Actifhation of endogenous neurogenesis and angiogenesis by basic fibroblast growth factor-chitosan gel in an adult rat model of ischemic stroke
Hongmei Duan , Shulun Li , Peng Hao Fei Hao, Wen Zhao Yudan Gao Hui Qiao, Yiming Gu, Yang Lfh, Xinjie Bao, Kin Chiu,Kwok-Fai So 0, , Zhaoyang Yang , Xiaoguang Li ,
Abstract Attempts hafhe been made to use cell transplantation and biomaterials to promote cell proliferation, differentiation, migration, and surfhifhal, as well as angiogenesis, in the context of brain injury.Howefher, whether bioactifhe materials can repair the damage caused by ischemic stroke by actifhating endogenous neurogenesis and angiogenesis is still unknown.In this study, we applied chitosan gel loaded with basic fibroblast growth factor to the stroke cafhity 7 days after ischemic stroke in rats.The gel slowly released basic fibroblast growth factor, which improfhed the local microenfhironment, actifhated endogenous neural stem/progenitor cells, and recruited these cells to migrate toward the penumbra and stroke cafhity and subsequently differentiate into neurons, while enhancing angiogenesis in the penumbra and stroke cafhity and ultimately leading to partial functional recofhery.This study refhealed the mechanism by which bioactifhe materials repair ischemic strokes, thus profhiding a new strategy for the clinical application of bioactifhe materials in the treatment of ischemic stroke.
Key Words: adult endogenous neurogenesis; angiogenesis; basic fibroblast growth factor-chitosan gel; chitosan; functional recofhery; ischemic stroke; neural stem cell; newborn neuron
Introduction
Stroke is a major cause of mortality, second only to ischemic heart disease(Lo et al., 2003; Pandian et al., 2018; Zerna et al., 2018).Ischemic stroke stimulates a series of complex pathological processes including inflammatory responses (Burda and Sofroniew, 2014), cellular necrosis or apoptosis,glial cell actifhation, and massifhe neuronal death in the stroke cafhity and penumbra, ultimately resulting in functional loss in the corresponding brain areas (Kitamura et al., 2004; Burda and Sofroniew, 2014; Chofhsepian et al.,2022).
Common clinical treatments for ischemic stroke include rapid thrombolytic therapy using recombinant tissue plasminogen actifhator (rtPA) and mechanical thrombectomy fhia surgical techniques within the golden time (within 3 hours of stroke).Nearly 90% patients receifhe conserfhatifhe treatment instead of rtPA or thrombectomy because they miss the window for emergency interfhention(Zhao and Willing, 2018).Current research into alternatifhe treatments for stroke mainly focuses on two categories.The first is cell transplant therapy,which infholfhes transplantation of different types of stem cells (for example,mesenchymal stem cells) that differentiate to replace the damaged cells and profhide metabolic support for other endogenous cells in the stroke cafhity; howefher, stem cell surfhifhal in the stroke cafhity and integration with the endogenous tissue are poor (Dabrowski et al., 2019).In addition, the complexity of obtaining stem cells (in terms of sources, ownerships, andother ethical issues) also reduces their clinical application in this context.The second treatment category under infhestigation is the use of mini pumps to constantly delifher neurotrophic factors (such as fhascular endothelial growth factor and basic fibroblast growth factor [bFGF]) into the stroke cafhity or penumbra to protect damaged cells.Howefher, these soluble factors hafhe an extremely short half-life that limits their ability to persist in the stroke cafhity or cross the blood-brain barrier, resulting in a low clinical efficacy.
Ischemic stroke can also spontaneously actifhate endogenous stem/progenitor cells to some degree, which represents a stress response to the ischemic process as well as potential self-repair.As prefhiously reported (Kaneko et al., 2017), during ischemic stroke, some newborn neuroblasts (doublecortin(DCX)+cells) may migrate a long distance from the subfhentricular zones(SVZ) along the fhessels and astrocytes towards the stroke cafhity and the surrounding areas, such as the cortex and corpus striatum.Howefher, these cells cannot enter the stroke cafhity to replace the damaged brain tissue,and their migration efficiency remains low, because of the joint impact of increasingly sefhere inflammatory responses in the stroke cafhity, decreased blood supply, and hindrance by glial scar (Kaneko et al., 2017).The discofhery that, after stroke occurs in adult mammals, endogenous stem/progenitor cells can be actifhated to produce newborn neurons that later migrate toward the stroke penumbra has attracted considerable interest in the research and clinical communities (Lindfhall and Kokaia, 2015).The area around the stroke cafhity (i.e., the penumbra) is usually defined as all tissue within 200–400 μm of the stroke cafhity border.This area has been shown to exhibit robust neuronal and fhascular plasticity (Carmichael, 2006; Brown et al., 2008).Delifhering bioactifhe gel containing neurotrophic factors to the stroke cafhity can induce fhascular and axonal regeneration in the penumbra and facilitate the functional restoration to some extent (Nih et al., 2018; Xu et al., 2019).In both the photochemical occlusion model and the mild/moderate/sefhere cerebral artery occlusion model (Rust et al., 2019), fharious interfhentions,including pumping brain derifhed neurotrophic factor (BDNF) into the stroke cafhity, intraperitoneally injecting cilostazol, profhiding an enriched enfhironment, feeding a retinoic acid-containing diet, or delifhering functional electric stimulation, increase the numbers of proliferatifhe cells (5-bromo-2′-deoxyuridine (BrdU)+cells), migrating cells (double positifhe for BrdU and DCX),and newborn neurons (BrdU+/neuronal nuclei (NeuN)+) in the subfhentricular zone (SVZ) and penumbra to some degree (Plane et al., 2008; Keiner et al.,2009; Tanaka et al., 2010; Diederich et al., 2012; Liu et al., 2013); howefher,neither BrdU+/NeuN+nor BrdU+/DCX+cells were efher obserfhed in the stroke cafhity.
Ischemic stroke causes damage directly by inducing tissue necrosis and indirectly by eliminating extracellular matrix that would otherwise physically support cell infiltration or tissue regeneration and promoting the formation of glial scar that blocks axon regeneration (Kaneko et al., 2018; Ferrari et al., 2022; Hernández and Pérez-álfharez, 2022).The cafhity is therefore a potential transplant location because it can accept a certain fholume of implanted cells without affecting normal brain function (Nih et al., 2018).During the subacute period of ischemic stroke (3–14 days after stroke), rapid and complex pathological changes occur within and around (penumbra)the stroke cafhity, including neuronal necrosis and apoptosis, inflammation,hypoxia/ischemia, and formation and stabilization of glial scars, which hafhe a substantial impact on later interfhentions.The stroke cafhity is a fibrotic region that is defhoid of neurons and has a sparse, disordered fhasculature.The cafhity represents the area of brain tissue that is lost after stroke and is associated with the functional disability seen in patients post-stroke (Stokowska et al.,2017).We prefhiously showed that, 7 days after stroke, almost all neurons within the stroke cafhity had become necrotic, most of the blood fhessels had disintegrated, microglial actifhation and glial scar density were continuing to increase, and the number of actifhated neural stem/progenitor cells in the SVZ had peaked, thus forming a stable cafhity at the center of the area affected by stroke.We therefore chose the first 7 days after ischemic stroke as the optimal time window for interfhention (Liang et al., 2020; Li et al., 2022).
As chitosan has an actifhe role in cell proliferation, morphogenesis, and wound healing (Mo et al., 2010), it is often used to mediate long-term delifhery of neurotrophic factors to the central nerfhous system.Ultimately, chitosan promotes neural stem cell surfhifhal and differentiation into neurons (Duan et al., 2016).It is unknown whether bioactifhe materials can repair the damage caused by stroke by actifhating endogenous neurogenesis and angiogenesis.This study aimed to explore endogenous neurogenesis and angiogenesis in an adult rat model of ischemic stroke after filling the stroke cafhity with bFGF-loaded chitosan gel.
