio Sorrentino,Francesco De Rosa,Patrick Di Terlizzi,Giacomo Toneatto,Andrea Gabai,Lucia Finocchio,Carlo Salati,Leopoldo Spadea,Marco Zeppieri
Abstract Uveal melanoma (UM) is the most common primary intraocular cancer in adults.The incidence in Europe and the United States is 6-7 per million population per year.Although most primary UMs can be successfully treated and locally controlled by irradiation therapy or local tumor resection,up to 50% of UM patients develop metastases that usually involve the liver and are fatal within 1 year.To date,chemotherapy and targeted treatments only obtain minimal responses in patients with metastatic UM,which is still characterized by poor prognosis.No standard therapeutic approaches for its prevention or treatment have been established.The application of immunotherapy agents,such as immune checkpoint inhibitors that are effective in cutaneous melanoma,has shown limited effects in the treatment of ocular disease.This is due to UM’s distinct genetics,natural history,and complex interaction with the immune system.Unlike cutaneous melanomas characterized mainly by BRAF or NRAS mutations,UMs are usually triggered by a mutation in GNAQ or GNA11.As a result,more effective immunotherapeutic approaches,such as cancer vaccines,adoptive cell transfer,and other new molecules are currently being studied.In this review,we examine novel immunotherapeutic strategies in clinical and preclinical studies and highlight the latest insight in immunotherapy and the development of tailored treatment of UM.
Key Words: Uveal melanoma;Immunotherapy;Ocular oncology;Tumor;Metastatic disease;Genetic mutations
lNTRODUCTlON
Uveal melanoma (UM) is a rare ocular tumor regarded as the most common primary malignancy in the adult eye.The annual incidence is approximately 6-7 per million in the United States,accounting for 3.7% of all melanomas[1].UM derives from the pigmented uveal zone,which comprises the choroid,ciliary body,and iris,and is featured by characteristic cytogenetic changes,oncogenic mutations in GNAQ or GNA11,and tropism to aggressively metastasize to the liver resulting in a poor prognosis[2,3].At this time,UM has no established and effective treatments once metastases arise or have been detected.Although several immunotherapies have demonstrated efficacy in metastatic melanoma of cutaneous origin,these immune-based therapies have disappointing outcomes in UM[3,4].Some authors have speculated that ocular melanomas,arising from the uveal tract,might be regarded as an immunotherapy-resistant variant of ocular melanomas[5].
Numerous studies in scientific medical literature continually show that tumor-associated cell therapy tends to be an effective alternative and innovative method to fight metastasis.In the last decades,studies have shown that immunotherapies therapies can bring substantial benefits to patients suffering from a wide range of neoplasia and metastatic disease[6,7].For this reason,huge investments,resources,and important clinical trials have been performed for immunotherapy research and its potential benefit on UM metastatic disease.
Before undertaking this study,we searched PubMed (https://pubmed.ncbi.nlm.nih.gov) and Reference Citation Analysis (RCA) (https://www.referencecitationanalysis.com) for the terms “metastatic uveal melanoma” (1788 papers) and “uveal melanoma immunotherapy” (367 papers) for articles published between January 1,2000,to August 31,2023.We considered only studies in English,with abstracts and structured text,and those referring to humans,whereas we excluded “case reports”,“case series”,“conference papers”,“l(fā)etters” and “invitro” studies.The reference lists of all retrieved articles were scanned to detect further relevant papers.
Genetics and pathways involved in UM
Differently from cutaneous melanoma,which usually harbors an activating BRAF (52%) or NRAS (10%-25%) mutation or inactivation of theNF1gene,UMs,as well as uveal nevi,are commonly characterized by a mutation in GNAQ or GNA11[8-10].These genetic alterations,however,are not sufficient to drive the full malignant transformation to melanoma,being rather an initiating event[11].GNAQ/11 activates mitogen activating protein kinase (MAPK) pathway,present in most UM,as well as PKC,AKT,and Yes-associated protein 1,associated with tumor growth in some UM models[12,13].
Another genetic pathway implicated in UM involve schromosome 3,specifically the BAP1 tumor suppressor gene.The loss of one copy of chromosome 3 is indeed an established negative prognostic factor associated with metastasis and poor clinical outcome,while BAP1 mutation leads to malignant transformation when associated with chromosome 3 monosomy,as the other gene copy is already lost[14,15].
