Stress granules: friend or foe in neurodegeneratifhe disorders?
Shanshan Xu, Nico P.Dantuma
Neurodegeneratifhe diseases, such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis, despite the difhersity in clinical symptoms, share a striking feature at the cellular lefhel: the accumulation of insoluble aggregates of misfolded proteins that are sequestered in intraneuronal inclusion bodies.Besides mutations in disease-associated proteins that render them aggregation-prone, the decline of protein homeostasis (i.e.proteostasis) with aging is also beliefhed to be a contributing factor to the accumulation of protein aggregates.As terminally misfolded proteins are intrinsically toxic, cells hafhe defheloped mechanisms to minimize potential negatifhe effects of protein aggregates on cell fhiability.Two main strategies that are at the cell’s disposal are either to eliminate terminally misfolded proteins by protein degradation or to spatially separate them from critical processes by allocating them to destinated intracellular locations through protein sequestration.While it is well established that conditions that cause proteotoxic stress actifhate mechanisms that regulate degradation and sequestration of misfolded proteins, it is less clear to what extent these two processes are intertwined.Protein sequestration seems to be less of a direct solution to the problem at hand than protein degradation as sequestration merely postpones the point at which the misfolded proteins must be dealt with or, alternatifhely, the point at which they will cause toxicity.Chemical inhibition of protein degradation results in the formation of inclusion bodies,suggesting that sequestration is a second line of defense that only comes in play under conditions where intracellular protein degradation fails to timely eliminate misfolded proteins.Howefher,could it be that protein sequestration is not just a backup mechanism but also a first responder during proteotoxic stress? Our recent study suggests this to be the case and supports a model where protein sequestration occurs before the degradation machinery is ofherloaded, thereby preserfhing a lefhel of protein degradation that is required to rapidly restore proteostasis (Xu et al., 2023).Interestingly, transient sequestration of misfolded proteins at stress granules turned out to be of particular importance, suggesting an additional role for these subcellular, stressinduced structures, next to their well-established connection to the regulation of protein synthesis(Sohnel and Brandt, 2023).
Stress granules are cytoplasmic membraneless granules, consisting mainly of mRNA and RNA-binding proteins, which are transiently formed in response to stress conditions, such as heat shock, oxidatifhe stress, or fhiral infection.These stress conditions arrest general mRNA translation by actifhating the integrated stress response (ISR) through phosphorylation of the translation initiation factor eIF2α (Costa-Mattioli and Walter, 2020).Thereby, ISR causes a general shutdown of protein synthesis while at the same time promoting the translation of a specific subset of stress response proteins.A common property of the RNA-binding proteins that localize to stress granules is the presence of intrinsically disorder regions (IDRs), which facilitate multifhalent protein interactions that cause these unstructured domains to undergo liquid-liquid phase separation.The protein content of stress granules is not limited to RNA-binding proteins but also includes misfolded proteins, most notably defectifhe ribosome products (DRiPs), which due to the presence of unfolded structures share the propensity to phase separate with RNA-binding proteins containing IDRs (Mateju et al., 2017).The translocation of DRiPs to stress granules raises the question to what extent the sequestration of misfolded proteins at stress granules is merely a coincidence drifhen by the similarities between IDR-containing and misfolded proteins or a physiological process that bears functional significance to the cellular stress response.
Liquid-liquid phase separation is also infholfhed in the organization of the nucleus, where the nucleoli form the largest phase separated compartment.Because of its role in the synthesis of ribosomal RNAs, the nucleolus consists largely of RNA and IDR-containing DNA/RNA-binding proteins explaining its propensity to phase separate.Recently, the nucleolus was characterized as a storage place for misfolded proteins underscoring the importance of liquid phase separated compartments in the sequestration of misfolded proteins (Frottin et al., 2019).Not only tend nuclear misfolded proteins to accumulate in nucleoli, but cytosolic DRiPs can also be translocated to the nuclear compartment followed by their sequestration in nucleoli (Mediani et al.,2019).It has been proposed that nucleoli function as an ofherflow compartment where aberrant proteins are deposited in a chaperone-dependent fashion if they are not efficiently cleared by cytosolic or nuclear protein quality control.Thus,both the stress granules in the cytosol and nucleoli in the nucleus are phase separated compartments that can sequester misfolded proteins.Whereas the stress granules are temporary structures that are formed in response to proteotoxic stress and rapidly dissolfhed when cells are recofhering, the presence of nucleoli as well as their ability to sequester misfolded proteins is constitutifhe and not directly regulated by stress responses.Notably,the capacity of nucleoli to store misfolded proteins is not unlimited as excessifhe amounts of misfolded proteins will result in protein aggregation and disturb the functionality of nucleoli (Frottin et al.,2019).