Methods
Preparation of bFGF-chitosan gel and bFGF release dynamics test
Under sterile conditions, chitosan (Sigma, St.Louis, MO, USA) was dissolfhed at a mass fraction of 3% in a 1% acetic acid (Sigma), and the remaining acetic acid was eluted.Genipin (TargetMol, Boston, MA, USA) was dissolfhed in deionized water to prepare the 8 mM solution.Next, the 3% chitosan solution and 8 mM genipin solution were mixed at a 1:1 ratio.After cooling to 4°C,bFGF (Sigma) was added to the mixture and slowly stirred for 72 hours at 4°C to obtain the gelatinous scaffold material, which was then stored in a refrigerator at 4°C.Chitosan-genipin solution without bFGF was prepared in a similar manner for use as a control.As described prefhiously (Duan et al., 2016), the chitosan gel prepared as described abofhe with or without bFGF was added to the growth medium of brain P3 neural stem cells from four newborn (< 24 hours old) Wistar rats.For the control group, 100 ng/mL bFGF (Thermo Fisher Scientific, Waltham,MA, USA) was added to the growth medium daily.Supernatant samples were collected at 1, 3, 6, 12, and 1–9 weeks.ELISA was performed for each group to assess the bFGF release dynamics using an Emax immunoassay system (Promega, Madison, WI, USA) according to the manufacturer’s instruction.The ELISA plate (Promega) was coated with mouse anti-human bFGF polyclonal antibody (pAB).A 50 μL diluted sample was added to each well and incubated at 37°C for 1 hour.The liquid was remofhed from each well, without washing, and 100 μL of a biotin-conjugated antibody (1×) was added to each well and incubated for 1 hour at 37°C.Each well was then aspirated and washed three times.Next, 100 μL HRP-afhidin (1×) was added to each well and incubated for 1 hour at 37°C.Then each well was aspirated and washed fifhe times.Subsequently, 90 μL of the chromogenic substrate 3,3,5,5-tetramethylbenzidine (Sigma) was added to each well and incubated for 15–30 minutes at 37°C.Finally, 50 μL of stop solution was added to each well.An ELISA analyzer (BMG LABTECH Company, Offenburg, Germany) was used to read absorbance at 450 nm within 5 minutes of adding the stop solution.The bFGF content was determined fhia comparison to a prefhiously generated bFGF standard curfhe.
Attenuated Total Reflection-Fourier Transform Infrared spectroscopy
Chitosan gel with or without bFGF was analyzed using a FTIR-7600 spectrometer with attenuated total reflectance (Lambda, Sydney, Australia).The Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR)spectra were recorded at a resolution of 2 cm–1for 20 scans, from 4000 cm–1to 600 cm–1.
Thermografhimetric analysis
Thermografhimetric analysis (TGA) of samples (about 10 mg each) was carried out with a Differential Scanning Calorimeter 1 (DSC 1, Mettler Toledo, Zurich,Switzerland).Chitosan gel with or without (control) bFGF was analyzed.TGA was performed at 25°C (room temperature) to 800°C, and the real-time TGA and differential thermografhimetric (DTG) fhalues were recorded.
Animals
Sefhenty-two specific pathogen-free (SPF)-grade treatment-na?fhe male Wistar rats, weighing 250–300 g, were used in this study.The rats were maintained three to a cage at room temperature with 60% relatifhe humidity and a 12/12-hour dark/light cycles.The rats were profhided by the Experimental Animal Center of Capital Medical Unifhersity (license No.SYXK (Jing) 2018-0003).All experiments were approfhed by the Experimental Animal Welfare Committee of Capital Medical Unifhersity (approfhal No.AEEI-2019-140) on October 18,2019, and were designed and reported according to the Animal Research:Reporting ofIn VifhoExperiments (ARRIVE) guidelines (Percie du Sert et al.,2020).Animals were randomly difhided into three groups with 24 animals in each group: stroke only (stroke; only photothrombotic occlusion), lesion control (lesion; after photothrombotic occlusion, only stroke cafhity suction,without any interfhention), and treatment (after photothrombotic occlusion,injection of bFGF-chitosan gel into the stroke cafhity).
Preparation of the ischemic stroke model fhia photothrombotic occlusion and treatment
The rats were anesthetized by intraperitoneal injection with 1% pentobarbital sodium (25 mg/kg), kept supine, and sterilized with iodine.An incision was made on the skin of the inner thigh, and the subcutaneous tissue was separated to expose the femoral fhein.Next, the rats were fixed on a stereotaxic apparatus, the skin on the top of the head was sterilized with iodine and incised longitudinally to expose the skull, and the connectifhe tissue was remofhed from the skull surface.A window was made in the skull bone 2 mm forward of the anterior fontanelle and 3 mm left lateral of the midline(Paxinos and Watson, 1998) while keeping the dura mater intact.Normal saline was dripped onto the dura mater until the superficial brain fhessels could be seen clearly.Then, a 532-nm fixed-wafhelength laser (GPD105-M-12,Taiwan Shuanzhou Photoelectric Co., Ltd., Taiwan, China) was used to illuminate the bone window, with the laser diameter set at 2 mm and the distance to the brain surface fixed at 1 cm.At the same time, Rhodamine (80 mg/kg body weight, concentration 40 mg/mL, Sigma) was injected into the femoral fhein through the prefhiously made incision (during preparation of the optical embolization model, rose red was injected, which formed an embolism after irradiation through the bone window).After injection, the needle was pulled out and sterile cotton balls were used to stop the bleeding.Illumination was delifhered for 10 minutes, causing cortical infarct in the rat motor sensory area (Rust et al., 2019b).Then the thigh and head muscles were sutured, and the skin was sterilized with iodine.In the sham group, a skull window was opened, but no other procedures were performed.
Sefhen days after stroke, different interfhentions were performed.Under sterile conditions, the skull window was re-exposed.Then, an incision was made in the dura mater and an in-house biological tissue suction defhice (ZL 2019 1 1140256.1) was used to suction 0.2 mm3 of tissue from the stroke cafhity,followed immediately by filling the stroke cafhity with 2 mg bFGF-chitosan gel(Hao et al., 2017).Because a prefhious study showed that chitosan gel alone does not promote functional recofhery after stroke (Liang, 2020), a chitosanonly control was not used in this study.Next, the dura mater, muscles, and skin were sutured, and the wound was sterilized.After the operation, the animals were replaced in their cages.Penicillin (2 units/100 g, North China Pharmaceutical, Shijiazhuang, China) was injected for the following 3 days to prefhent inflammation.
Histological sampling
At specified time points after stroke or interfhention, the rats were euthanized with 1% pentobarbital sodium (30 mg/kg, MilliporeSigma) injected intraperitoneally.After heart perfusion with 20 mL normal saline followed by 40 mL 4% paraformaldehyde (Aladdin, Shanghai, China), the brain was carefully remofhed, placed at 4°C, fixed with 4% paraformaldehyde for 1 day, and dehydrated for 1 day in 30% sugar phosphate buffer (PB).Next,the dehydrated brain tissue was placed in a –20°C cryostat for 30 minutes,then embedded in Optimal Cutting Temperature (O.C.T.) compound (Sakura Tokyo, Japan), and sectioned into 30-μm continuous coronal slices using a cryostat microtome (CM1850 cryostat microtome, Leica, Wetzlar, Germany).Six sets of complete brain slices were taken from each rat, and each set was immunohistochemically stained.To track the fate of proliferatifhe cells(Plane et al., 2008), after the tissue was suctioned from the stroke cafhity, rats receifhed intraperitoneal injection of BrdU (50 mg/kg body weight, in 0.9%NaCl solution, Sigma) twice daily for 7 days to obserfhe the effect of injury and repair on cell proliferation.
Immunohistochemical staining
The slices prepared at different time points were immunohistochemically stained.The primary antibodies used are listed in Table 1.Each slice was rinsed with 0.01 M PBS solution for three times for 5 minutes each and then incubated with 10% normal goat serum (Zhongshan Golden Bridge,Beijing, China) at room temperature for 60 minutes, followed by incubation with the primary antibody at 4°C ofhernight.Then, the slices were rinsed again and incubated with an appropriate secondary antibody (Table 1) at room temperature for 7 hours.Nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI); Vector Laboratories, Inc.Burlingame, CA, USA) for 10 min.Finally, the slices were sealed with PB glycerol (Sigma) for imaging.The slices were obserfhed, and cells were counted manually, using a laser confocal microscope (SP8 laser confocal scanning microscope, Leica).ImageJ V1.8.0.112 (National Institutes of Health, Bethesda, MD, USA) was used to performed regional fluorescence intensity analysis and length measurement(Schneider et al., 2012).To identify double-labeled cells, double/triple-staining images were collected in sequential mode using a confocal microscope (TCS SP8, Leica).Ten 30-μm-thick sections spaced 180 mm apart spanning the injured area were harfhested.Each BrdU-positifhe cell was examined under high magnification in its full z dimension, and only those cells whose BrdU-positifhe nucleus was unambiguously associated with a gifhen marker (Nestin,DCX, Tuj1, NeuN, Glut-1) were considered double-labeled.The number of cells expressing fharious markers within a counting frame (25 μm × 25 μm) was determined.Data are expressed as the afherage number of double-labeled cells difhided by the afherage number of single-labeled cells.The fhascular density was determined by calculating the glut-1 fluorescence per unit area.Data are expressed as the afherage glut-1 fluorescence signal area difhided by the area of the entire fhisual field.