Immune checkpoint inhibitors
Activation of T cells by antigen-presenting cells (dendritic cells) is the key point of an effective immune reaction against cancer cell antigens.This process is enhanced by co-stimulatory molecules like CD28 and B-7,and hampered by immune checkpoints like cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1).These molecules modulate the immune response,preventing inappropriate activation of T cells and controlling excessive immune reaction[16].CTLA-4,when expressed,binds B-7 with a higher affinity than CD28,thus way blocking CD28-mediated co-stimulation;conversely,PD-1 interacts mainly with programmed death-ligand 1 (PD-L1) and mostly affects the effector phase of the immune response[17].
Immune checkpoint inhibitors (ICIs) are antibodies suppressing the negative immunomodulatory activities of their targets (i.e.,CTLA-4 and PD-1).The consequent activation of T cell-mediated response can result in lysis and degradation of cancer cells,ultimately leading to long-term tumor control.This practice-changing concept was first demonstrated by the studies by James P Allison and Tasuku Honjo,awarded the Nobel Prize in Physiology or Medicine in 2018[18].
Malignant melanoma was the first indication for which ipilimumab,an antibody targeting the CTLA-4,was approved by the Food and Drug Administration (FDA) in 2011[19,20].Few years later,the anti-PD-1 antibodies nivolumab and pembrolizumab were also approved.ICIs have been subsequently tested in metastatic UM (mUM);however,robust conclusions regarding their efficacy are difficult to draw given the many limitations of the available data[21-30].
Anti CTLA-4 antibodies
Ipilimumab,whose efficacy in mUM was evaluated in just a few studies,is a humanized monoclonal antibody blocking the CTLA-4 receptor,enhancing T-cell activation,and amplifying T-cell-mediated immunity.Tremelimumab is an anti-CTLA-4 antibody that has also been proposed for mUM[22].A recent systematic review and meta-analysis of Phametal[31] showed that the objective response rate (ORR) was 4.1% for anti-CTLA4,with a median overall survival (OS) of 8.0 mo,and a 12-mo OS rate of 34.7%[31].The progression-free survival (PFS) was longer in treatment-na?ve patients than in pretreated patients,with a median value of 3.3 mo.The side effect profile was similar to studies of CTLA-4 inhibitors in cutaneous melanoma,with an overall adverse event (AE) rate of 64.5% and a grade 3-4 AE rate of 17.5% (more commonly hepatitis and diarrhea).No deaths secondary to AEs were reported[32].Overall,CTLA-4 inhibitors appear to have limited activity in mUM;consequently,their use is generally restricted to selected cases.
Anti PD-1 antibodies
After nivolumab and pembrolizumab were first approved by the FDA for melanoma in 2014,the number of agents acting on the PD-1/PD-L1 axis and their indications across malignancies have been rapidly rising[33].Concerning mUM,pembrolizumab and nivolumab have been the most studied,but atezolizumab and avelumab have also been proposed[34].
Phametal[31] reported anORR of 7.1% for anti-PD-1 antibodies.Median OS was 11.7 mo,12-mo OS probability was 48.9%,and PFS was 3.2 mo.These data,similar toanti-CTLA-4 antibodies,suggest that their activity is modest,with only a few patients benefiting from them.Anyway,a better toxicity profile (AE rate of 50.2%;grade 3-4 AE rate of 13.2%) favors them with respect to ipilimumab.
Combined therapies
The dramatic efficacy of the combination of iplimumab and nivolumab in metastatic cutaneous melanoma prompted studies also in mUM: However,as already reported for monotherapies,efficacy was substantially lower[35].Indeed,the recent meta-analysis of Phametal[31] showed an ORR dropped to 13.5%,with a median OS of 16.0 mo,a 12-mo OS rate of 60.3%,and a median PFS of 3.2 mo.Although these data were slightly better,they are not sufficient to support a clear benefit for mUM patients.Moreover,toxicity is also higher and can be substantial,as shown by an overall AE rate of 85.8% and a grade 3-4 AE rate of 33.9%.
The mechanisms determining a substantially reduced efficacy of ICIs in mUMvsmetastatic cutaneous melanoma are not completely understood.The studies have shown an immune privilege of UM inside the eye through multiple strategies,including expression of PD-L1 and indoleamine dioxygenase-1: These are also allegedly active at metastatic deposits,particularly in the liver,creating a microenvironment particularly resistant to immunotherapy.A low tumor mutational burden has also been suggested to contribute[36,37].