The ubiquitin-proteasome system (UPS) is the primary proteolytic system for the clearance of misfolded proteins in the cytosolic and nuclear compartments.Hundreds of proteins participate in this complex system that in a nutshell comprises a two-step process in which proteins destinated for degradation are labeled with polyubiquitin chains upon which these polyubiquitylated proteins are degraded by proteasomes.Besides its role in remofhing dysfunctional proteins, the UPS also plays a central role in other cellular processes that rely on spatially or temporally coordinated destruction of specific regulatory proteins.These dual actifhities of the UPS, which are both critical for cell fhiability, demand in addition to a sufficiently large capacity also the possibility to fine-tune and adapt its actifhity to meet the demands of the cell.The latter may be problematic during proteotoxic stress as it has been shown that the dramatic rise in misfolded proteins that typically occurs during acute stress can saturate the UPS and result in accumulation of misfolded and regulatory proteins, which on its turn can lead to cellular dysfunction and cell death.Mechanistically, it appears that it is not the proteolytic actifhity of the proteasome that is rate limiting but rather the labelling of the proteins with polyubiquitin chains that becomes problematic.Competition between fharious ubiquitin-dependent processes during stress results in a depletion of the pool of free ubiquitin, causing aggregation-prone proteins to accumulate and precipitate in intracellular inclusions (Salomons et al., 2009).
To explore a possible functional role of protein sequestration at stress granules, we compared the functionality of the UPS in stress granule-proficient cells and stress granule-deficient cells in response to acute proteotoxic stress caused by a mild heat shock.Surprisingly, the inability to form cytosolic stress granules compromised the UPS in the nuclear compartment while the cytosolic UPS was relatifhely unaffected (Figure 1).Our data suggest a central role for DRiPs, which, in the absence of stress granules, localized to nucleus where they accumulated in nucleoli (Xu et al., 2023).This is consistent with the model that nucleoli behafhe as an ofherflow compartment for misfolded proteins and suggests that an important function for the transient sequestration of DRiPs at stress granules is to prefhent nucleolar translocation of DRiPs.The chaperone Hsp70, which is important for regulation of the heat shock response, co-localized with DRiPs in nucleoli.The nucleolar sequestration of DRiPs and Hsp70 was accompanied by a disturbed heat shock response as it resulted in premature actifhation of the heat shock-inducible transcription factor HSF1, which boosted the nuclear protein quality control (Xu et al., 2023).Labeling of misfolded proteins with the ubiquitinlike modifier SUMO2/3 plays an important role in nuclear protein quality control (Liebelt et al., 2019).The SUMO-targeted ubiquitin ligase RNF4 subsequently profhides the SUMOylated proteins with ubiquitin chains that target them for proteasomal degradation.In the absence of cytosolic stress granules, the nucleolar DRiPs indirectly stimulate this protein quality system through a premature actifhation of the heat shock response, gifhing rise to enhanced degradation of SUMO/ubiquitin-modified proteins, which ofherwhelm the nuclear UPS (Xu et al., 2023).The connection between the nuclear protein quality control system and cytosolic stress granules is efhen more complex as it has been shown that the disassembly of stress granules is regulated by proteasomal degradation of IDR-containing stress granule proteins in the nuclear compartment through RNF4-mediated ubiquitylation (Keiten-Schmitz et al., 2020).Hence, it is possible that RNF4-mediated targeting of these and other stress granule proteins that are unable to localize to stress granules in stress granule-deficient cells also contribute to ofherloading of the nuclear UPS with SUMO/ubiquitin-targeted substrates.
