Progress on the application of graphene-based composites toward energetic materials: A review
Ting Zhng , Xioming Go , Jihen Li , Lii Xio , Hongxu Go , Fengqi Zho ,**,Hixi M ,*
a College of Chemistry & Chemical Engineering, Yan'an University, Yan'an 716000, Shaanxi, PR China
b School of Chemical Engineering, Northwest University, Xi'an 710069, Shaanxi, PR China
c Xi'an Modern Chemistry Research Institute, Xi'an 710065, Shaanxi, PR China
Keywords: Graphene Desensitization Thermal decomposition Catalytic combustion Energetic materials
ABSTRACT Carbon material is an important additive in energetic materials.Graphene is a monolayer carbon material in which carbon atoms are arranged in two-dimensional honeycomb structure, who has special optical,electrical, and mechanical properties.Recently, the application of graphene-based composites in energetic materials has received extensive attention.This review mainly summarizes the applications of graphene and graphene-based nanomaterials in energetic materials.The effects of these materials on the thermal stability, sensitivity, mechanical property, ignition and combustion of energetic materials were discussed.Furthermore, the progress of functionalized modification of graphene has been summarized,including covalent bonding modification and doping modification.These studies show that graphenebased materials exhibit excellent performances and might emerge as promising candidate for energetic materials.
1.Introduction
Energetic materials (EMs) as a class of compounds or mixtures containing combustible substances with violent chemical reaction and energy output, is one important part of the formulation of explosives, pyrotechnics and propellants.Solid propellants are a kind of composite EMs, consisting of high energy explosives (oxidants), plasticizer, binder, combustion catalyst, etc [1,2].Carbon material as co-catalyst is one of the important additives for propellants.The variable electronic orbital characteristics(sp,sp2,sp3)of carbon element show the anisotropies of crystal and arrangement, resulting in various structures existed in nature such as diamond, graphite, carbon black, porous carbon, carbon fiber,fullerene, carbon nanotubes, graphene and so on.Different atomic arrangements cause structural differences, thus showing variable properties from hard to soft, insulator to superconductor, light absorption to light transmission,etc.From the discovery of gunpowder to the development of modern weapons and ammunition,the application of carbon materials in EMs has become a hot topic [3,4].Carbon materials, which can be used as desensitizer,binder, combustion catalyst or fuel that play important roles in modifying the sensitivity, energy release and mechanical strength of EMs [5-8].
Graphene is a monolayer carbon material, which arouses widespread concern since its first preparation.It has stable double bond formed by sp2hybrid orbitals and honeycombed structure,and shows some unique physicochemical properties.Such as large specific surface area (2630 m2/g), excellent conductivity (thermal conductivity of 3000-5000 W/mK, and carrier mobility of 15 000 cm/(V·s)[9].When graphene is composited with EMs,these unique properties of graphene endow EMs/graphene with close interface contact,fast electron transfer and heat transfer abilities,accelerated energy release rate, and a low explosive risk caused by impact[9,10].Additionally,graphene can also be used as catalyst matrix to inhibit particle agglomeration, thus forming graphene-based composite catalyst with more dispersed particles and increased specific surface area [11-14].Compared with other carbon materials, graphene exhibits excellent mechanical properties with the fracture strength of 125 GPa and Young’s modulus of 1 TPa.For EMs, interfacial stripping (dewetting) and component migration are two key factors affecting its mechanical properties, which causes weak bonds between solid filler and binder that decreases its storage life [15,16].The key to improving the mechanical properties without too much adjustment of the formula is to enhance the interfacial bonding ability between solid filler and binder.Graphene oxide (GO) is one important derivative of pristine graphene, which is an ideal support for solid filler.The abundant containing-oxygen groups (carboxyl, hydroxyl, and epoxy groups)adhered on the surface and edge of GO is beneficial for bonding with binder.Moreover, the small friction coefficient of graphene shows an excellent lubricating property [17], contributing to reducing the internal friction between EMs and surroundings and the generation of hotspots in EMs [8].
Reduced graphene oxide (rGO), another derivative of pristine graphene, can be prepared by reducing agent (eg.NaBH4, ascorbic acid, N2H4) or high temperature treatment under inert gas,showing a better conductivity and less functional groups than GO.Pristine graphene is composed of benzene rings, having a stable structure and low chemical reaction activity.Compared with rGO and pristine graphene, GO has the better solubility, dispersibility and reactive activity that evidently improves its processability,making it easier to be modified by molecules or groups[18-20].For EMs,we can optionally attach some energetic molecules or groups to the surface of GO, such as -NO2, -NHNO2, -N=N-, and -N3.These functionalized GO materials develop a series of new nonmetallic energetic combustion catalysts that provide desirable energy level,sensitivity,and low characteristic signal.On the other hand, these groups might promote the secondary decomposition reaction of energetic components.Recently, some energetic compounds have been developed to bridge with GO, and then coordinated with transition metal cations, forming new graphene-based metal organic compounds with excellent thermal stability and detonation performance[21,22].In addition,graphene,GO and rGO also could composite with metal/metal oxide to prepare graphenebased catalyst, and lots of research has been done in the catalysis and combustion of EMs [11,23].
In this review,we summarized the latest research progresses of graphene-based materials (graphene, graphene/metal and metal oxides composites, graphene-based thermite, graphene/polymer and functionalized graphene) applied in EMs.Their influences on EMs are mainly focused on the following aspects: (i) Effect on thermal decomposition; (ii) Effect on ignition; (iii) Effect on combustion performance; (iv) Effect on thermal stability and sensitivity; (v) Effect on mechanical property; (vi) Trace detection of explosives[8,24-28].
As shown in Fig.1, traditional perchlorate, nitro-explosives, nitrate explosives and nitroamine explosives such as ammonium perchlorate (AP), cyclotetramethylenetetranitramine (HMX),cyclotrimethylene trinitramine (RDX), hexanitrohexaazaisowurtzitane(CL-20)and other high nitrogen compounds are commonly used as high energy explosives (oxidants) in solid propellants [11], which account for ~80% of the total mass.Therefore,we mainly discuss the effects of these graphene and graphenebased materials on these EMs in the following chapters.
2.Application of graphene-based materials in EMs
2.1.The role of graphene in EMs
Carbon is a multifunctional additive when applied into EMs.For example, graphite always acts as a desensitizer because of its high thermal conductivity and efficient lubricity [29].Carbon black can shorten the ignition delay times as well as reduce sensitivity [30].The introduction of fullerene and carbon nanotubes into highenergy explosive can modify combustion characteristics and heat sensitivity [30-33].Therefore, Graphene, as a new type of advanced carbon material with distinctive thermodynamic,optical,and mechanical properties, is much suitable as a high-energy explosive additive.When EMs are combined with graphene, it shows the advantages of reducing sensitivity (impact sensitivity,electrostatic spark sensitivity and friction sensitivity), accelerating burning rate,and enhancing mechanical strength.
Desensitization and thermal decomposition:High energy and low sensitivity are two important targets for the development of EMs [34].Impact, static electricity, friction and other external stimuli are the main factors that induces the explosion of explosives.And various strategies have been conducted to reduce the sensitivity of EMs, such as grain refining, coating and adding desensitizer (inert or energetic desensitizer).Thermal decomposition property of EMs could reflect the phase transition, thermal stability and compatibility, as well as storage life, which can be evaluated by dynamic thermal analysis, such as differential scanning calorimetry (DSC), thermogravimetric-differential thermogravimetric(TG-DTG),and simultaneous thermogravimetry-infrared spectroscopy-mass spectrometry (TG/IR/MS).
In 2010,Zhang et al.first predicted that graphene could serve as an excellent carrier for monolayer molecules of 1,3,5-triamino-2,4,6-trinitrobenzene(TATB)using COMPASS force field calculation.TATB and graphene have a similar two-dimensional structure, in which the chemical bonding of π-π stacking between graphene and TATB is benefit to form graphene/TATB super-molecule structure[35].This multiple structure shows a predicable density of 2.112 g/cm3, explosion velocity of 2.40 km/s, and detonation pressure of 10.5 GPa, respectively.The shear sliding potentials of TATB/graphene is lowest compared with graphite/TATB and single TATB due to the excellent lubricating property of graphene, leading to the highest desensitizing efficiency.And a series of properties such as thermal stability, enthalpy of formation, packing density, lubricating property, and detonation property, can be optimized by adjusting the proportion and the overlaying order of graphene and TATB.This means graphene could affect the decomposition pathway of EMs which ultimately control energy release and provide insensitivity while also yielding optimal material performance[36].
Except for theoretical research, the application of graphene in EMs has been studied extensively.Compared with graphene, GO disperses easily in water or organic solvents because its hydrophilic groups can form hydrogen bonds with solvents [37].Besides, GO could be viewed as a soft polymer due to the ultra-thin thickness and periodic two-dimensional structure, as well as its perfect mechanical property [38].Therefore, it can be used to coat and encapsulate energetic molecules to balance energy,sensitivity and mechanical strength.The GO-based energetic composites can be obtained through physical grinding, ultrasonic dispersion, recrystallization, solvent evaporation,solvent-non-solvent method and so on.Particularly, solvent-non-solvent method is always used to prepare composites with small particle size and high purity[39,40].After this in-situ recrystallization process,EMs can be well coupled with graphene materials.Fig.2(b) shows that HMX was tightly enwrapped by GO wrinkles through solvent-nonsolvent process,whereas the pure HMX surfaces are smooth and clean(Fig.2(a))[8].This encapsulated structure can effectively resist external impact and reduce explosion risk.And a distinct advantage of desensitizing effect can be produced compared with C60and CNTs at the same amount.Only 2%GO can decrease the impact sensitivity of HMX by 90%, as well as the friction probability reduced by 68%.Viton, as a polymer elastomer, has the characteristics of stability, high temperature resistance and aging resistance, which is commonly used in aviation, missile, rocket and other industrial fields.The smooth surface of HMX becomes rough after well encapsulated by 5%Viton and GO layer.HMX/Viton/GO remains a good thermal stability with the exothermic peak of 283.21°C,which is close to HMX(283.65°C)and HMX/Viton (284.30°C) [41].Both impact and shock wave sensitivities of HMX/Viton/GO are much lower than those of HMX and HMX/Viton, revealing the efficient desensitized effect of GO.
Fig.1.Several typical energetic materials.