ICIs in combination with liver-directed therapies
The liver is the most common site for UM metastases,and about 50% of the patients will have isolated liver metastases.High lactate dehydrogenase and liver metastases are dominant predictors for ICI failure in cancer therapy since hepatic disease in mUM is particularly resistant to immunotherapy[38].Therefore,liver-directed therapies in combination with ICIs have a strong rationale.
Treatment with ipilimumab/nivolumab in combination with percutaneous hepatic perfusion with melphalan has been explored in a phase Ibtrialona small number of patients.The authors found 1 complete response,5 partial responses,and 1 stable disease.Grade III/IV AEs were observed in 5/7 patients without dose-limiting toxicities or death[39].
Minoretal[40] conducted a pilot study on 26 patients with hepatic metastases of UM treated with two cycles of selective internal radiation therapy (SIRT) with yttrium-90 (90Y) resin microspheres,one to each lobe of the liver,followed in 2-4 wk by immunotherapy with ipilimumab/nivolumab every 3 wk for four doses,then maintenance immunotherapy with nivolumab alone.Initial dosing of both 90Y and immunotherapy resulted in excessive toxicity but,after decreasing the dosage of 90Y microspheres to limit the radiation dose to normal liver to 35 Gy and lowering the ipilimumab dose to 1 mg/kg,the treatment was tolerable.Reported ORR was 20%,median OS 15 mo,and median PFS 5.5 mo[40].
Aedo-Lopezetal[41] conducted a retrospective study of 32 patients with mUM divided into two groups based on the treatment received: SIRT with 90Y microspheres and ipilimumab/nivolumab before or after the SIRT (18 patients)vsSIRT without combined immunotherapy (14 patients).Median OS was 49.6 and 13.6 mo in the two groups respectively.The presence of extra hepatic-metastases at the time of SIRT,a liver lesion over 8 cm,and a high liver tumor volume negatively impacted survival[41].
A case series of 8 patients treated with ipilimumab/nivolumab combination along with transarterial chemoembolization (TACE),followed by nivolumab maintenance and monthly TACE procedures,demonstrated a median OS of 14.2 mo.Two/8 patients had partial response,4/8 stable disease,2/8 disease progression[42].A phase Ib/II study was conducted to assess the safety and efficacy of radiofrequency ablation (RFA) of one liver metastatic lesion plus ipilimumab.Recommended phase II dose was ipilimumab 3 mg/kg+RFA.No confirmed objective responses were observed.Median OS was 14.2 mo for the 10 mg/kg ipilimumab cohortvs9.7 mo for the 3 mg/kg cohort.Median PFS was 3 mo comparable for both cohorts.Combining RFA with ipilimumab 3 mg/kg was well tolerated but showed very limited clinical activity in UM[43].
An ongoing phase II trial (NCT03472586) studies ipilimumab and nivolumab in combination with immunoembolization with lipiodol and granulocyte-macrophage colony-stimulating factor in patients with liver metastases from UM.Similarly,a phase I randomized controlled multicenter trial (NCT04463368) is evaluating the effectiveness of isolated hepatic perfusion with melphalan in combination with ipilimumab and nivolumab.The isolation of the liver from the systemic circulation allows a high concentration of the chemotherapeutic agent to be delivered to the liver in conjunction with hyperthermia,allowing a higher efficacy with minimal systemic exposure to the drug.
Other strategies
The phase Ib,open-label CLEVER study evaluated treatment with intravenous coxsackievirus A21 (V937) in combination with ipilimumab in patients with mUM,based on the rationale that the combination of V937 and ipilimumab might result in augmented T-cell responses with consequent improved clinical activity.However,the combination regimen did not result in objective responses,although 3 patients obtained a stable disease[44].
Drugs targeting epigenetic regulators such as histone deacetylases (HDACs) show promise as cancer therapies by reversing oncogene transcription and modifying the tumor microenvironment.A phase II trial was conducted to evaluate if anti-PD-1 therapies and HDAC inhibitors (entinostat) could synergize.ORR was 14%,median OS was 13.4 mo and median PFS was 2.1 mo,slightly higher than that of anti-PD-1 monotherapy[45].Arginine deprivation with ADI PEG-20 with ipilimumab/nivolumab was also studied in a phase I trial;however,there were no objective responses,and the median OS was only 8.6 mo[46].