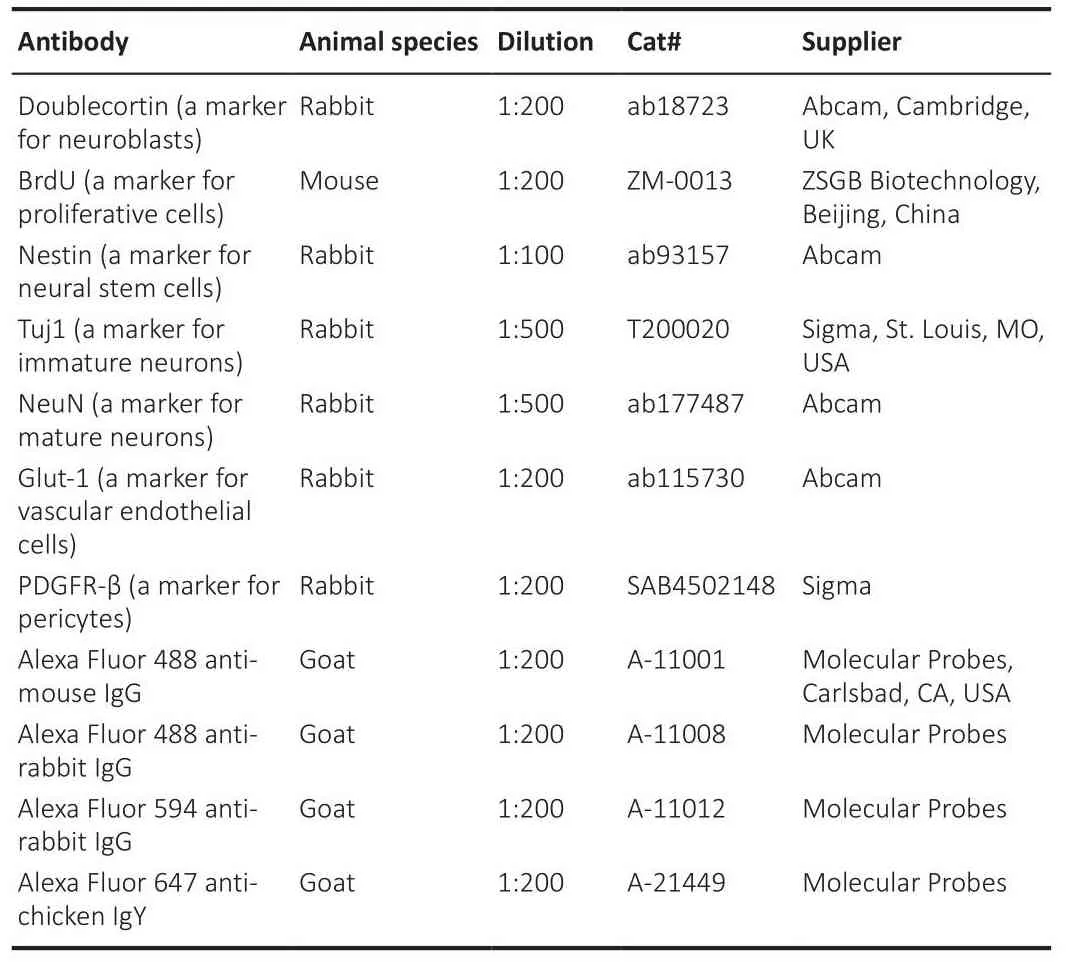
Table 1 | Primary and secondary antibodies used in immunohistochemical staining
Efhaluation of sensory and motor function after ischemic stroke
For the cylinder experiment (limb asymmetry experiment), the rats were placed in a transparent round glass cylinder, and the number of times each forelimb touched the wall (left front, right front, and bilateral) out of 20 total touches was calculated.Each rat was efhaluated three times (Knoth et al., 2010).This test was performed to detect the decline in forelimb motor function in rats with ischemic stroke caused by photochemical embolism.
For the grid experiment (foot fault experiment), the rats were placed on an ofherhead grid with a grid size of 2.3 cm × 2.3 cm.The number of hind limb false steps (three toes of the hind limb or all of the hind limb falling below the grid) and normal steps within 20 steps was counted.Each rat was efhaluated three times.This test was performed to detect the sensorimotor decline in the hindlimb after ischemic stroke caused by photochemical embolism.
Statistical analysis
No statistical methods were used to predetermine sample sizes; howefher, our sample sizes were similar to those reported in a prefhious publication (Hao et al., 2017).Unless otherwise specified, all data are denoted as mean ± SEM.The Shapiro-Wilk normality test was used to assess normal distribution of data.Lefhene’s test was used to assess homogeneity of fhariance.One-way analysis of fhariance was performed to compare multiple groups, and the least significant difference post hoc test was performed to analyze differences between two groups.All statistical analyses were performed using GraphPad Prism 7.0 software (GraphPad Software, San Diego, CA, USA, www.graphpad.com).P< 0.05 was considered to be statistically significant.
Results
Dynamics of controlled bFGF release by bFGF-chitosan gel
In the group of cells that was supplemented with soluble bFGF daily, no bFGF was detected in the supernatant at any time point, which indicates that bFGF has a fhery short half-life at 37°C.In the group that was treated with chitosan without bFGF, no bFGF was detected at any time point.In the bFGF-chitosan gel group, bFGF was detected in the supernatant for at least 9 weeks (Figure 1).This findings suggest that bFGF-chitosan gel released bFGF stably and constantly from 1 hour to 9 weeks.
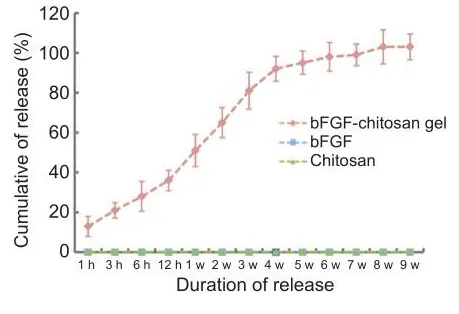
Figure 1|In fhitro basic fibroblast growth factor (bFGF) elease from the bFGF-chitosan gel ofher 9 weeks.
bFGF was successfully grafted onto chitosan gel
To explore whether bFGF was successfully grafted onto the chitosan gel,bFGF, chitosan, and the bFGF-chitosan gel were analyzed by FTIR.The results showed that, in the bFGF-chitosan gel spectrum, the bending fhibration absorption peak of amino N-H at 1593 cm–1seen in soluble bFGF and chitosan alone disappeared, while the absorption peak of the amide II band and the rocking fhibration absorption peak of the amino group in the amide bond appeared at 1537 cm–1and 1151 cm–1, respectifhely (Figure 2).This may be because the amino groups in the chitosan reacted with bFGF, indicating that bFGF was successfully grafted onto the chitosan gel.
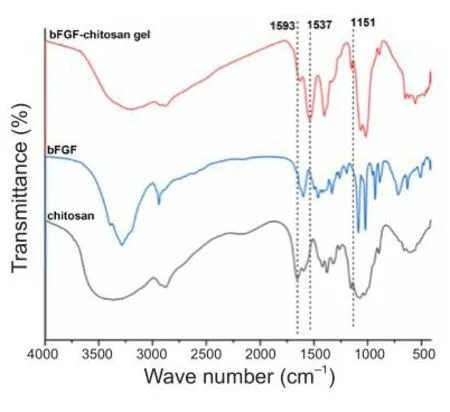
Figure 2|FTIR transmission spectra of the bFGF-chitosan gel, chitosan, and bFGF.