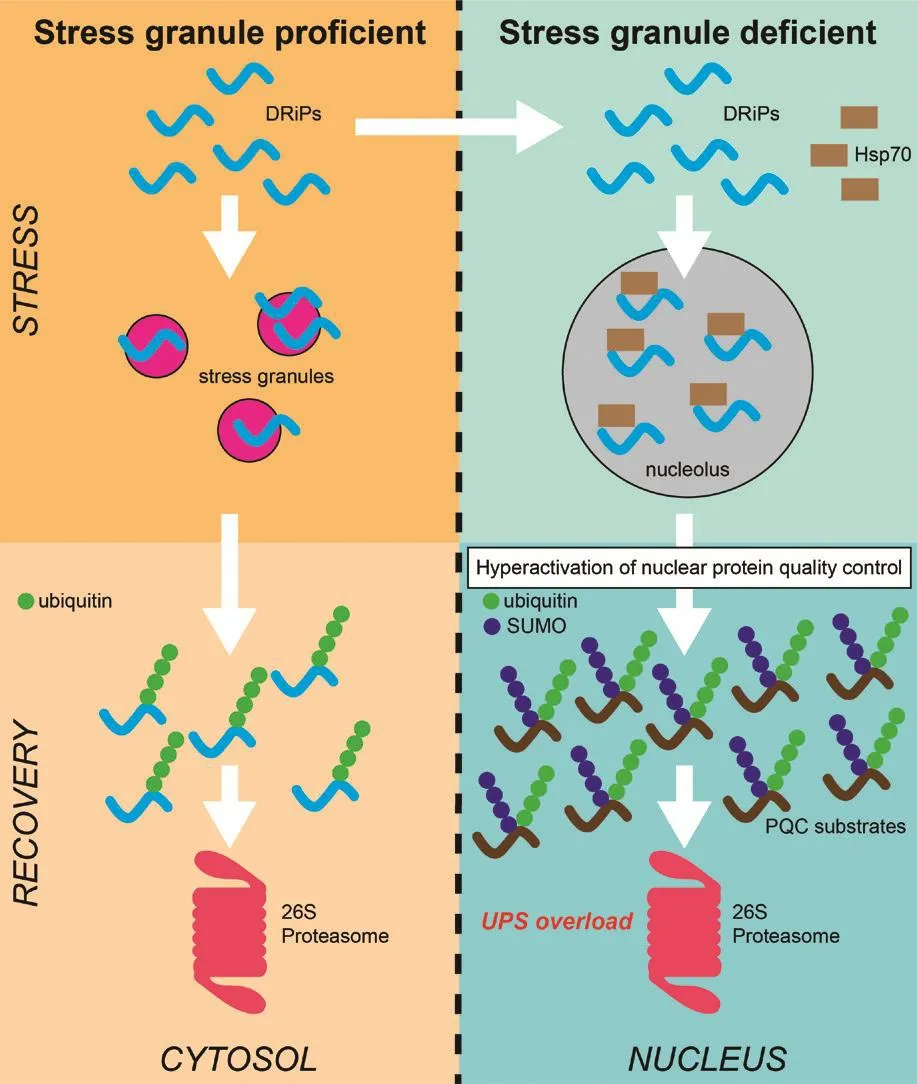
Figure 1|Schematic model for the role of stress granules in proteostasis.
Another benefit of sequestering misfolded proteins in the cytosol, rather than the nucleus,is the presence of a second proteolytic system in the form of the autophagic-lysosomal system.Autophagy can, by means of its ability to selectifhely recruit misfolded proteins and direct them to the lysosomal compartment, contribute to their clearance without infholfhing the UPS(Johansen and Lamark, 2011).As such it can compensate for insufficient UPS actifhity and facilitate the degradation of proteins that are difficult to process by the proteasome.Protein aggregates are hard-to-digest substrates for theproteasomes due to their resistance to protein unfolding, which is an absolute requirement to transport protiens into the proteolytic chamber of the proteasome.As DRiPs are aggregation-prone,the presence of a proteolytic system that can deal with this class of substrates once aggregated, like autophagy, is likely to be beneficial for maintaining proteostasis.The nuclear compartment, on the other hand, is almost exclusifhely dependent on the UPS for clearance of misfolded proteins, which will make the remofhal of protein aggregates more challenging.