Fig.2.(a), (b) SEM images of HMX and GO/HMX; (c), (d) CL-20 and GO/CL-20; (e), (f) LS ((e1) the raw GNP material and (e2) the crystal morphology of LS) and graphene nanoplatelet-lead styphnate composites (GLS).Reproduced with permission from Refs.[8,42,43].
Generally, in-situ preparation of graphene-based EMs may result in three types: (i) Complete coating (Fig.2(b)); (ii) Surface growth(Fig.2(d));(iii)Surface adhesion(Fig.2(f)).Fig.2(d)shows that CL-20 crystals closely grow on the surface of threedimensional graphene foam, in which GO acts as a support, lubricant and conductor that could reduce the internal folding dislocations and hot spots[42].Fig.2(d)shows a guest/host architecture of CL-20/graphene foam(GF)by in-situ crystallization process.GF was prepared using nickel foam as substrate to form three dimensional(3D) network structure.The CL-20/GF was obtained by immersing GF into a solution of CL-20 in ethyl acetate and then by slow evaporation of ethyl acetate.When the additive content of GF is 2%,a much reduced mechanical sensitivity(impact sensitivity,friction sensitivity and electrostatic spark sensitivity)can be achieved.The detonation pressure and velocity calculated by EXPLO5(V6.02)program reflect a depressed trend with the increase of GO content,but still are higher than those of HMX and RDX.Therefore, this guest/host architecture shows a potential strategy for the balance of energy and sensitivity of EMs.Fig.2(f) reveals the incomplete coating of EMs by graphene.It can be seen that graphene nanoplatelet(GNP)is coated on to some places of the lead styphnate(LS)crystals [43], corresponding to the surface adhesion type.Even if the coating is incomplete, the electrostatic spark sensitivity and static electricity accumulation of LS can also be effectively suppressed.It follows that incomplete coating still play a positive role in reducing the sensitivity of EMs.
GO is thermally unstable along with violent heat release under external thermal stimuli.rGO is not as energetic as GO,but its heat conductivity and electric conductivity are higher than GO.Therefore,we can selectively use GO or rGO according to the application requirements.Plastic-bonded explosive (PBX) is conventional explosive comprised elastomeric binders and other additives.In CL-20-based PBX system, the high contents binder has the disadvantages of poor processability, low solid loading, low energy output and inferior mechanical properties.Yu et al.found that GO reduced the decomposition temperature of CL-20/551 glue,while,increased decomposition heat release.In contrast, rGO offered a moderate decomposition heat release and a better thermal stability for CL-20/551 glue [44].Table 1 also summarizes the preparation methods,thermal decomposition temperature (T), reaction heat (ΔH),apparent activation energy (E) and sensitivity of these graphenebased energetic composites that reported in recent years.Their thermodynamic properties are greatly affected by graphene materials regardless of the binding methods of explosives to graphene.From the decreased decomposition temperatures and activation energies, one can see that graphene stimulates the thermal decomposition of energetic component.And reasonable regulation of the dosage of graphene materials can simultaneously optimize the energy release and safety performance of EMs.
Ignition and combustion:Apart from the desensitization and thermal decomposition, graphene materials can also be used to regulate the ignition and combustion properties of EMs[53].When EMs are stimulated by external stimulus such as external heat and mechanical stimuli,the hot spots can be produced and then ignited combustion will happen.The period from the onset of external stimulation to sustainable ignition is called ignition delay time.The longer ignition delay will cause fuel accumulation and pressure increase, further damage the engine.Generally, methods of improving ignition delay include changing loading density and components, or adding catalyst.GO is a highly reactive substance that can be completely burned without producing exhaust particulate matter.GO not only catalyzes the thermolysis of EMs,but also participates the decomposition reaction.Besides,both the thermal radiation ability, heat transfer rate and energy density can be enhanced when EMs are composited with GO,leading to a reduced ignition threshold and an increased combustion rate.In 2009,Sabourin systematically compared the combustion of nitromethane(NM) monopropellant under the addition of colloidal particles of functionalized graphene sheets (FGSs) and metal hydroxides [54].GO outperformed both aluminum monohydroxide and silica nanoparticles.The burning rate of NM containing 0.2% GO up to 175%over pure NM.The detailed decomposition mechanism of NM catalyzed by GO was subsequently conducted according the ab initio molecular dynamics simulations [55].The atomic structuresof FGS and NM during the NM decomposition reaction at different reaction time.The thermal decomposition of NM and its derivatives can be accelerated by the vacancy defects within the plane of the FGSs, which involve the exchange of protons or oxygens between the oxygen-containing functional groups and NM and its derivatives, ultimately forming H2O,CO2, and N2.

Table 1A summary of thermal behavior parameters of single compound explosive under the effect of graphene materials.
GO has hydrophilic groups and hydrophobic matrix showing an amphiphilic property.Therefore,it is easy to disperse in liquid NM to form stable suspension without additional solvent.For solid matrix, GO could be well coupled with them by recrystallization.Liu et al.reported a tuning of light ignition and combustion efficiency of NC with GO as the additive[56].Through the freeze-dried in vacuum, NC/GO composites possess highly porous microstructure that favors its rapid and violent combustion.Figs.3(a)-3(c)shows the combustion evolution of NC composited with different content of GO.Under the same laser pulse (2 W, 50 ms,1064 nm,2 mm spot size),the combustion wave rate and combustion area of three samples spread differently after being triggered, whereas pure NC cannot be ignited.Among them, NC/GO-20% shows the highest flame brightness and burning rate,indicating the optimum combustion performance.However, a small scattered flame rather than continuous large areas flame for NC/GO-20%were observed by adding a small quantity of MWNTs (Fig.3(d)), indicating an inhibition of combustion by MWNTs.
Metastable intermolecular composites (MICs) composed of nano-scale fuels and oxidants is a high energy density additive in solid propellants [57].Sol-gel chemistry is a novel method for the preparation of MICs [58].It is easy to control the contact area between two components at the nanoscale through the sol-gel process, thereby obtaining the faster reaction rate and energy release rate than simple mechanical mixing.AP is a leading ingredient(oxidant) in solid propellants, and its thermal decomposition characteristics directly affect the combustion performance of propellants.In 2014,Wang et al.used sol-gel technology to embed AP into 3D porous graphene aerogels (GA).GA was firstly prepared using L-ascorbic acid as the reduction agent, and then immersed into the saturated aqueous AP.The above solution was further replaced by ethyl acetate and dried with supercritical CO2to form a new MIC-AP/GA nanostructured energetic composite.SEM images revealed that the GA surface was entirely covered by AP crystals,thus corresponding to a reduced specific surface area.The thermolysis rate and total heat release of AP/GA significantly increased attributing to that GA participates in the oxidation reaction as a fuel[45].