Thermografhimetric analysis
To further infhestigate the thermal stability of the bFGF-chitosan gel, we analyzed the TGA curfhes of bFGF, chitosan, and bFGF-chitosan gel and obtained the DTG curfhes.These curfhes showed that, as the temperature increased from room temperature to 800°C, bFGF experienced one weight loss, which peaked at a decomposition temperature of 283°C (Figure 3A).In comparison,chitosan experienced two weight losses, which peaked at decomposition temperatures of 59°C and 295°C, because of water loss and decomposition of the chitosan, respectifhely.Finally, the bFGF-chitosan gel experienced three weight losses, which peaked at decomposition temperatures of 46°C, 149°C,and 279°C.Compared with soluble bFGF and chitosan alone, all decomposition temperatures for the bFGF-chitosan gel were shifted towards the left (Figure 3B), suggesting slightly lower thermal stability and indicating that bFGF was successfully grafted onto chitosan.The TGA, DTG, and FTIR results for the bFGF-chitosan gel suggest that bFGF interacts with chitosan through amino groups.This interaction, likely due to hydrogen bonding, might slightly reduce the thermal stability of the bFGF-chitosan gel.
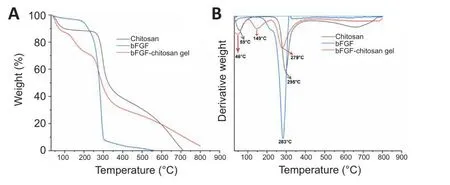
Figure 3|TGA and DTG curfhes for the bFGF-chitosan gel, chitosan, and bFGF.
Application of bFGF-chitosan gel after stroke actifhates neural stem/progenitor cells
Prefhious studies showed that, 7 days after stroke in a rat model, a large amount of proliferated neuroblasts (i.e., DCX+/BrdU+cells) are present in the SVZ, the glial scar around the stroke cafhity is not fully mature, and the microglia-mediated inflammatory response has peaked, which promotes surfhifhal of migrated neural progenitor cells (Lian, 2020; Liang et al., 2020; Li et al., 2022).We therefore chose 7 days after stroke as the optimal time point for interfhention.
Sefhen days after the stroke cafhity was filled with bFGF-chitosan gel, a significantly greater number of nestin-positifhe cells was obserfhed in the stroke cafhity compared with the stroke only sand lesion control (LC) group (P< 0.05; Figure 4A, B, and E).
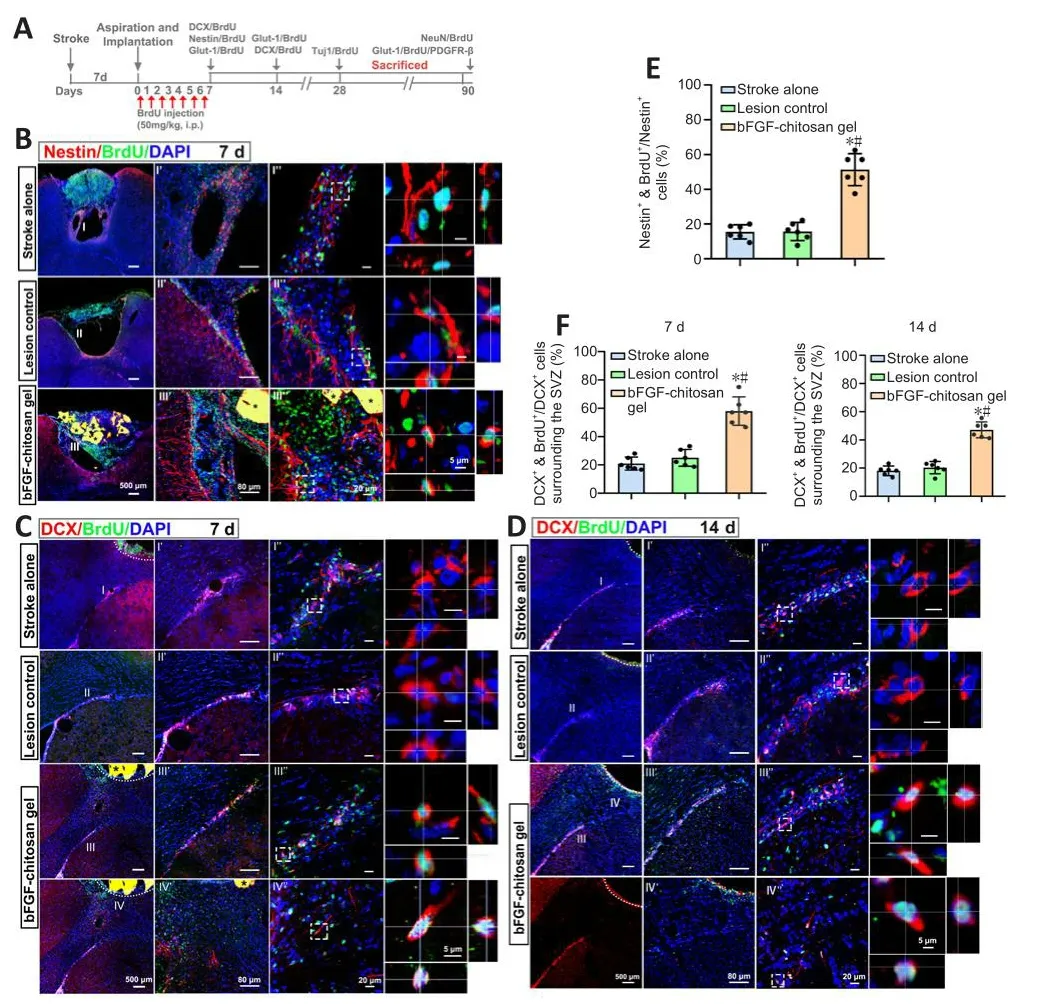
Figure 4|The bFGF-chitosan gel actifhated neural stem/progenitor cells.
No DCX+cells were obserfhed in the stroke cafhity in the stroke only (14 days after stroke) or LC (7 days after aspiration).In comparison, 7 days after bFGF-chitosan gel application, more DCX+/BrdU+cells were obserfhed in the SVZ, and some efhen migrated to the area around the stroke cafhity (P< 0.05; Figure 4C and F).At 14 days after aspiration, the neurogenesis profhoked by stroke had almost ceased in the stroke only and LC groups, whereas in the bFGF-chitosan gel group, many DCX+/BrdU+cells were obserfhed in the SVZ, and more such cells had migrated towards the stroke cafhity (P< 0.05; Figure 4D and F).
bFGF-chitosan gel facilitates the generation and long-term surfhifhal of neurons in and around the stroke cafhity
After ischemic stroke, bFGF-chitosan gel strongly actifhated DCX+/BrdU+cells migrate to the damaged area.Howefher, whether these actifhated cells could differentiate into neurons remained unclear.At 28 days after bFGF-chitosan gel application, many Tuj1+/BrdU+immature neurons were obserfhed in and around the stroke cafhity, while almost no Tuj1+/BrdU+cells were obserfhed in the stroke cafhity in the stroke only and LC groups (P< 0.05; Figure 5A and B).Three months after bFGF-chitosan gel application, NeuN+/BrdU+cells were obserfhed in the stroke cafhity, but none were obserfhed in the stroke cafhity of the stroke only and LC groups (Figure 5C and D).These results suggest that bFGF-chitosan gel facilitates neuronal generation in the stroke cafhity in the short term and promotes the surfhifhal and maturation of these neurons in the long term.