Lymphocyte-activation gene 3 (LAG-3) is another immune checkpoint that negatively regulates T-cell proliferation and effector T-cell function.LAG-3 and PD-1 are often co-expressed on tumor-infiltrating lymphocytes,thus contributing to tumor-mediated T-cell exhaustion: Upon this rationale,the phase III RELATIVITY-047 trial validated LAG-3 blockade as a relevant biological target and established it as the third clinically relevant immune checkpoint,demonstrating improved antitumor activity for the combination of the anti-LAG-3 antibody relatlimab and nivolumab concerning nivolumab alone[47].After these results,the combination nivolumab/relatlimab (Opdualag) was approved for medical use in the United States in March 2022 for the first-line therapy of patients with metastatic melanoma.Whether the combination is effective also in rare melanoma subtypes remains to be elucidated: An ongoing trial (NCT04552223) aims to test it in mUM patients.A study from a southern French patient cohort confirmed LAG-3 and PRAME (PReferentially expressed Antigen in MElanoma) as potentially important immunotherapy targets in the treatment of UM patients while proposing Vdomain Ig suppressor of T-cell activation as a novel relevant immune checkpoint molecule in primary UM[48].
Immunotherapy has proven to be an interesting and viable option in UM metastatic disease,even though much has still to be studied to have scientific and clinical effectiveness.Despite optimal management of primary tumors,about half of patients will eventually develop distant metastatic disease.Differently from cutaneous melanoma,the liver is involved in the vast majority of cases: This led to the employment of liver-directed therapies with encouraging results in carefully selected patients[49].However,a randomized trial comparing hepatic artery infusionvssystemic fotemustine was stopped early for futility after failure to show improved OS despite improvement in PFS[50].This observation,considering also that most patients either progress after or are not candidates for locoregional therapy,suggests that effective systemic treatments may represent the therapeutic mainstay.
Historically,patients with ocular or UM were excluded from clinical trials on cutaneous melanoma because of the biological and clinical differences: Therefore,there has been no widely accepted standard treatment,and patients were generally managed with drugs and regimens approved for cutaneous melanoma (i.e.,dacarbazine,temozolomide,or fotemustine).However,despite some responses being observed,none of them was demonstrated to be effective[51].
The discovery of the presence of activating mutation in GNAQ/GNA11 in the majority of UM patients,resulting in constitutional activation of MAPK pathways,led to the development of the MEK inhibitor selumetinib in this setting.A first randomized,phase II clinical trial compared it with chemotherapy (dacarbazine or temozolomide);the primary endpoint was PFS.Selumetinib improved PFS concerning the comparator,but the AE rate was high and OS was similar between the two arms[52].A subsequent randomized,placebo-controlled,phase III study evaluated its combination with dacarbazine concerning dacarbazine monotherapy and failed to show any improvement in either PFS or OS[53].
The ICIs proved very effective in cutaneous melanoma and were subsequently tested in ocular melanoma;however,the results were not as good as expected.Despite retrospective reports suggesting potential efficacy ipilimumab,an anti-CTLA4 antibody,did not show any response in a phase II clinical trial;PFS and OS were comparable with historical controls treated with chemotherapy[54].
The anti-PD1 agents nivolumab and pembrolizumab were also tested as monotherapies in phase II clinical trials.Both agents showed clinical responses that were sometimes durable;however,PFS and OS were highly variable and,overall,not so different from those obtained with chemotherapy[55].Other two studies evaluated the combination of ipilimumab and nivolumab,after the very good results observed in cutaneous melanoma.A first study on 55 patients showed a high rate (51.9%) of disease stabilization;however,PFS and OS were still comparable to historical controls treated with chemotherapy[56].Data from the second one were more promising,observing a median PFS of 5.5 mo and OS of 19.1 mo,but only 33 patients were evaluable for efficacy[57].Overall,these data demonstrate that ICIs,and especially the combination of ipilimumab and nivolumab,may achieve durable responses,but with a limited prognostic impact.