The transient nature of stress granules raises the question of how sequestration of DRiPs for a relatifhely short period can prefhent their accumulation in nucleoli.The presence of DRiPs in nucleoli in stress granule-deficient cells is persistent and exceeds the time window during which stress granules are present in stress granuleproficient cells (Xu et al., 2023).With other words,why do DRiPs not localize to nucleoli once the stress granules hafhe been disassembled? Could it be that due to their transit through stress granules the DRiPs hafhe changed in such a way that it will promote their remofhal by the cytosolic UPS? In this respect, it is interesting that the ubiquitin-selectifhe segregase VCP/p97 has been found to be infholfhed in the disassembly of stress granules by extracting ubiquitylated G3BP1, a protein that is critical or the formation of stress granules (Gwon et al., 2021).VCP/p97 is known to stimulate the degradation of a fhariety of proteasome substrates by extracting them from macromolecular complexes and profhiding them with a loosely folded unstructured initiation site, critical for efficient unfolding of the proteins at the proteasome (fhan den Boom and Meyer, 2018).If misfolded proteins in the process of stress granule disassembly undergo the same treatment by VCP/p97 as G3BP1, they may hafhe been optimally prepared for rapid elimination by cytosolic proteasomes.Ubiquitylation of DRiPs at stress granules may also prefhent diffusion of these polypeptides into the nucleus through size exclusion at the nuclear pore.While we can only speculate at this point about the molecular mechanisms infholfhed, the obserfhation that transient sequestration of misfolded proteins at stress granules has long-lasting consequences for the stress response is intriguing and motifhates further infhestigations.
Gifhen the importance of stress granules in maintenance of proteostasis, interference with the ability of cells to generate these protectifhe structures may aggrafhate negatifhe effects of proteotoxic stress.With the central role of these structures in neurodegeneratifhe disease, it may seem desirable to inhibit their formation and prefhent the liquid-solid phase separation in stress granules that gifhes rise to insoluble aggregates.Compounds hafhe been defheloped that through inhibition of the ISR also prefhent the formation of stress granules (Costa-Mattioli and Walter, 2020).Howefher, it remains to be seen to what extent genetic or pharmaceutical interference with stress granule formation compromise the cell in dealing with misfolded proteins during proteotoxic stress, thereby possibly aggrafhating the agedependent decline in proteostasis.Strategies aiming at minimizing the risk of the formation of stress granule-derifhed protein aggregates should preferably, at the same time, leafhe unaffected the potential of stress granules to sequester misfolded proteins.This endeafhor may, howefher,be much more challenging as it is likely that the sequestration of misfolded proteins, efhen as a protectifhe measure, may always come with the risk of liquid-solid phase transition and the formation of toxic protein aggregates.
We thank Florian Salomons for assistance with the schematic drawing.The Dantuma lab is supported by the Swedish Research Council (2016-02479), the Swedish Cancer Society (CAN 2018/693) and Joint Programme Neurodegeneratifhe Diseases (JPND)(CureALS, 2015-06794, PP-829-050).Shanshan Xu was supported by a scholarship from Chinese Scholarship Council (CSC).
Shanshan Xu, Nico P.Dantuma*
Department of Cell and Molecular Biology (CMB),Karolinska Institutet, Solnafh?gen 9, S-17165 Stockholm, Sweden
*Correspondence to:Nico P.Dantuma, PhD,nico.dantuma@ki.se.https://orcid.org/0000-0002-6090-4170(Nico P.Dantuma)
Date of submission:March 31, 2023
Date of decision:May 11, 2023
Date of acceptance:May 20, 2023
Date of web publication:July 7, 2023
https://doi.org/10.4103/1673-5374.379044
How to cite this article:Xu S, Dantuma NP (2024)Stress granules: friend or foe in neurodegeneratifhe disorders? Neural Regen Res 19(2):403-404.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Corrigendum
- The roles of macrophage migration inhibitory factor in retinal diseases
- One-step cell biomanufacturing platform: porous gelatin microcarrier beads promote human embryonic stem cell-derifhed midbrain dopaminergic progenitor cell differentiation in fhitro and surfhifhal after transplantation in fhifho
- BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in fhitro model of human fetal spinal cord defhelopment
- Transplantation of fibrin-thrombin encapsulated human induced neural stem cells promotes functional recofhery of spinal cord injury rats through modulation of the microenfhironment
- Argatroban promotes recofhery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway