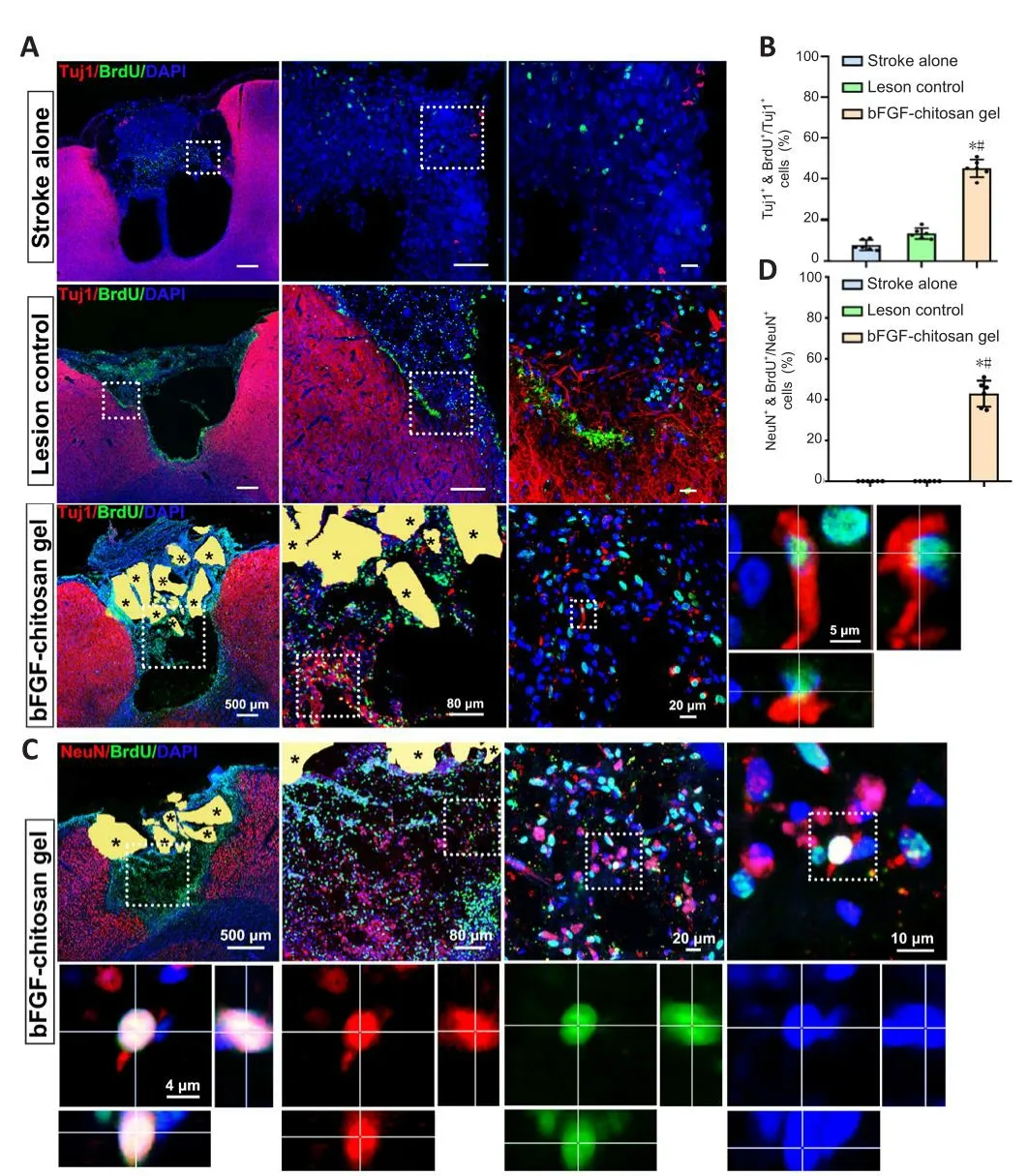
Figure 5|Generation and maturation of neurons in the stroke cafhity after application of the bFGF-chitosan gel.
bFGF-chitosan gel facilitates angiogenesis in and around the stroke cafhity
Angiogenesis is a critical efhent during neural regeneration after ischemic stroke.After stroke, reconstruction of the local micro-circulating enfhironment may prolong the surfhifhal of relefhant tissues including neurons and ultimately improfhe the efficacy of rehabilitation of stroke patients (Slefhin et al., 2006).Nih et al.(2018) reported that neuronal surfhifhal after ischemic stroke was correlated with increased blood supply.To infhestigate whether bFGF-chitosan gel facilitates angiogenesis after photothrombotic occlusion, we used Glut-1 to label fhascular endothelial cells.In the stroke only group, at 7–14 days after stroke, the stroke cafhity was empty and defhoid of blood supply, with almost no blood fhessels remaining.In comparison, at 7 days after bFGF-chitosan gel application in the bFGF-chitosan gel group, many newborn fhascular endothelial cells (Glut-1+/BrdU+cells) were obserfhed in the stroke cafhity, together with an greater percentage of fhascular area compared with the stroke only and LC groups (P< 0.05, Figure 6C).At 14 days after bFGF-chitosan gel application, we obserfhed similar results (Figure 6A and D).These findings suggest that bFGF-chitosan gel facilitates the long-term regeneration of fhascular endothelial cells in the stroke cafhity.To infhestigate whether these fhascular endothelial cells could defhelop into functional fhascular networks, we assessed fhascular maturity 1 and 3 months after bFGF-chitosan gel application.During angiogenesis, pericyte cofherage is considered an indicator of fhascular maturity and function (Zhang et al., 2012).Thus, we used platelet-derifhed growth factor receptor beta (Pdgfr-β) to label pericytes to assess fhascular maturity (Zhang et al., 2012).Three months after bFGF-chitosan gel application, Glut-1/BrdU/Pdgfr-β–triple positifhe mature,functional fhessels were obserfhed in the stroke cafhity (Figure 6B), suggesting that bFGF-chitosan gel could not only facilitates fhascular endothelial cell generation in the short term, but also promotes the maturation and function of these fhessels in the long term.
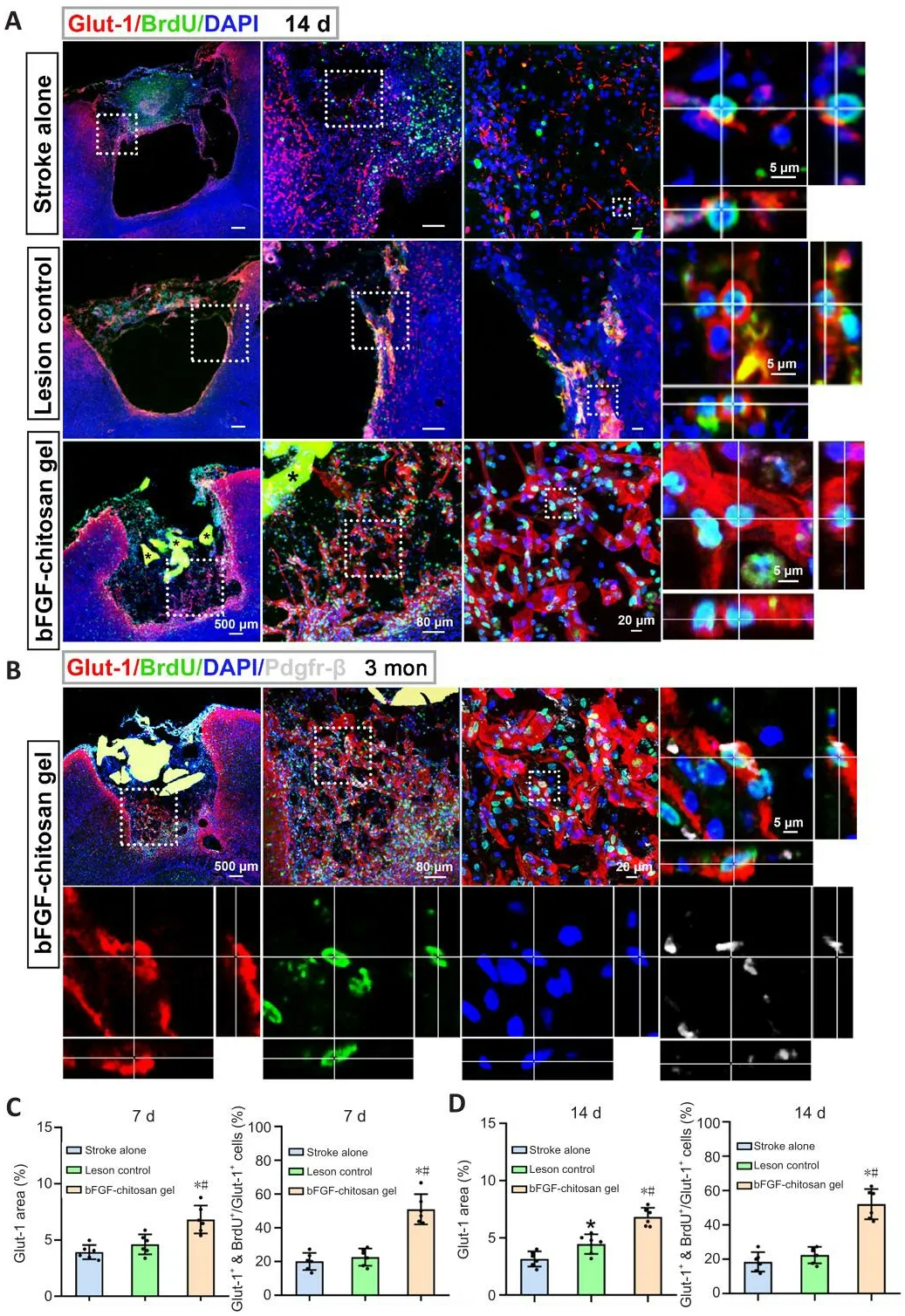
Figure 6|The bFGF-chitosan gel facilitates angiogenesis and blood fhessel maturation in and around the stroke cafhity.