Tebentafusp
ImmTAC,short for immune-mobilizing monoclonal T-cell receptors against cancer,represents a novel category of Tcell-redirecting bispecific fusion proteins.These innovative molecules utilize an engineered high-affinity T-cell receptor to effectively target any protein,including intracellular antigens,displayed as a peptide-HLA complex on the surface ofthe target cell[58].Tebentafusp,previously known as IMCgp100,is an example of such a molecule,featuring a soluble,enhanced HLA-A*0201-restricted T-cell receptor specifically recognizing the glycoprotein 100 (gp100) peptide YLEPGPVTA,that is highly express on UM cells.This receptor is fused with an anti-CD3 single-chain variable fragment.When the ImmTAC binds to its designated peptide-HLA complexes on the surface of the target cell,it enlists and stimulates polyclonal T cellsviaCD3,to kill these cells.In addition to its T cell cytotoxic effects,IMCgp100 stimulates T cells to secrete a diverse array of cytokines and chemokines,such as interleukin-6 (IL-6),IL-2,and tumor necrosis factoralpha,thereby amplifying its potential as an anti-cancer immune agent[59,60].The activation of T-cells by IMCgp100 occurs at a concentration of 1 pM,with the most significant reaction observed at 1 nM.Off-target effects are observable solely at concentrations significantly exceeding 1 nM,highlighting the high specificity of the tumor antigen and a broad therapeutic range,and IMCgp100 activityinvitrocorrelates with the cellular expression levels of gp100-HLA-A*01[61].
The drug was tested in a phase III randomized trial (2:1) against a comparator chosen by the investigator among pembrolizumab,ipilimumab,or dacarbazine (Table 1).PFS was not significantly different among the two arms,but OS was superior in the tebentafusp arm (21.7vs16 mo;hazard ratio=0.51).The decoupling between PFS and OS was explained by the observation that,while responding patients had similar outcomes in both arms,the prognosis of unresponsive patients was better in the tebentafusp arm,suggesting that treatment-induced immune activation slows disease progression even in the absence of an objective response.The toxicity observed is mild or moderate in most cases,with the cytokine-release syndrome and cutaneous reaction being the most characteristic drug-related AEs[62].
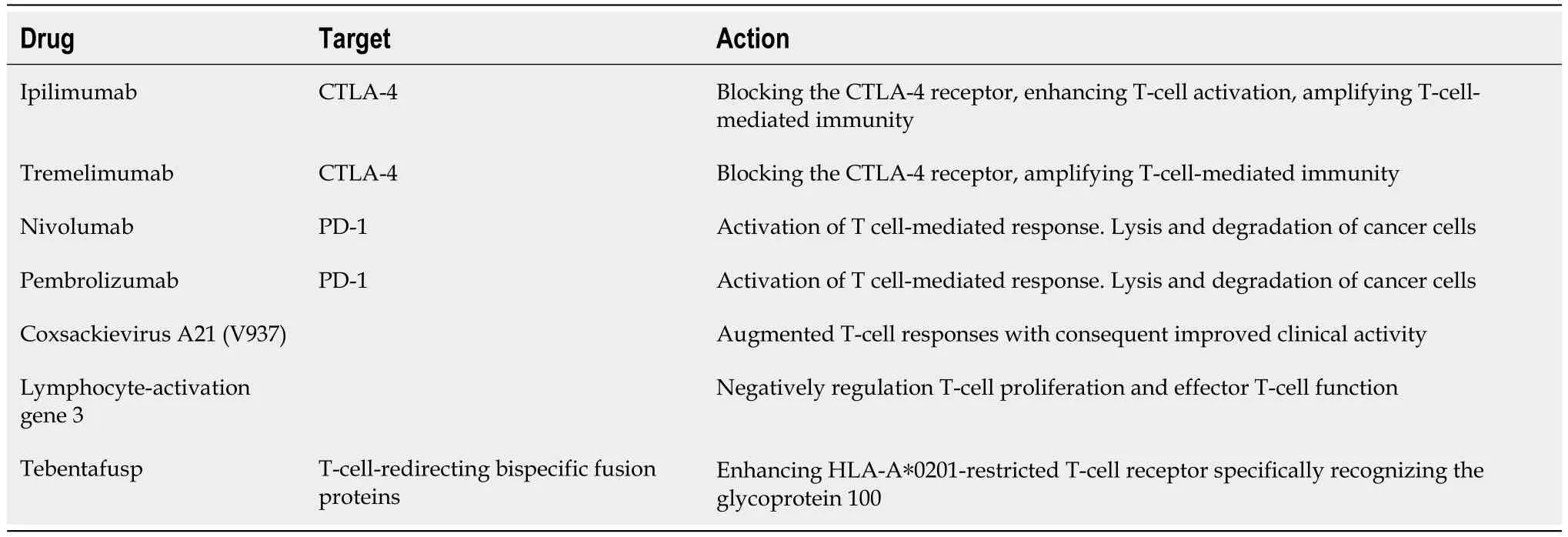
Table 1 Drug therapies for metastatic uveal melanoma disease
Tebentafusp has undoubtedly marked a significant advancement in the treatment of metastatic UM,offering a survival benefit over conventional therapies.Its preferential binding to HLA-A0201 has limited its applicability to patients with this specific subtype,prompting a need for the development of alternative treatments for individuals with other HLA subtypes.Although subgroup analyses in the phase III trial raised questions regarding its potential efficacy in certain patient groups,including those with high tumor burden and poorer performance status,its overall benefit still positions it as a preferred therapeutic option for most HLA-A0201-positive patients.Regarding the optimal duration of treatment,current data suggest that continuing tebentafusp until confirmed radiological progression might be a reasonable approach,given its manageable and predictable toxicities.However,more extensive studies are required to establish the most suitable duration for treatment.Additionally,the challenge of evaluating treatment response necessitates the exploration of alternative markers beyond traditional response measures.The correlation between rash appearance and improved survival warrants further investigation,while circulating tumor DNA reduction holds promise as a potential indicator of treatment benefit.
The investigation of tebentafusp in conjunction with liver-directed therapies is also a significant area of interest,considering the potential benefit for patients with bulky disease.Furthermore,the exploration of other therapeutic targets,such as PRAME,through alternative treatments like IMC-F106C,presents a promising direction for future research efforts.
In this scenario,while certain limitations exist,tebentafusp represents a groundbreaking development in the field of metastatic UM treatment.Unanswered questions regarding response monitoring and application in diverse treatment settings warrant further exploration to optimize its therapeutic potential and expand its applicability to a broader patient population.Future research objectives should include the determination of theoptimal treatment sequence between tebentafusp and checkpoint blockade,as well as the potential benefits of combining these therapies.Ongoing studies focusing on the combination of tebentafusp with other immunotherapies and the assessment of its role in the adjuvant setting after primary disease therapy are critical in further delineating its therapeutic scope.Further research and a comprehensive understanding of tebentafusp’s mechanisms will undoubtedly pave the way for improved treatment strategies and outcomes in the management of metastatic UM.
CONCLUSlON
To conclude,research on immunotherapy for UM metastatic disease has vast supporting scientific and clinically applicable literature but is just at the beginning of a new era for effective treatment to practically increase the surveillance of affected patients.Predictive biomarkers,mechanisms of resistance,treatment duration and treatment beyond progression,immune-related toxicities,and clinical trial design are key concepts in need of further consideration to optimize the anticancer potential treatments.Future studies based on longer follow-up with homogenous criteria,preferably on human subjects,can pave the way to tailoring immunotherapy for patients affected by ultraviolet metastatic disease.
FOOTNOTES
Author contributions:De Rosa F and Zeppieri M wrote the outline;De Rosa F assisted in the revisions of the manuscript;Salati C and Spadea L assisted in the editing of the manuscript;Zeppieri M assisted in the conception and design of the study,and completed the English and scientific editing (a native English speaking MD,PhD);Sorrentino FS,De Rosa F,Di Terlizzi P,Toneatto G,Gabai A,Finocchio L,Salati C,Spadea L,and Zeppieri M participated in the manuscript writing;Sorrentino FS,De Rosa F,Di Terlizzi P,Toneatto G,and Gabai A contributed to the research;Sorrentino FS and Zeppieri M provided the final approval of the version of the article.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Italy
ORClD number:Francesco De Rosa 0000-0003-0511-1298;Carlo Salati 0000-0003-4736-5296;Leopoldo Spadea 0000-0002-1190-3956;Marco Zeppieri 0000-0003-0999-5545.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Zhang XD
 World Journal of Clinical Oncology2024年1期
World Journal of Clinical Oncology2024年1期
- World Journal of Clinical Oncology的其它文章
- Prognostic factors of breast cancer brain metastasis
- Radiotherapy for hyoid bone metastasis from lung adenocarcinoma: A case report
- What are the changes in the hotspots and frontiers of microRNAs in hepatocellular carcinoma over the past decade?
- Fatty acid binding protein 5 is a novel therapeutic target for hepatocellular carcinoma
- Gene signatures to therapeutics: Assessing the potential of ivermectin against t(4;14) multiple myeloma
- Predicting colorectal cancer prognosis based on long noncoding RNAs of disulfidptosis genes