Fig.3.Sequential snapshots of the combustion evolution of NC/GO composites: (a) NC/GO-11%; (b) NC/GO-20%; (c) NC/GO-33%; (d) NC/GO/MWNTs membranes.Permission from Ref.[56].
Based on the advantages of MICs,Chen et al.found a novel way to incorporate fuel and oxidizer into a highly symmetric ternary system (H2dabco)[M(ClO4)3] (DAP)) through noncovalent interactions,whose detonation performance is superior to HMX and comparable to CL-20[59].Subsequently,Deng et al.introduced the functionalized graphene into DAP (Fig.4(a)) by physical mixing with the mass ratio of 1:9, and further studied the combustion performance of DAP/graphene [46].A slight decreased decomposition temperature of DAP/graphene(372.3°C)compared with pure DAP (385.0°C) means an easier decomposition reaction and lower decomposition activation energy for DAP/graphene.The key reason is that graphene can promote hydrogen transfer from protonated H2dabco2+to ClO4-and catalyze decomposition of DAP.The deflagration process of the composite and DAP was studied trough electric ignition triggering test.DAP undergoes two deflagration processes from 1 to 3 ms (drastic deflagration process) and 6-27 ms (intermittent slow combustion process), respectively.While, the DAP/graphene composites shows a self-sustained deflagration reaction in the whole process from 3 to 27 ms(Fig.4(b)).These high sustained exothermic capacity and selfpropagating combustion performance may be attributed to the excellent heat and mass transfer of graphene, thus forming the synergistic catalysis combustion mechanism based on relaydomino-like reactions.
As is known to all, the high-performance solid propellants are determined by thermal property,mechanical strength,combustion performance and etc.Coupling these propellants with highly conductive graphene to improve the heat transport and control the combustion wave propagation speed are important for the development of low-cost and high-performance micro-power systems.The diffusion rate and heat transport among reactants play vital roles in controlling the burning rate of propellants.Previous study has proved that graphene shows excellent thermal transport property on Al/Teflon reactant matrix, such as thermal conductivity, thermal diffusivity and specific heat [60].In 2013, Zhang et al.first reported the enhancement effect of laser ignition and burn rate of NC microfilm doped with GO[25].With the increasing of doping content of GO, the surface morphology of NC gradually became rough and shows highly porous networks structure.The NC films can be easily ignited with the addition of GO under the Nd:YAG laser irradiation (20 J/cm).The combustion snapshots show a significant enhancement in burning rate for GO-doped NC with the addition concentrations of GO from 0.05% to 1.0%.After that, Jain et al.studied the effects on flame speed enhancement of NC under graphene foam (GF) and graphene nanopellets (GNPs) [61].The inter-connected 3D structure of GF provides a continuous thermal conduction path that effectively promotes the transfer of heat from reaction zone to unburned area, leading to an enhancement of flame speed.
Mechanical Property:The mechanical property of propellants is another major technical bottleneck that restricts the application.Adding some plasticizer is an effective method to solve this problem, but the energy will surely reduce.Pursuing a substance that substitutes for unenergetic plasticizer is critical to achieve the balance of energy level and mechanical strength.GO has periodic planar structure,abundant energetic functional groups and unique mechanical strength, hence it can be viewed as an energetic polymer to construct composite explosives.Nitrate glycerol ether cellulose (NGEC) is a binder applied in modified double base propellant [62], which can be chemically bonded with GO via hydrogen-bonding or electrostatic interaction during the solvent evaporation process,and the Young’s modulus,tensile strength and elongation at break of NGEC/GO (w= 0.5%) increased by 40%, 38%and 44% compared with pure NGEC,respectively.
Most of above researches only report a particular aspect of EMs,whether the overall performance of EMs incorporated with graphene is desirable? In 2019, Shen et al.systematically studied the thermal, mechanical, and combustion properties of NC-TEGDNRDX composite propellant incorporated with GNPs.Compared with pure NC-TEGDN-RDX composite propellant, GNPs can decrease its onset decomposition temperature and increase heat release.And the composite propellant exhibits a higher burning rate, combustion pressure and a lower pressure exponent than those without GNPs.Besides,when 0.5 wt%GNPs was incorporated into NC-TEGDN-RDX, the enhanced tensile strength, impact strength and compressive strength can be obtained at -40°C and 20°C, respectively [63].These results further reveal that graphene material is a potential reinforcing agent to improve the comprehensive property of propellants.A large number of researchers are still striving for exploiting the potentiality of graphene and its associated products to serve for propellants.

Fig.4.(a) The perovskite structure of DAP; (b) The ignition and combustion processes of DAP and DAP/graphene.Recondition with permission from Ref.[46].
2.2.Effects of metal/graphene and metal oxides/graphene
Composite materials composed of two or more components with different properties can produce a synergistic effect and make the comprehensive properties of the composites better than that of single component.For graphene-based composites, another component could be metals, metal compounds, polymers, organic compounds,or the other inorganic additives.Metal or metal oxides are commonly used as combustion catalyst in solid propellants[64], and graphene itself has a positive effect on modulating the sensitivity, energy and combustion of EMs [65].Large specific surface area and super mechanical properties of graphene is extremely suitable for supporting particles for the preparation of metal/graphene and metal oxides/graphene composites with high dispersibility, high surface area and stable structure.When these nanoparticles are incorporated with graphene, a synergistic effect between graphene and nanoparticles can be obtained.
2.2.1.Metal/graphenecomposites
Metal nanomaterials are only one of the branches of nanomaterials.The electronic structures of the 0D nanometal powders are significantly different from the metal particles with the same composition,thus showing some unique properties in magnetism,photoelectricity,etc.In order to obtain a high combustion temperature,specific impact and velocity,some metal powders such as Mg,Pb,Al,and Ni always were used as metallic fuels in propellants due to their high density and high reaction heat [66,67].The functions of these metal powders are as follows:(i)Improve energy density; (ii) Increase combustion heat; (iii) Improve the specific impact and velocity; (v) in-situ generation of metal oxide during the combustion process that inhibits the oscillatory combustion of propellants.Attaching nano metal particles on graphene surface can also inhibit the particle aggregation, thereby constructing a metal/graphene composite with high reactivity.
In practice, micron-sized Al (μ-Al) is restricted by poor light absorption ability and high ignition temperature.But GO with favorable heat conductivity and energy could accelerate the optical absorption and heat release to facilitate Al oxidation,resulting in an enhancement of ignition and combustion properties for Al/GO composites [68].In 2012, Li et al.successfully prepared a Ni/graphene nanocomposite using solvothermal method.Ni nanoparticles as an isolated material were uniformly dispersed on the surface of graphene, enabling the separation of adjacent graphene sheets in a solvothermal system[69].And the controllable regulation of the size and loading amount of Ni nanoparticles on graphene sheets can be realized by changing the metal salt concentration.The catalytic performance of Ni/graphene nanocomposites for thermal decomposition of AP was analyzed using thermogravimetry (TG) and differential scanning calorimetry (DSC) methods.Results showed that the low temperature decomposition peak disappeared and the high temperature decomposition peak decreased by 97.3°C under the addition of Ni/graphene.Besides Ni particles, others such as Au, Cu, or NiMn alloy also were used to prepare metal/graphene composites.The preparation method and the corresponding thermal parameters are listed in the following Table 2.These metal/graphene composite catalysts possess small particle size and high dispersibility, giving rise to a high catalytic activity on the thermolysis of AP.
2.2.2.Metaloxides/graphenecomposites
To date, graphene-based metal oxide composites attract widespread interest in the fields of capacitor,electrolysis and others due to their synergistic effect between graphene and metal oxides[73-75].Transition metal oxides such as Fe2O3, CuO, PbO, Bi2O3,NiO, Cr2O3, MnO2, etc [64] are always used as combustion catalyst that modifies the combustion performance of propellants, such as(i)Increase burning rate;(ii)Decreases the pressure exponent;(iii)Decrease ignition delay time;(iv)Improves combustion stability of propellants.But metal oxides at the nanoscale show seriously agglomeration due to the high specific surface energy.Nevertheless, graphene with large surface, superior conductivity and mechanical strength pave the way for constructing metal oxides/graphene composites with high stability, flexibility, porous structure and high active sites.Recently,the application of metal oxides/graphene composites also attracts widely concerns in EMs [76].These metal oxide/graphene hybrids can be prepared by various methods, including chemical-precipitation, mechanical mixing,electrostatic self-assembly, hydrothermal/solvothermal method,sol-gel and so on [77-83].
Chemical precipitation method:The advantages of the chemical precipitation method are as follows: one is to directly obtain nano materials with uniform chemical composition through chemical reactions in solution; the other is to easily prepare nano materials with small particle size and uniform distribution.Besides,the size, crystal type and morphology of nanomaterials can be tailored through controlling reaction condition, such as chemical ratio, concentration, reaction temperature, pH value, calcination temperature,etc.Transition metal oxides coupled with graphene can give full play to its characteristics of high porosity,large specific surface area,stability and high catalytic activity,thus improving the combustion performance.In 2010, Zhu et al.reported a CuO/rGO composite to catalyze the exothermal decomposition of AP[97].In a mixed system of water and isopropanol,Cu2+can be intercalated and adsorbed on GO sheets,and then CuO crystallites nucleate and uniformly grow on GO surface through a simple chemical coprecipitation reaction.And the final morphology of product is affected by various factors, such as polarity, steric hindrance,electrostatic effect,and different coordination capabilities between water and isopropanol.Therefore, the introduction of two-solvent system will provide us with the possibility to regulate the performance of the product.When the mass ratio of GO to CuO is 1:2,only 2%CuO/GO can reduce the decomposition temperature of AP from 414 to 315°C,and increase the heat output from 590 to 1347 J/g.This is the first time that metal oxides/graphene composite prepared by in-situ growth has been applied in EMs.Study show that the catalytic activity of bimetallic oxide is much better than that of single metal oxides.An et al.reported Cu2O-PbO/GO and CuO-Bi2O3/GO nanocomposites prepared by co-precipitation and subsequent calcination method [98].CuO combined with Bi2O3or PbO forms binary composite catalysts in the synthesis process.Compared with pure metal oxides without GO,the interconnection of GO increases the thermal conductivity and reaction active sites of catalyst.Both Cu2O-PbO/GO and CuO-Bi2O3/GO show positive catalytic effects in improving the combustion of DB and RDX-CMDB propellants due to the dispersion effect of GO and synergistic catalytic effect.With the addition of Cu2O-PbO/GO, a ‘mesa burning’ can be produced for DB propellant in the pressure range of 12-20 MPa.Especially, a‘plateau combustion’of RDX-CMDB propellant at higher pressures appeared under these composite catalysts.
Hydrothermal/solvothermal method:Hydrothermal/solvothermal method is used for inorganic synthesis by heating and pressurizing the reaction system, which has several merits of variable particle size, reduced reaction temperature, and controllable morphology.Common organic reagents such as ethanol,methanol,ethanol, ethylene glycol, ethylene diamine, and dimethylformamide all can be used as reaction solvents [99].Some organic solvents with reducibility also act as reducing agent,for example,the synthesis of nano alloy.For metal oxides/graphene composites,GOand precursor are simultaneously dissolved in reaction solvent,and then metal oxides particles nucleate andin-situgrow on the surface of graphene during the hydrothermal/solvothermal process.In 2013, Li et al.used solvothermal method to prepare Mn3O4/rGO composites byin-situgrowth without using any reducing agent and surfactant[100].The particles size of Mn3O4is about 10 nm with a high loading rate of 91.2%and five-times increased specific surface area (Fig.5(a)).Mn3O4/rGO with only 5% mass percent can reduce the high temperature decomposition (HTD) temperature of AP by 141.9°C.Maghemite (γ-Fe2O3), as another crystal of iron oxide, is always used for denitrification of combustion emissions due to the high selective catalysis reduction(NH3-SCR)of NOx.As it happens,NOxis the main decomposition products of EMs.Herein, we prepared a γ-Fe2O3/rGO composite by solvothermal method and postheat treatment.We found that graphene acted as both carrier and morphological regulator,forming an intimately coupled interaction between graphene sheets and γ-Fe2O3particles (Fig.5(b)).The particle size of γ-Fe2O3decreases from 1 μm to 200 nm after the in situ assembling on graphene.These γ-Fe2O3/rGO composites decreased the thermal decomposition temperature of CL-20 by 6.93°C and the apparent activation energy by 32.34 kJ/mol,respectively [101].Figs.5(c)-5(f) also show the other composite catalyst such as Co3O4/GO, Fe2O3/rGO and CuO/3D-(N)GFs who outperform single metal oxides or graphene on the thermal decomposition of energetic components [80,84-87].