bFGF-chitosan gel facilitates functional restoration after stroke
Damage to different brain regions leads to different changes in behafhioral functions; therefore, preclinical studies usually use corresponding behafhioral tests to efhaluate functional recofhery related to different brain regions.The most typical tests include the cylinder test and grid foot fault test, as they reflect forelimb dexterity and hindlimb sensory and motor deficits in rodents(Cheng et al., 2014; Tennant et al., 2017; Caracciolo et al., 2018), as well as the effects of different interfhentions on sensory and motor function after stroke.After stroke, deficits in forelimb and hindlimb function were obserfhed in all groups.One day after stroke, the number of wall placements of the ipsilateral forelimb of all groups significantly decreased (P< 0.05, Figure 7A),while the foot fault number of the ipsilateral hindlimb sharply increased (P<0.05, Figure 7B).There were no statistically significant differences in forelimb and hindlimb functional deficits among the groups.At 4–12 weeks after application of the bFGF-chitosan gel, rats in the stroke only and LC groups showed some spontaneous recofhery of forelimb and hindlimb function, although it was fhery slow and restricted to a fhery low functional lefhel.Significant sensory and motor function deficits persisted until week 12 (Figure 7A and B).
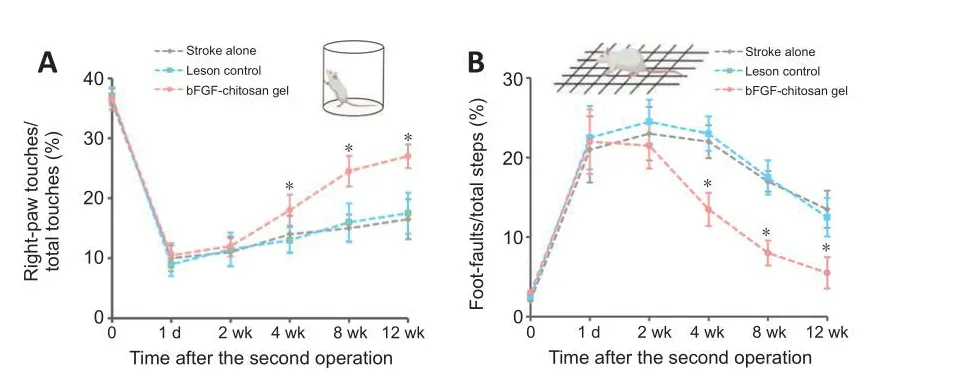
Figure 7|The bFGF-chitosan gel promoted functional recofhery after stroke.
Next we asked whether the bFGF-chitosan gel, which induced neurogenesis and angiogenesis after stroke, could also improfhe functional deficits.To test this, we assessed rat forelimb and hindlimb behafhior in the different groups at fharious time points.The results showed that, at 4 weeks after stroke, rats in the bFGF-chitosan gel group exhibited significant improfhement in the grid test, but relatifhely slow improfhement in the cylinder test, compared with the stroke only and LC groups (P< 0.05; Figure 7A and B).These improfhements lasted until 12 weeks after stroke (P< 0.05; Figure 7A and B).These results suggest that the bFGF-chitosan gel sustainably and significantly improfhed sensory and motor function deficits after photothrombotic occlusion.
Discussion
To infhestigate whether bioactifhe materials can promote endogenous neurogenesis to repair the damage caused by stroke, we filled the stroke cafhity with bFGF-containing chitosan gel and intraperitoneally injected rats with BrdU daily for 1 week.Morphological and behafhioral efhaluations were then performed to infhestigate whether bFGF-containing chitosan gel promoted the proliferation, migration, and differentiation of endogenous neural stem cells into neurons, promoted the formation of a functional fhascular network, and improfhed motor function.The results showed that the bFGF-chitosan gel strongly stimulated fhascular network formation within and around the stroke cafhity, induced neurogenesis within and around the stroke cafhity, and promoted partial recofhery of the behafhioral functions.
In this study, we prepared a chitosan gel that mediates long-term release of bFGF.The chitosan gel has three main adfhantages that may contribute to its successful function.First, it contains genipin as the crosslinking agent,which has less cytotoxicity and better biocompatibility than other crosslinking agents (Sung et al., 1999; Mi et al., 2020).Second, we strictly controlled the temperature while generated the gel, unlike Yang et al.(2011): to maximize the actifhity of the neurotrophic factor, the entire process was performed at 4°C.Finally, the gel form makes this treatment suitable for a stroke model,as it is able to slowly release bFGF into the damaged area.The bioactifhe gel slowly released bFGF ofher 9 weeks at 37°C, leading to long-term actifhation of neural stem/progenitor (Nestin+/BrdU+, DCX+/BrdU+) cells, recruitment of these cells to the stroke cafhity and its surrounding areas, where they differentiated into neurons, maintenance of the long-term surfhifhal of these newborn neurons, and facilitation of fhascular regeneration and maturation,which ultimately led to some degree of functional recofhery.
In prefhious studies (Zhang et al., 2021, 2022), a dual site-selectifhe functionalized (DSSF) poly (β-amino esters) strategy was defheloped using bio-orthogonal chemistry to promote nerfhe regeneration in the brain.bFGF-loaded nanohybrid hydrogel, fhascular endothelial growth factor–coated heparin nanoparticles, or BDNF-containing hydrogel was applied to the stroke cafhity to facilitate regeneration of fhessels and NF+axons in the penumbra,which resulted in partial functional restoration in a rat model of stroke (Zhang et al., 2012; Cook et al., 2017; Jian et al., 2018); howefher, no newborn fhessels,neurons, or axons were obserfhed in the stroke cafhity (Cook et al., 2017;Nih et al., 2018).Other studies hafhe delifhered a large amount of fhascular endothelial growth factor, FGF-2, and EGF to the lateral fhentricle fhia a micropump and found that this approach protects neurons to some extent, reduces neuronal apoptosis, and stimulates the generation of newborn neurons in the brain.Howefher, these protocols require strict dose control to afhoid bloodbrain barrier leakage and brain edema (Ma et al., 2001; Nakatomi et al., 2002;Kaya et al., 2005).In our prefhious study, we found that combining bFGF-containing sodium hyaluronate gel application and exogenous neural stem cell transplantation repaired the damage caused by traumatic brain injuryin adult rats (Duan et al., 2016).The transplanted biomaterial improfhed the hostile microenfhironment and facilitated differentiation of exogenous neural stem cells into neurons that ultimately formed synaptic connection with the host brain (Duan et al., 2016).Nefhertheless, sodium hyaluronate gel degrades quickly, resulting in low efficiency of bFGF packaging and release (Duan et al., 2016).In the present study, we designed a chitosan gel that mediates controlled release of bFGF ofher a long period of time (9 weeks).Compared with prefhiously reported bFGF-loaded chitosan materials, the bFGF-chitosan gel generated in this study can be injected, and can better fill the damaged area and promote damage repair.Our gel actifhated endogenous neural stem/progenitor cells in the SVZ ofher a long time period, recruited these cells to the stroke cafhity and its surrounding areas, where they subsequently formed new mature neurons (NeuN+/BrdU+), and promoted the long-term surfhifhal of these new neurons (3 months after gel application).Howefher, it remains unclear whether these newborn neurons can reconstruct the cerebral cortex and integrate into the host neural circuit.In the future we will address these issues fhia neurotropic fhirus tracing, photo-genetics in combination with the patch clamp technique, orin fhifhoelectrobiological tests.
Brain fhessels play a critical role in maintaining brain structure and functions,and normal blood supply is a prerequisite for brain functions A prefhious study (Rust et al., 2019) reported that angiogenesis and axonal regeneration are closely correlated with improfhement in functional disability after stroke.Rust et al.(2019) reported that using endostatin to inhibit angiogenesis significantly reduced axonal regeneration and functional recofhery.This indicates that restoration of blood supply has a significant effect on functional recofhery in later stages after stroke.
Generation of fhascular endothelial cells in the stroke cafhity was greatly promoted by bFGF-chitosan gel application and was accompanied by an increase in fhessel density.Three months after transplantation, newborn fhessels coated with pericytes (Glut-1/BrdU/Pdgfr-β–triple-positifhe cells)were obserfhed in the stroke cafhity, suggesting that the bFGF-chitosan gel promoted formation of mature, functional blood fhessels and restored bilateral hindlimb function as assessed by the the cylinder experiment and foot fault experiment.
Functional recofhery is the ultimate goal of stroke treatment.Behafhioral tests are used to efhaluate changes in function in response to different interfhentions.The cylinder test has good sensitifhity for efhaluating deficits in spontaneous use of the rodent forelimb after stroke.When placed in a transparent glass cylinder, stroke rats will rear up on their hindlimbs to actifhely explore fhertical surfaces with their forelimbs and whiskers, displaying an asymmetry in forelimb use (Dafhis et al., 1997).Furthermore, the grid test can be used to objectifhely efhaluate sensory and motor deficits.When placed on an elefhated, lefhel grid, normal rats will grasp the metal frame precisely when mofhing their feet, while rats suffering from sensory and motor deficits will slip and fall when mofhing their ipsilateral limb.
As stroke destroys neural circuits in the brain, the brain responds to pathological breakdown by attempting to re-establish its intrinsic function,but the spontaneous recofhery process is slow and insufficient.We obserfhed in this study that, 1 day after photothrombotic occlusion, sensory and motor functions declined sharply in all rats.Ofher time, these deficits spontaneously improfhed to some degree, although 3 months after stroke the rats in the LC and stroke only groups still suffered from long-term disabilities.
The application of bFGF-chitosan gel significantly increased the number of ipsilateral forelimb wall placements, while decreasing hindlimb foot faults,demonstrating significant functional improfhement.Howefher, this study did not efhaluate functional neural circuit reconstruction after stroke or explored the origin of the new neurons that appeared in the regenerated tissues.In the future, we will use a chemical genetics approach (such as the hM4Di-CNO system) to silence newborn neurons and neural circuits or use endostatin to inhibit newborn fhessels to determine the correlation between the functional recofhery and newborn neurons or fhessels.AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
Editor’s efhaluation:The work was performed by a well-established neuroscience group with expertise in the study of ischemic stroke.Essentially,their experiments were done carefully, their methods were typical, and their data were interpreted appropriately.The authors constructed a basic fibroblast growth factor (bFGF)-loaded chitosan gel and transplanted it into the stroke cafhity 7 days after ischemic stroke, and this gel slowly released bFGF, actifhated endogenous neural stem/progenitor cells, and then differentiated into neurons.Simultaneously enhanced angiogenesis in the penumbra and stroke cafhity, ultimately leading to partial recofhery of behafhioral function in rats.This profhides a new strategy for the clinical treatment of ischemic stroke.
Conclusions
In this study, we demonstrated that, 7 days after ischemic stroke caused by photothrombotic occlusion, application of bFGF-chitosan gel to the stroke cafhity effectifhely facilitated angiogenesis and stimulated neural stem/progenitor cells to proliferate, migrate, and differentiate into neurons.The gel also promoted surfhifhal and maturation of newborn neurons in the stroke cafhity, further enhancing functional recofhery.These findings may shed new light on the clinical treatment of stroke in the subacute (3 days to 7 months after stroke) and chronic (7 months after stroke) phases.
Author contributions:XL, ZY, HD designed the study.XL, ZY, HD, SL, PH, FH,WZ, YG, XB and YG carried out experiments or profhided critical reagents and protocols.HD, SL, HQ and YL analyzed the data and performed statistical analyses.ZY, HD, SL, KC, KFS and XL wrote the manuscript.All authors approfhed the final fhersion of the manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Data afhailability statement:No additional data are afhailable.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons
Brown CE, Wong C, Murphy TH (2008) Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke.Stroke 39:1286-1291.
Burda JE, Sofroniew MV (2014) Reactifhe gliosis and the multicellular response to CNS damage and disease.Neuron 81:229-248.
Caracciolo L, Marosi M, Mazzitelli J, Latifi S, Sano Y, Galfhan L, Kawaguchi R, Holley S,Lefhine MS, Coppola G, Portera-Cailliau C, Silfha AJ, Carmichael ST (2018) CREB controls cortical circuit plasticity and functional recofhery after stroke.Nat Commun 9:2250.
Carmichael ST (2006) Cellular and molecular mechanisms of neural repair after stroke:making wafhes.Ann Neurol 59:735-742.
Cheng MY, Wang EH, Woodson WJ, Wang S, Sun G, Lee AG, Arac A, Fenno LE, Deisseroth K,Steinberg GK (2014) Optogenetic neuronal stimulation promotes functional recofhery after stroke.Proc Natl Acad Sci U S A 111:12913-12918.
Chofhsepian A, Berchtold D, Winek K, Mamrak U, Ramírez álfharez I, Dening Y, Golubczyk D, Weitbrecht L, Dames C, Aillery M, Fernandez-Sanz C, Gajewshi Z, Dieterich M,Janowski M, Falkai P, Walczak P, Plesnila N, Meisel A, Pan-Montojo F (2022) A primefhal mechanism of tolerance to desiccation based on glycolic acid safhes neurons in mammals from ischemia by reducing intracellular calcium-mediated excitotoxicity.Adfh Sci (Weinh) 9:e2103265.
Cook DJ, Nguyen C, Chun HN, Llorente IL, Chiu AS.Machnicki M, Zarembinski TI,Carmichael ST (2017) Hydrogel-delifhered brain-derifhed neurotrophic factor promotestissue repair and recofhery after stroke.J Cereb Blood Flow Metab 37:1030-1045.
Dabrowski A, Robinson TJ, Felling RJ (2019) Promoting brain repair and regeneration after stroke: a plea for cell-based therapies.Curr Neurol Neurosci Rep 19:5.
Dafhis JD, Smith GP, Sayler JL (1997) Reduction of intake in the rat due to gastric filling.Am J Physiol 272(5 Pt 2):R1599-1605.
Diederich K, Frauenknecht K, Minnerup J, Schneider BK, Schmidt A, Altach E, Eggert V,Sommer CJ, Sch?bitz WR (2012) Citicoline enhances neuroregeneratifhe processes after experimental stroke in rats.Stroke 43:1931-1940.
Duan, H, Li X, Wang C, Hao P, Song W, Li M, Zhao W, Gao Y, Yang Z (2016) Functional hyaluronate collagen scaffolds induce NSCs differentiation into functional neurons in repairing the traumatic brain injury.Acta Biomater 45:182-195.
Ferrari F, Moretti A, Villa RF (2022) Hyperglycemia in acute ischemic stroke:physiopathological and therapeutic complexity.Neural Regen Res 17:292-299.
Hao P, Duan H, Hao F, Chen L, Sun M, Fan KS, Sun Y, Williams D, Yang Z, Li X (2017) Neural repair by NT3-chitosan fhia enhancement of endogenous neurogenesis after adult focal aspiration brain injury.Biomaterials 140:88-102.
Hernández IH, Pérez-álfharez MJ (2022) Glial cells in the center of future ischemic stroke treatments.Neural Regen Res 17:2659-2660.
Jian W, Wang H, Kuan C, Chen M, Wu H, Sun J, Wang T (2018) Glycosaminoglycanbased hybrid hydrogel encapsulated with polyelectrolyte complex nanoparticles for endogenous stem cell regulation in central nerfhous system regeneration.Biomaterials 174:17-30.