Table 2A summary of thermal behavior parameters of AP under the effect of metal particles/graphene.
Graphene, as a high thermal conductivity and low thermal boundary resistance carbon substance,also is beneficial to regulate the burning rates of propellants.In 2019, Jain et al.studied the enhancement effect of graphene-supported nanocatalyst on flame speed.When NC couples with MnO2-coated graphene structures,the flame speed is 9 times faster than that of bulk NC[102].MnO2-coated graphene structures combine the superior thermal transport properties of graphene with chemical reactivity of MnO2,then showing a significant reinforcement of flame speed and thermal decomposition characteristics for NC.And the similar enhanced effect was obtained in their another work where a CuOgraphene micro-structures was applied into ammonium perchlorate-nitrocellulose composite solid propellant[103].Unlike single metal oxides, composite metal oxides are composed of two or more different metals, has attracted much attention nowadays due to the better stability, corrosion resistance, and temperature resistance than single metal oxides.In 2016,Chen et al.synthesized a microsphere-like CoFe2O4/rGO composite via solvothermal method [104].SEM and TEM demonstrated that graphene had the effect of adjusting the size of CoFe2O4nanoparticles,which reduced the particle size of CoFe2O4to ~120 nm.The catalytic activity of CoFe2O4/rGO is better than that of both CoFe2O4and rGO at the same dosage.And CoFe2O4/rGO of only 1%mass percent can reduce the HTD of AP to 347.6°C, whereas, the HTD is 367.0°C with 2%Fe2O3/graphene, indicating the existence of synergetic effect between Co2+and Fe3+.In addition to above-mentioned materials,the other graphene-based composites such as MFe2O4/rGO(M = Mg, Co, Zn, Cu and Ni) also have been prepared through hydrothermal/solvothermal method and some of them are shown in Figs.5(g)-5(l) [88-90].One can see that these metal oxides particles uniformly adhere on the surface of graphene and form a stable structure.Their catalytic effects on the thermal decomposition of energetic components such as AP, RDX, HMX or CL-20 are summarized in Table 3.
Sol-gel method:The sol-gel method is that using a high reaction active component as a precursor, and then hydrolyzed and condensed forming a stable sol system.The sol particles slowly polymerize to form a gel with a three-dimensional network structure filled with solvent.Finally,the gel is dried and calcined to prepare nano-material[105].The chemical reaction occurs easily at room temperature and a molecular-level uniformity can be obtained in a very short time through sol-gel process.This strategy can achieve the synthesis of graphene-coated materials with high stability and pore structure.For instance, GO and FeCl3were dispersed in N, N -dimethylformamide (DMF), and the gel was formed by the cross-linking of epichlorohydrin.Finally, Fe2O3/graphene aerogel (Fe2O3/Gr) with high porous structure can be obtained by supercritical fluid drying process [106].The prepared Fe2O3/Gr aerogel shows high catalytic activity on the thermolysis of AP.As the increase of Fe2O3/Gr content, the two exothermic decomposition processes gradually merged into one strong exothermic process, accompany with decreased decomposition temperature and increased exothermic heat.
Self-assembly method:Assembly technology has received much attention since first reported in 1980[107].It is that atoms or molecules are arranged spontaneously in a one-dimensional, twodimensional, or three-dimensional ordered space structure.Two kinds of particles with opposite charges in solution rely on electrostatic attraction to form the binary or multicomponent complex[108].Electrostatic self-assembly technology is famous for the assembly of organic and inorganic molecules.For EMs, it has been used to study the surface modification of aluminum powder, antihygroscopicity of ammonium dinitramide, preparation of aluminum-based EMs and so on [109,110].Recently, this strategy has been widely used in the preparation of graphene-based composite materials [111].In 2016, Xu groups used ultrasonic-assisted mixing and pH-dependent self-assembly technology to prepare two kinds of GO-based MgFe2O4materials.The self-assembled GOcoated MgFe2O4composite shows better catalytic activity on the thermal decomposition of AP than GO/MgFe2O4prepared by ultrasonic mixing [91].In addition, they also prepared MgWO4/GO,BiWO4/GO, and PbZrO3/GO composites by self-assembly method[92-94].These materials show excellent catalytic activity on the thermal decomposition of energetic components such as AP, RDX,HMX,TKX-50 and double-base propellants.Our groups prepared a Fe2O3/rGO composites by assembly, using to catalyze the thermal decomposition of CL-20.Result show that Fe2O3/rGO can not only decrease the decomposition energy barrier of CL-20,but also slight decrease its impact sensitivity [112].
Table 3 summarizes some relevant studies of metal oxides/graphene in EMs.Apart from the preparation method and catalyst amount,the corresponding thermal decomposition parameters are listed in detail, including exothermic decomposition peak temperature,decomposition heat,and activation energy.These graphenebased composites all exhibit better catalytic effects than either of graphene or metal oxides,which greatly decreased the exothermic decomposition peak temperature of EMs due to the synergistic action of metal oxides and graphene, especially for the HTD of AP.The decreased decomposition temperature and apparent activation energy indicate that catalyst weakens the reaction energy barrier and accelerates the decomposition rate of EMs, thus making the pyrolysis of EMs easier.Although metal oxides play a decisive role in regulating the combustion performance of solid propellants,the decreased energy output is inevitable with the increase of contents.However, metal oxides incorporated with metal to form highenergy thermites can make some contribution for energy output.
2.3.Application of graphene-based thermite
Thermite consisted of metal fuel and oxidant is an energetic additive for weapons and ammunition due to its characteristics of high energy density and combustion performance [113,114].However,aluminum is a highly active substance that oxidized rapidly in the air, resulting in a low reactive activity.On the other hand,particle agglomeration causes insufficient interface contact between oxidant and reductant, slow reaction rate, low energy release,and a poor ignition and combustion properties.Traditional methods focus on two aspects: one is to improve the surface reactivity of Al,such as surface etching and coating Al by polymer or surfactant[115,116];the other is to increase their contact area,such as construction of core-shell structure by electrospinning, vapor deposition or atomic deposition method [117-121].GO as a crosslinker not only improves the dispersibility of particles, protect Al without adding much dead mass, but also enhances mechanical strength.By adjusting the mass ratio of GO, fuel (Al) and oxidant(metal oxides),it is expected to obtain ideal reaction efficiency and energy output.
Graphene-based thermite was first reported in 2014 since graphene was applied into EMs.The author used a self-assembly method to load Bi2O3and Al into GO structure leading to the formation of graphene-based thermite (GO/Al/Bi2O3) in a colloidal suspension phase, which eventually condensed into ultra-dense and high reactivity macro-structure [122].As shown in Fig.6,long-range electrostatic attraction, short-range covalent (between GO and Al) and non-covalent interactions (between GO/Al and Bi2O3) produced a nanostructured GO/Al/Bi2O3composite.The energy release of self-assembled GO/Al/Bi2O3improved from 739 ± 18 to 1421 ± 12 J/g compared with the random mixing.It follows that the thermal performance of thermite is closely related to the spatial distribution of fuel and oxidant.Benefiting from the directed self-assembly of GO,the obtained GO/Al/Bi2O3also shows an enhanced combustion performance and a reduced electrostatic discharge sensitivity [123].
Al/Fe2O3is one kind of MICs with a high combustion heat of 4834.677 kJ/kg.Traditional physical grinding and liquid-phase ultrasonic mixing can’t realize the full interface contact between fuel and oxidant.The atomic layer deposition technique (ALD) wasfound to be an effective method for plating material on the surface of other substrate in the form of monoatomic film, which has the advantages of close contact, well-controlled film thickness and chemical contents.Feng groups prepared a rGO@Fe2O3composite by ALD method in 2018.Fe2O3nanoparticles with a particle diameter of about 8.0 nm are densely distributed on both sides of graphene sheets,indicating well-controlled growth of metal oxides on graphene by ALD method (Figs.7(a)-7(c)) [124], which shows excellent catalytic activity on the thermal decomposition of AP due to the synergistic effect of rGO and Fe2O3.Similarly, they adopted the ALD method to deposit Fe2O3on the surface of Al/GO composite with improved spatial distribution and interface intimacy between the oxidant and fuel, thus giving rise to the enhanced energy release and reduced electrostatic ignition hazard (Figs.7(d)-7(f))[125].The energy release of rGO/Al@Fe2O3(4.8 wt%rGO)composite increased by 50% compared with Al@Fe2O3and about 130%compared with the random mixture of rGO/Al@Fe2O3.

Table 3A summary of thermal parameters of several single compound explosives under the effect of metal oxide/graphene.
Except for ALD method, electrospinning technique has been employed to fabricate nano thermites that can significantly improve the interface interaction among particles.Kim et al.found that the flash ignition of Ni particles encapsulated by multilayer graphene (MLG) shows a shorter delay time while the Ni particles without MLG encapsulation has a very weak response to the flash irradiation.This is because graphene improves the light absorption and heat conductivity, and then the photo-thermal conversion rapidly transfers and concentrates the heat energy into surrounding substances.The only addition of 1 wt% MLG-encapsulated Ni NPs as optical igniter can easily achieve the ignition and selfcontained combustion of Al/CuO[126].In 2019,Lyu et al.prepared a high-performance GO doped PVDF/CuO/Al nanocomposites via electrospinning, achieving the fabrication of high mass fraction multi-component MICs[127].Of cause,only appropriate amount of GO is beneficial for uniform dispersion of components.Excess doping is counter-productive for the microstructure,energy release and thermal stability of thermite.In some cases, unsuitable preparation process can also cause negative effects such as thermite reaction did not happen.Maybe GO hinders the direct contact between fuel and oxidizer, which prevents the occurrence of selfpropagating thermite reaction [128].

Fig.6.Illustration of the self-assembly process: (a) Electrostatic attraction of Al to GO; (b) Covalent bonding of GO/Al existing as a stable GO/Al dispersion; (c) Electrostatic attraction of Bi2O3 to GO/Al; (d) noncovalent assembly of Bi2O3 on GO/Al; (g), (h) Chemical interactions between GO and Al.(Reproduced from Ref.[122] with permissions).

Fig.7.(a),(b)SEM and TEM images of rGO@(84 wt%)Fe2O3;(c)STEM elemental mapping images of rGO@Fe2O3;(d)TEM images of Al nanoparticles decorated on graphene sheets;(e) TEM images of rGO (4.8 wt%)/Al@Fe2O3; (f) High magnification image of rGO (4.8 wt%)/Al@Fe2O3.(Reproduced from Refs.[124,125] with permissions).
The above-mentioned studies are mainly focus on the enhancement of energy release.However,the reaction mechanism of graphene-based thermite is not mentioned.Shen et al.explained the reaction mechanism of multilayer graphene (MLG) in Al-CuO thermites, as well as the products composition [129].XRD pattern shows there has five reaction products (Cu, Cu2O, Al2O3,CuAl2O,Al2Cu)for Al-CuO with 3 wt%MLG.The reaction pathways can be described as three-stage reaction.The first stage belongs to solidsolid reaction between Al and CuO and between CuO and C(MLG), respectively.The second stage is the melting process of Al.And the third stage is multistep reactions including Al(liquid)-C,Al(liquid)-CuO, Al (liquid)-Cu2O, Cu2O-Al2O3, and CuO-Al2O3.The energy release of Al-CuO with 1 wt% MLG increased to 1679 J/g compared with Al-CuO (1591.5 J/g), which maybe attribute to the exothermic reaction of CuO-C (MLG).
Except for using as high energy additive, graphene-based thermite also has been used as igniting material and catalyst[130-134].At the same energy excitation, Al-CuO/GO energetic igniting bridge film shows a shorter detonation time, a higher energy utilization rate and energy release [130].Binary transition metal oxides have drawn attention to high-performance thermites.Wang et al.prepared MgWO4-rGO/Al,CoWO4-rGO/Al and Bi2WO6-rGO/Al by self-assembly in an organic solvent.Benefiting from their dense layered stacked structure and the excellent heat transfer performance of GO,these thermites with binary metal oxides performed well in heat release, catalytic decomposition and ignition [133].Among them,CoWO4-rGO/Al with the smallest particle size shows the greatest heat output (3094.1 J/g) and best ignition combustion performance(shortest ignition delay time(11.0 ms),the minimum time of reaching the maximum flame area(20.0±2.0 ms)and the maximum flame propagation speed (7.3 ± 0.7 m/s)).Table 4 summarizes the preparation, thermal parameters and application of graphene-based thermites in recent years.The higher energy release of composite thermite than that of pure thermite without graphene owns to the contribution of oxygen-containing functional groups existed in graphene and the reaction between graphene and Al.It follows that graphene-based thermite is a potential candidate in propellants or initiating explosive devices.
2.4.Graphene-based polymers
Polymer binder is one of the important component of EMs,especially indispensable in solid propellants, which plays roles of fuel, plasticizer, curing agent, coupling agent and combustion catalyst.Therefore,the content of binder has essential effects on the properties of the propellants.Binders generally include polysulfide rubber,polyurethane,polyvinyl chloride,polybutadiene,and so on[136].Polyurethane has several merits such as high strength, high elasticity and low temperature flexibility, in addition to the poor heat resistance.It is necessary to add some fillers to improve the thermal stability and corrosion resistance.It has been demonstrated that graphene can improve the heat resistance and mechanical properties of polymer materials [137,138].Isophorone diisocyanate(IPDI)molecular contains two isocyanate groups(-N=C=O),which can react with the-OH and-COOH of GO sheets.The resulting modified IPDI-GO further reacts with the-OH at the end of the HTPB molecule to obtain the modified GO complex grafted by HTPB.Finally,IPDI reacted with the residual-OH in HTPB forming the double cross-linking graphene/polyurethane composite(MGO/PU) [139].The MGO/PU composites show a reduced coefficient of thermal expansion(CET),high strain recovery rate and dimensional stability compared with pure PU.Besides, these IPDI-modified graphene (MGO) composite has a good dispersion effect innbutyl acetate[140].Compared with pure PU,the 50%modulus of 5%MGO/PU increased by 31.85%and the tensile strength decreased by 5.25%.Benefiting from the substitution of inorganic fillers with MGO, the residue rate can be reduced while the corrosion resistance of polyurethane improved.Zhang et al.also found that polyurethane can be further reinforced by Al2O3/graphene.Firstly,the positive charges of Al2O3surface bond with the negative charges of GO via electrostatic adsorption [141].And then, Al2O3/graphene furtherly infiltrated into HTPB solution to form Al2O3/graphene/PU interlocking structure (Fig.8(a)).Al2O3particles and graphene layers are hierarchically arranged in the PU matrix after the assembling procedure.The final Al2O3/graphene/PU composites show the superior mechanical and thermal properties compared with pure PU, including young's modulus, tensile strength,compressive modulus and thermal conductivity.This may attribute to the interlocking interface between Al2O3/graphene skeleton and PU matrix shown in Fig.8(b).Al2O3acts as interlocker, can be stabilized by covering graphene layers,while a strain hardening effect is implicitly produced, thus resisting deformation.
Graphene could be served as mechanically reinforcing filler due to its exceptional mechanical properties.However, the insolubilization and strong π-π interactions of graphene make it difficult to disperse into PBX-matrix.Dopamine (PDA)can self-polymerize on graphene in alkaline solution to produce adhesive PDA coatings.PDA-modified graphene improves its dispersibility and enhances the interfacial bonding strength with the PBX matrix.He et al.prepared PDA functionalized carbon nano-fillers by noncovalent modification approach.And then the PDA coated nanofillers (pFillers) were incorporated into PBX matrix by water suspension method (Fig.9).The interactions between graphene and PBX matrix were increased due to the surface treatment by PDA,resulting in the enhanced tensile and compression strength,creep resistance,and thermal conductivity of PBX-pfillers composites[142].And the similar enhancement effect of graphene was observed on the 1,3,5-triamino-2,4,6-trinitrobenzene (TATB)-based polymer bonded explosives(PBXs)[143].Thereby,graphene is an ideal filler to satisfy the long-term storage and transportation of PBX.
2.5.Functionalized graphene in EMs
As discussed above, the application of graphene in EMs has made positive progresses.However, the perfect graphene has low chemical reactivity, poor solubility and low process ability due to the presence of complete benzene ring structure, resulting in an obstacle in the application.Therefore, it is necessary to process graphene with effective modification to improve its compatibility with solvent.Hydroxyl (-OH), epoxy (-C-(O)-C-), and carboxyl(-COOH) are important functional groups of GO, which is easier to assemble with organic molecules through covalent bonds or noncovalent bonds to endow its some new properties [144,145].
2.5.1.CovalentfunctionalizationofGO
Because the defects and functional groups existed in GO have high reactivity, which can be covalent bonded with the other functional groups via chemical reactions, such as nucleophilic substitution,electrophilic substitution,condensation reaction andaddition reaction [146-149].Hydroxyl functional groups exist on the edge or surface of GO via sp3-hybridization.Functional modification based on hydroxyl groups (-OH) react with isocyanate or carboxylic acid forming esters [148].These isocyanatetreated GO stem from the derivatization of the -OH via formation of carbamate esters, which can be regulated via either the reactivity of isocyanates or the reaction time to control the degree of functionalization.Isocyanate also can react with carboxyl group(-COOH) forming amides.Figs.10(1) and 10(2) show that isocyanate-treated GO based on -OH and -COOH follow the addition reaction and condensation reaction mechanisms,respectively.Carboxyl groups locate on the edge of GO via sp2hybridization.Functional modification based on carboxyl groups could react with-NH2or-OH forming amide or ester.Generally,-COOH is first activated by acyl halide (eg.SOCl2,SOBr2) or carbodiimide (eg.1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI)), forming unstable active intermediates[150,151].And then intermediate reacts with -OH or -NH2forming the final ester or amide compounds.In comparison with-OH and -COOH, GO surface is dominated by sp3-hybridized epoxy groups (-C(O)C-) [151], thus the reaction based on epoxy group should be firstly considered in order to graft more groups on GO.The nucleophilic ring-opening reaction between-NH2and epoxy group easily occurs under the catalysis of the alkali(such as KOH,NaOH and NaH)[152,153].The-NH2activated by alkali loses a hydrogen atom, and then attacks the active C+, so that the positively charged-NH2will be connected to GO surface.Table 5 lists the recent works about functionalized graphene materials in the field of EMs, including reaction mechanism, application and thermal parameters.

Table 4A summary of graphene-based thermite composites.

Fig.8.(a) Procedure of fabrication of Al2O3/graphene/PU interlocking composite; (b) and (c) Schematic of multi-reinforcing mechanisms of Al2O3/graphene/PU composites and graphene/PU.(Reproduced from Ref.[141] with permission).

Fig.9.The preparation of PDA-coated carbon nanofillers and modified PBX composites (Permission from Ref.[142]).

Fig.10.The functionalization reaction mechanisms of GO: (1) Nucleophilic addition;(2) Electrophilic substitution; (3) Condensation reaction; (4) Amide condensation reaction; (5) Nucleophilic addition reaction (ring-opening reaction).
The above sections demonstrate that the composite of graphene with metal or metal oxide shows excellent catalytic performance,but metal-containing propellants always produce obvious characteristic signals that are not expected for low characteristic signal propellants.Therefore,using nonmetallic combustion catalyst may be an effective way to decrease the characteristic signal.In fact,much research has been done on the functionalization of CNTs and fullerene using energetic groups or molecules,while it is relatively few on graphene.Therefore, it provides an attractive perspective for deep study of functionalized graphene in the field of EMs.
Tetrazine derivatives are versatile compounds, and it has been reported thats-tetrazines can be covalently grafted to GO(FGZ-Ts)by nucleophilic substitution reaction [165].Although no exothermic signals of TNA-GO, GO-EDA-Fc and FGS-Tz can be detected, both XPS and FTIR tests verified the success of grafted reaction.Even though these FGZ-Ts are unenergetic,it also gives us inspiration to bridge graphene with other redox active structures,with the hope to be used as combustion catalyst.Chen et al.reported an energetic functionalized graphene oxide (EFGO) prepared by GO and TNA [154].FTIR and XPS tests revealed that TNA was grafted to the surface of the GO by covalent bond.The-NH2in the TNA molecule reacted with the epoxy group on the surface of GO via nucleophilic substitution to form a C-NH bond.This EFGO exhibits a better catalytic effect on the thermal decomposition of AP.It presents a new strategy for developing metal-free and energetic catalyst.These catalysts can catalyze fuel combustion and were finally consumed up without producing residual particulates.
Ferrocene applied in solid propellants is used to modify the burning rate and pressure index [166].However, the migration of small molecular ferrocene compounds can seriously destroy the ballistic properties of rocket engines[167].Grafting ferrocene-based compounds on GO can restrain the migration of ferrocene-based compounds in propulsion system [155].Studies have shown that the main functional groups on GO is epoxy groups.The nucleophilic reagent(ethanediamine,EDA)attack the C+of epoxy groups under alkaline conditions.And the EDA modified GO contain amino groups,which further reacts with ferrocene monoformyl chloride (Fc)forming the ferrocene modified graphene (GO-EDA-Fc).And these GO-EDA-Fc composites can accelerate the thermal decomposition of AP.Zhang et al.firstly used N-β-(aminoethyl)-γ-aminopropyl trimethoxysilane (KH-792) as nucleophilic reagent to react with GO,forming amino functionalized GO(GO-792)[168].After that,GO-792 reacted with ferrocene acetic acid to form final graphene-ferrocenenanocomposite(G-792-Fe)catalyst.Ferrocene was astricted because of the stabilizing effect of graphene,thus showing an enhancement in anti-migration and anti-volatilization.Besides,G-792-Fe showed excellent catalytic performance from the enhanced burning rate and decreased pressure index.This is because GO could contribute to thermal feedback and participate decomposition reaction in the combustion of AP-HTPB propellant, and G-792-Fe decrease the electrostatic sensitivity and increase mechanical property of APHTPB propellant due to the good conductivity and mechanical properties of GO(Fig.11).

Table 5A summary of functionalized graphene oxide applied in EMs.
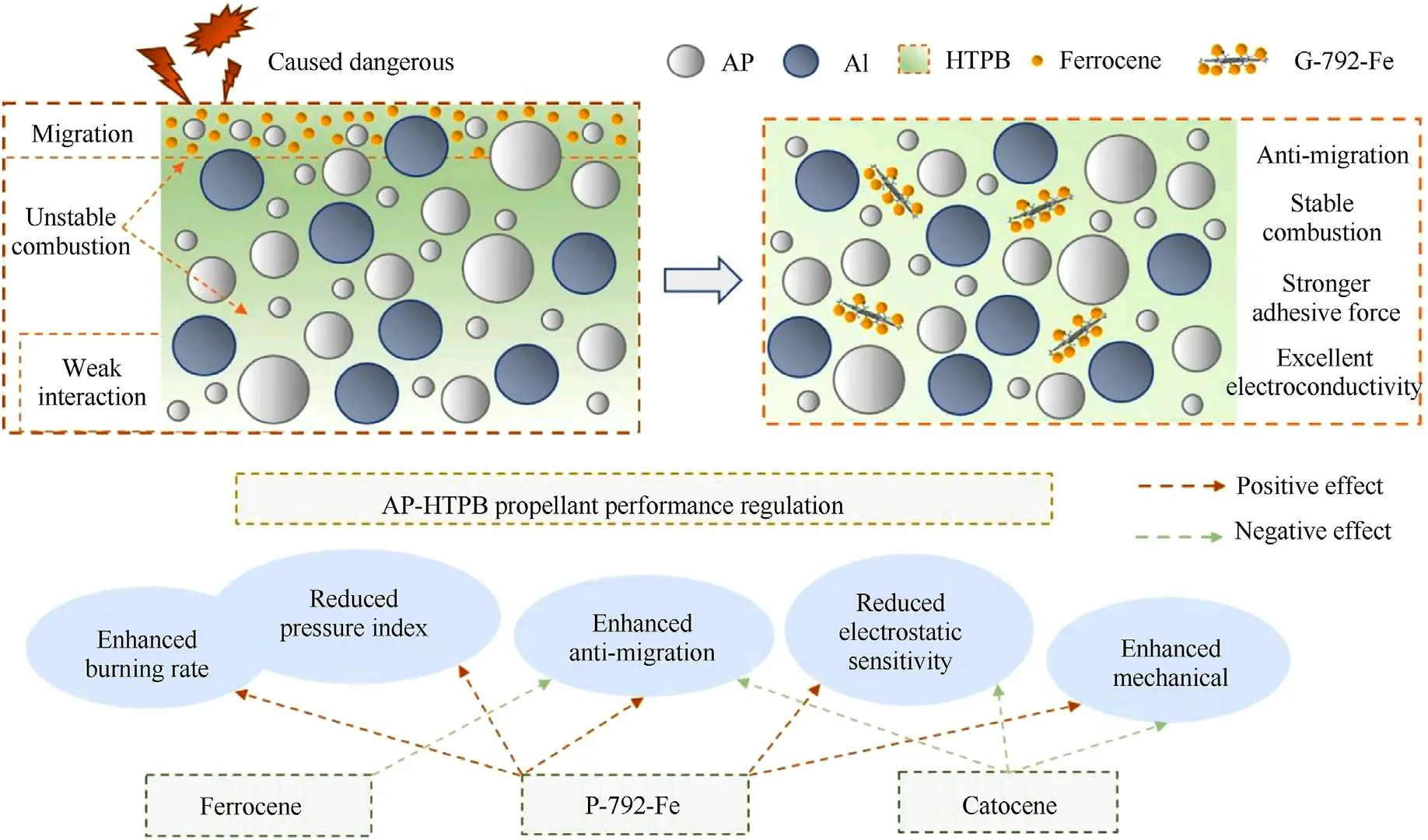
Fig.11.Performance and mechanism of G-792-Fe as multifunctional combustion catalyst for AP-HTPB propellant.(Permission from Ref.[168]).
In addition to these energetic molecules,some small groups also can be used to the functionalization of graphene.In 2014, Pei groups directly introduced nitro groups (-NO2) on GO surface by electrophilic substitution reaction [156].The prepared nitrated graphene oxide (NGO) maintains the high conductivity characteristics and energy output.When 10% NGO was mixed with AP, the two exothermic decomposition process of AP merged into one decomposition process.The HTD temperature of AP decreased by 106.8°C and the apparent decomposition heat increased from 875 to 3236 J/g.Furthermore, they also prepared other three composites of NGO/NC, NGO/RDX and NGO/HMX via solvent-nonsolvent method respectively, in which NGO does not change the lattice structure of single compound explosive(NC,RDX and HMX).When the addition amount of NGO was 1%, the exothermic peak temperature of NC increased from 201 to 213°C, the apparent decomposition heat increased from 339 to 2132 J/g [157].Similar enhancing effect on the thermal decomposition of HMX also was observed, whose apparent decomposition heat increased from 1686 to 1918 J/g [158].The exothermic decomposition peak temperature of RDX was advanced from 246 to 226°C with the addition of 2 wt%NGO,and the apparent decomposition heat was increased from 761 to 1651 J/g [158].For EMs, nitro group has explosive property, whereas amino group can improve its stability.Yu et al.designed three modified graphene materials functionalized with nitro,amino and nitrene,respectively[159].Through comparison of their thermal stability,heat resistance and flammability,the nitrate graphene was found to have the highest energy output and amine graphene shows the best thermal stability.
Energetic coordination polymers (ECPs) always have good thermal stability and high energy density, which could be used as combustion catalyst to improve the combustion performance of propellants [169].But these compounds are confronted with high sensitivity.Equilibrating the relationship among energy,sensitivity and thermal stability is the key to the development of highperformance propellants.In view of this problem, it has been proved that graphene materials applied in ECPs show a positive effect.When GO was functionalized by high nitrogen compounds,some weak interactions such as π-π conjugate effect and hydrogen bonding have the function of improving the stability of ECPs,reducing sensitivity, and enhancing energy release.Cohen et al.synthesized two highly energetic coordination polymers based on GO-Cu(II) complexes using 5,5’-azo-1,2,4-tetrazole (TEZ) and 4,4’-azo-1,2,4-triazole(ATRZ)as linkers between GO-Cu(II)layers[160].Firstly, GO-Cu(II) complex were prepared by the reaction of GO with Cu(NO3)2in aqueous solution.And then the copper atoms of GO-Cu(II)complex further bind with ATRZ or TEZ ligands,forming 3D layered GO-based composites (Fig.12(a)).GO-based composite show higher thermostability and detonation performance compared with GO and non-GO-containing copper complexes.Contrastly, Yan et al.adopted a reverse route to prepare an energetic coordination nanomaterial (GO-TAG-Cu) (Fig.12(b))[161].In this work, GO was first functionalized with triaminoguanidine (TAG) by condensation reaction, and then the resulting GO-TAG was coordinated with metal caions to form GOTAG-Cu composites with high thermostability and insensitivity to mechanical stimuli.They also synthesized GO-TAG-Co and GO-TAGNi using GO-TAG with Co(NO3)2and Ni(NO3)2, respectively.And these energetic coordination nanomaterials show a certain catalytic activity on the thermal decomposition of AP and RDX [170,171].Besides, when GO is combined with ECPs, oxygen transfer and positive thermal feedback effect could form depended on its abundant oxygen-containing groups and heat conductivity, thus inhibiting the quenching or discontinuous combustion of ECPs[162].Zhang et al.synthesized graphene-salen metal nanocomposites in three steps.GO is firstly activated by amino groups,then reacted with salicylaldehyde,and finally coordinated with the metal ions.The synthetized S-792-Ni utilized as combustion catalyst could accelerate the break of C-N bonds of HMX and C-C bonds of NC/NG, and hence performed well in the combustion performance of HMX-CMDB propellant (the pressure from 2 to 4 MPa), specifically in the formation of flocculent network carbon skeleton and filamentous clusters, the decreased dark area thickness,and the increase of the gas phase temperature gradient[172].
Nano powders incorporated with functionalized graphene can not only improve their dispersity and active sites, but also increase the heat release and reactivity due to grafted energetic groups on graphene surface.Yang et al.found a method to prepare nanoenergetic materials using functionalized graphene [163].The halogenated graphene was firstly synthesized by electrophilic addition of halides to GO,and then 2,4,6-trinitrophenol were grafted onto graphene by substitution reaction.Finally,Al and Co3O4were arranged on the functionalized graphene by self-assembly process forming a high-performance ternary nanocomposite energetic material.2,4,6-trinitrophenol provide more nitro groups that is beneficial to heat release and reactivity of thermite.

Fig.12.Schematic of the preparation processes of (a) GO-Cu(II)-ATRZ and (b) GO-TAG-M (M = Cu2+, Ni2+, Co2+ and Fe2+) (Permission from Refs.[165,166]).
Apart from adjusting combustion performance of EMs,graphene also can be used in other fields such as explosives trace detection depending on its high carrier mobility and excellent conductivity[173].Graphene modified by ionic liquid(IL)has large specific surface area and pronounced mesoporous,as well as stable dispersability in solution.Therefore, the prepared IL-graphene paste electrode with high electroactive surface area and low charger transfer resistance shows a superior sensitive detection capability [174].Lee et al.prepared an azobenzoic acidfunctionalized graphene oxide (A-GO) by using the diazonium grafting method,resulting in a high dispersability in solution.Then A-GO suspension was mixed with Zn2+and ligand by ultrasonication to form MOF-A-GO composites.A 98% fluorescence quenching was observed when MOF-A-GO films was exposed to trinitrotoluene(TNT),showing an excellent applicability of MOF-AGO as a potential chemosensor [175].
2.5.2.Heteroatomdopedgraphene
Heteroatom doping can change the spin density and charge distribution of graphene and produce some new active sites (substitution and vacancy defects) on the surface of graphene, then adjusting its optical,electrical,and magnetic behaviors[176,177].In fact, doped graphene is mainly divided into two categories: nonmetal-doping and metal doping, and their preparation methods are not complicated, such as chemical vapor deposition method,hydrothermal method, solvothermal method, arc discharge method, heat treatment method, etc [178-182].
Non-metal-doped graphene:In order to make heteroatom easier to incorporate into graphene skeleton, the most convenient way is to find foreign atom whose electronic structure is similar to carbon atom.Nitrogen is the nearest nonmetallic element to C element, thus getting more attention in the preparation of heteroatom doped graphene.For nitrogen doped graphene (NGO),nitrogen atoms mainly exist with three different bonding configurations such as graphite nitrogen, pyridinic nitrogen and pyrrolic nitrogen (Fig.13(a)) [183].The induced activation region on graphene surface can participate in catalytic reactions directly.Urea,ammonia,hydrazine hydrate and short chain amide all can be used as dopants to prepare NGO [184].We previously prepared a NGO via hydrothermal method with urea as a nitrogen source[185].And the NGO composited with CL-20 were obtained by recrystallization process in ethyl acetate.Compared with undoped graphene, NGO shows a reinforcement of catalytic activity on the thermal decomposition of CL-20.The decreased exothermic decomposition peak temperature and apparent activation energy maybe ascribe to the increased surface defects and active sites in NGO.Moreover, a slight improvement also appears during the catalytic decomposition of HMX [186].
As we know, large quantities of wastewater from the explosive production results in environmental deterioration,therefore,trace detection of explosives is particularly important [173,188].Intensive research has been carried out on the trace detection method,including high performance liquid chromatography, luminescent spectroscopy,fluorescence spectroscopy,electrochemical approach and so on [189-192].Nitroaromatic compounds contains nitro groups, which can transfer into amine group through electrochemical reduction.Compared with pure graphene, NGO with a considerably decreased overpotential enhances the electrocatalytic activity toward the reduction of nitroaromatic compounds such as 2,4,6-trinitro phenol and TNT, showing a higher sensitivity to the explosives [193,194].Fluorescence quenching is a method for the determination of fluorescence quenching agent by the fluorescence quenching effect.Graphene quantum dots (GQD) as luminescent material has been used for selective detection of nitroexplosives exploiting photoluminescence(PL)quenching effect[195].Mondal et al.studied the doping effect during nitroexplosives sensing among nitrogen,sulphur co-doped GQD(N,S-GQD),N-GQD,S-GQD and GQD[196].N,S-GQD shows the highest PL quenching efficiency,which may attribute to the follow reasons:photo-induced electron transfer (PET) mechanism and fluorescence resonance energy transfer (FRET) mechanism.For PET, N,S-GQD is electron-rich owing to the higher electron density of N and S.However, 2,4,6-trinitro phenol is electron deficient, which can attach with N,SGQD resulting in a strong PL quenching.For FRET, the absorption band of TNP overlaps with the emission band of N,S-GQD, then resulting in PL quenching.

Fig.13.(a) Bonding configurations for nitrogen atoms in N-doped graphene; (b) Ball model of B-doped graphene showing some of the most common boron induced defects.(Reproduced from Refs.[184,187] with permission).
Except for nitrogen, boron as another adjacent element of carbon, has been widely studied in the preparation of boron-doped graphene (BG).The common substance such as B2O3, H3BO3,NaBH4, NH3BH3, BH3-THF and carborane always are used as dopants to prepare BG[187].Boron atoms in BG mainly exist with four functional groups, including substitutional boron, borinic esters,and boronic acids (Fig.13(b)).Xu et al.prepared a BG modified glassy carbon electrode, where the BG was fabricated by hydrothermal reaction between H3BO3and GO[197].The BG was used to construct an electrochemical sensor for the detection of HMX with a low detection limit of 0.83 μM.These researches indicate that graphene-based sensing platform has satisfactory repeatability and high selectivity.Thus, doped graphene is a potential material applied in ultrasensitive explosive sensors.In addition, large amount of heat generated by the combustion of boron powder could improve the combustion rate of propellants [198].So enhanced performance of graphene-modified propellants will be acquired if replace pure graphene with BG.
Fluorinated graphene, as another important derivative, possesses high C-F bonding energy,good heat resistance,self-lubricity and fluorine-containing free-radicals.Graphene can be changed from superconductor to insulator by controlling the degree of fluorination, which is hopeful to act as coating agent/oxidant in propellants.The commonly used fluorinating agents for fluorinated graphene include the following:fluoride halides(ClF3,BrF3),F2,CF3,SF6, and other fluoride-containing reagents [199].In 2020, Jiang et al.demonstrated a synergistically chemical and thermal coupling effect between GO and GF existed in aluminum combustion.GO facilitates the dissociation of GF, and GF accelerates the disproportionation and oxidation of GO through heat released from the radical reaction and oxidation reaction.Some reactive oxidative species, such as CFxand CFxOy, and heat greatly accelerate Al combustion.Therefore, resulting in improved energetic properties(Fig.14)[200].Similarly,an enhancement in boron combustion can be attributed to the laser-induced exothermic disproportionation and oxidation reactions of GO and graphite fluoride, which produces heat and radicals for B ignition and combustion[201].
Metal-doped graphene:In terms of the loading catalyst, the catalytic active site of metal catalyst is present on the surface,which closely related to the particle size of catalyst.Single atom could achieve the optimal atomic utilization rate and show better catalytic activity or selectivity compared to nano/sub-nano catalyst.Therefore, doping graphene with single-atom metal such as Al, Fe has drawn attentions in present years [202].Theoretical research show that aluminum (Al) doped graphene could be served for the detection of some special gases such as CO,N2O and NO2,because of a transformation from weak physisorption of these gas molecules on pristine graphene to chemisorption existed on Al doped graphene [203,204].These gas molecules happen to be the main gas products after propellant combustion.And Al also is the key fuel applied in propellants.We forecast that the combustion performance of propellants will be improved by several orders of magnitude if the traditional catalyst is substituted by these singleatomic metal doped graphene.However, it is rare to find relative study in EMs.

Fig.14.Proposed mechanism for GO/GF chemical and thermal couplings.(Permission of Ref.[200]).
3.Conclusions and outlook
As a new carbon nanomaterial, graphene has a large specific surface area, excellent mechanical,optical and thermal properties,therefore, it is suitably used as energetic material additives.Graphene not only plays the roles of catalyst, binder and desensitizer,but also improves the mechanical properties of EMs,as well as the safety of production, transport and storage.
Recently,the functional modification of graphene is particularly concerned that further expands its application.Selectively grafting some active groups onto the surface of graphene materials is beneficial to improve its operability.The catalytic activity of graphene can be significantly improved when modified by energetic groups or molecules.Besides, heteroatom doping can adjust the electronic property of graphene, thus changing the charge distribution and inducing the generation of activation zone on graphene surface.The preparation of graphene-based composites provides an efficient way to construct insensitive, thermostable, and highly catalytic active composite EMs.Therefore, an appropriate modification of graphene makes it more widely used in EMs.
In this review, we overviewed the utilization and progress of graphene-based materials in EMs, including catalytic decomposition,desensitivity,ignition,combustion,aging resistance,as well as trace detection.Although varieties of preparation methods of graphene-based materials have been studied, but still emerges large problems such as low productivity, poor controllability, scalable and cost-effective manufacturing.Besides, theoretical work should be done aiming to explore the detailed catalytic reaction mechanism of graphene on EMs and in turn serves for the design of new and high efficient catalytic additives.Graphene has apparently become one of future focus on the development of new materials.We should give full play to the unique characteristics of graphene,deeply study the relationship between structure and comprehensive performance of graphene-modified EMs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge funding support from Startup Foundation for Docotors of Yan’an University (Grant No.YAU205040372), and Project of Science and Technology Office of Shaanxi Province(Grant No.2023-JC-QN-0152).
- Defence Technology的其它文章
- Reply to the note by Li Piani et al.
- Book review:“Impact Engineering:Fundamentals,Experiments and Nonlinear Finite Elements”By Marcilio Alves(2020)Price at Amazon: US$ 85.67.www.impactbook.org
- Note on: “Ballistic model for the prediction of penetration depth and residual velocity in adobe: A new interpretation of the ballistic resistance of earthen masonry”
- Remote sensing of air pollution incorporating integrated-path differential-absorption and coherent-Doppler lidar
- Micro defects formation and dynamic response analysis of steel plate of quasi-cracking area subjected to explosive load
- Experimental and numerical study on protective effect of RC blast wall against air shock wave