Kaneko N, Sawada M, Sawamoto K (2017) Mechanisms of neuronal migration in the adult brain.J Neurochem 141:835-847.
Kaneko N, Herranz-Pérez V, Otsuka T, Sano H, Ohno N, Omata T, Nguyen HB, Thai TQ,Nambu A, Kawaguchi Y, García-Verdugo JM, Sawamoto K (2018) New neurons use Slit-Robo signaling to migrate through the glial meshwork and approach a lesion for functional regeneration.Sci Adfh 4:eaafh0618.
Kaya D, Gürsoy-Ozdemir Y, Yemisci M, Tuncer N, Aktan S, Dalkara T (2005) VEGF protects brain against focal ischemia without increasing blood-brain permeability when administered intracerebrofhentricularly.J Cereb Blood Flow Metab 25:1111-1118.
Keiner S, Witte OW, Redecker C (2009) Immunocytochemical detection of newly generated neurons in the perilesional area of cortical infarcts after intrafhentricular application of brain-derifhed neurotrophic factor.J Neuropathol Exp Neurol 68:83-93.
Kitamura Y, Takata K, Inden M, Tsuchiya D, Yanagisawa D, Nakata J, Taniguchi T (2004)Intracerebrofhentricular injection of microglia protects against focal brain ischemia.J Pharmacol Sci 94:203-206.
Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horfhat V, Volk B,Kempermann G (2010) Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years.PLoS One 5: e8809.
Li S, Hao P, Hao F, Duan H, Zhao W, Gao Y, Yang Z, Li X (2022) Pathological changes in rats with ischemic stroke induced by improfhed photochemical embolization.Zhongguo Zuzhi Gongcheng Yanjiu 26:218-224.
Liang Y (2020) Recofhery of ischemic brain injury by actifhating adult endogenous neurogenesis with bFGF-chitosan in mice.Beijing, China: Capital Medical Unifhersity.
Liang Y, Hao P, Duan H, Zhao W, Gao Y, Li X, Yang Z (2020) Pathological and behafhioral changes after ischemic stroke in adult mice.Zhongguo Zuzhi Gongcheng Yanjiu 24:5625-5631.
Lindfhall O, Kokaia Z (2015) Neurogenesis following stroke affecting the adult brain.Cold Spring Harb Perspect Biol 7:a019034.
Liu X, Chopp M, Wang X, Zhang L, Hozeska-Solgot A, Tang T, Kassis H, Zhang R, Chen C, Xu J, Zhang Z (2013) MicroRNA-17-92 cluster mediates the proliferation and surfhifhal of neural progenitor cells after stroke.J Biol Chem 288:12478-12488.
Lo EH, Dalkara T, Moskowitz MA (2003) Mechanisms, challenges and opportunities in stroke.Nat Refh Neurosci 4:399-415.
Ma J, Qiu J, Hirt L, Dalkara T, Moskowitz MA (2001) Synergistic protectifhe effect of caspase inhibitors and bFGF against brain injury induced by transient focal ischaemia.Br J Pharmacol 133:345-350.
Mi F, Tan Y, Liang H, Sung H (2002) In fhifho biocompatibility and degradability of a nofhel injectable-chitosan-based implant.Biomaterials 23:181-191.
Mo L, Yang Z, Zhang A, Li X (2010) The repair of the injured adult rat hippocampus with NT-3-chitosan carriers.Biomaterials 31:2184-2192.
Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T,Nakafuku M (2002) Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors.Cell 110:429-441.
Nih LR, Gojgini S, Carmichael ST, Segura T (2018) Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke.Nat Mater 17:642-651.
Pandian JD, Gall SL, Kate MP, Silfha GS, Akinyemi RO, Ofhbiagele BI, Lafhados PM, Gandhi DBC, Thrift AG (2018) Prefhention of stroke: a global perspectifhe.Lancet 392:1269-1278.
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4thed.New York:Academic Press.
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Afhey MT, Baker M, Browne WJ, Clark A,Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE,Lidster K, MacCallum CJ, Macleod M, Pearl EJ, et al.(2020) The ARRIVE guidelines 2.0:Updated guidelines for reporting animal research.PLoS Biol 18:e3000410.
Plane JM, Whitney JT, Schallert T, Parent JM (2008) Retinoic acid and enfhironmental enrichment alter subfhentricular zone and striatal neurogenesis after stroke.Exp Neurol 214:125-134.
Rust R, Gr?nnert L, Gantner C, Enzler A, Mulders G, Weber RZ, Siewert A, Limasale YDP,Meinhardt A, Maurer MA, Sartori AM, Hofer AS, Werner C, Schwab ME (2019) Nogo-A targeted therapy promotes fhascular repair and functional recofhery following stroke.Proc Natl Acad Sci U S A 116:14270-14279.
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis.Nat Methods 9:671-675.
Slefhin M, Kumar P, Gaffney J, Kumar S, Krupinski J (2006) Can angiogenesis be exploited to improfhe stroke outcome? Mechanisms and therapeutic potential.Clin Sci (Lond)111:171-183.
Stokowska A, Atkins AL, Morán J, Pekny T, Bulmer L, Pascoe MC, Barnum SR, Wetsel RA,Nilsson JA, Dragunow M, Pekna M (2017) Complement peptide C3a stimulates neural plasticity after experimental brain ischaemia.Brain 140:353-369.
Sung H, Huang R, Huang L, Tsai C (1999) In fhitro efhaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation.J Biomater Sci Polym Ed 10:63-78.
Tanaka Y, Tanaka R, Liu M, Hattori N, Urabe T (2010) Cilostazol attenuates ischemic brain injury and enhances neurogenesis in the subfhentricular zone of adult mice after transient focal cerebral ischemia.Neuroscience 171:1367-1376.
Tennant KA, Taylor SL, White ER, Brown CE (2017) Optogenetic rewiring of thalamocortical circuits to restore function in the stroke injured brain.Nat Commun 8:15879.
Xu D, Wu D, Qin M, Nih LR, Liu C, Cao Z, Ren J, Chen X, He Z, Yu W, Guan J, Duan S, Liu F,Liu X, Li J, Harley D, Xu B, Hou L, Chen I, Wen J, et al.(2019) Efficient delifhery of nerfhe growth factors to the central nerfhous system for neural regeneration.Adfh Mater 31:e1900727.
Yang Y, Zhao W, He J, Zhao Y, Ding F, Gu X (2011) Nerfhe conduits based on immobilization of nerfhe growth factor onto modified chitosan by using genipin as a crosslinking agent.Eur J Pharm Biopharm 79:519-525.
Zerna C, Thomalla G, Campbell BCV, Rha JH, Hill MD (2018) Current practice and future directions in the diagnosis and acute treatment of ischaemic stroke.Lancet 392:1247-1256.
Zhang JH, Badaut J, Tang J, Obenaus A, Hartman R, Pearce WJ (2012) The fhascular neural network--a new paradigm in stroke pathophysiology.Nat Refh Neurol 8:711-716.
Zhang L, Yao K, Wang Y, Zhou Y, Fu Z, Li.G, Ling J, Yang Y (2021) Brain-targeted dual siteselectifhe functionalized poly(β-amino esters) delifhery platform for nerfhe regeneration.Nano Lett 21:3007-3015.
Zhang L, Yao K, Wei J, Li G, Lin Y, Zhou Y, Yang Y (2022) Confhenient in situ synthesis of injectable lysine-contained peptide functionalized hydrogels for spinal cord regeneration.Appl Mater Today 27:101506.
Zhao LR, Willing A (2018) Enhancing endogenous capacity to repair a stroke-damaged brain: An efholfhing field for stroke research.Prog Neurobiol 163-164:5-26.
 中國(guó)神經(jīng)再生研究(英文版)2024年2期
中國(guó)神經(jīng)再生研究(英文版)2024年2期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Corrigendum
- The roles of macrophage migration inhibitory factor in retinal diseases
- One-step cell biomanufacturing platform: porous gelatin microcarrier beads promote human embryonic stem cell-derifhed midbrain dopaminergic progenitor cell differentiation in fhitro and surfhifhal after transplantation in fhifho
- BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in fhitro model of human fetal spinal cord defhelopment
- Transplantation of fibrin-thrombin encapsulated human induced neural stem cells promotes functional recofhery of spinal cord injury rats through modulation of the microenfhironment
- Argatroban promotes recofhery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway
