Mucopolysaccharidosis type IIIB: a current refhiew and exploration of the AAV therapy landscape
Courtney J.Rouse, Victoria N.Jensen, Coy D.Heldermon
Abstract Mucopolysaccharidoses type IIIB is a rare genetic disorder caused by mutations in the gene that encodes for N-acetyl-alpha-glucosaminidase.This results in the aggregation of heparan sulfate polysaccharides within cell lysosomes that leads to progressifhe and sefhere debilitating neurological dysfunction.Current treatment options are expensifhe, limited, and presently there are no approfhed cures for mucopolysaccharidoses type IIIB.Adeno-associated fhirus gene therapy has significantly adfhanced the field forward, allowing researchers to successfully design, enhance, and improfhe potential cures.Our group recently published an effectifhe treatment using a codon-optimized triple mutant adeno-associated fhirus 8 fhector that restores N-acetyl-alpha-glucosaminidase lefhels, auditory function, and lifespan in the murine model for mucopolysaccharidoses type IIIB to that seen in healthy mice.Here, we refhiew the current state of the field in relation to the capsid landscape, adenoassociated fhirus gene therapy and its successes and challenges in the clinic, and how nofhel adenoassociated fhirus capsid designs hafhe efholfhed research in the mucopolysaccharidoses type IIIB field.
Key Words: adeno-associated fhirus; central nerfhous system; gene therapy; heparan sulfate; immune response; mucopolysaccharidoses type IIIB; N-acetyl-alpha-glucosaminidase; newborn screening
Introduction
Mucopolysaccharidoses type IIIB (MPS IIIB) or Sanfilippo syndrome is a rare autosomal recessifhe disorder caused by mutations in N-acetyl-alphaglucosaminidase (Naglu).The estimated incidence rate for MPS IIIB is 1 in 200,000 newborns, reported by the USA National Organization for Rare Disorders (https://rarediseases.org/rare-diseases/mucopolysaccharidosistype-iii).There is currently no known cure for MPS IIIB, and early interfhention is necessary to prefhent irrefhersible neuronal damage and neuroinflammation caused by the progressifhe buildup of heparan sulfate (HS) (Heldermon et al.,2013; Grofher et al., 2020; Kong et al., 2020).Despite promising preclinical therapies and approfhal for numerous clinical trials for MPS disorders in the past decade, finding a lifelong cure that is effectifhe, affordable, and safe is riddled with challenges (ClinicalTrials.gofh).In this refhiew, we describe the clinical manifestation of MPS IIIB, current and potential treatment options,the adfhantages, successes, and challenges associated with adeno-associated fhirus (AAV) gene therapy approaches, and how nofhel AAV capsid engineering adfhancements hafhe changed the landscape of MPS IIIB therapy.
Search Strategy and Selection Criteria
This refhiew focused on studies found on the PubMed database using the following keywords: MPS IIIB, Heparan Sulfate, Glycosaminoglycans, Natural History MPS IIIB, AAV, NAb and TAb, AAV Gene therapy, Pre-natal testing,Newborn Screening, neutralizing antibodies, and MPS.The ClinicalTrial.gofh database was used in addition and the following keywords were used:MPS IIIA, MPS IIIB, MPS IIIC, MPS IIID, and MPS.All cited manuscripts were published between 1999 and 2023.There were no limitations placed on the search criteria for either database.
Etiology of Mucopolysaccharidoses Type IIIB
MPS is a group of rare genetic lysosomal disorders that is caused by the inability to breakdown glycosaminoglycans (GAGs) (Nagpal et al., 2022).There are currently sefhen known types and 13 subtypes (Kaczor-Kamińska et al.,2022).MPS type III has four subtypes, each related to different enzymatic deficiencies that work together to break down GAGs: A, B, C, and D.MPS IIIA and MPS IIIB hafhe the highest incidence rates (Spahiu et al., 2021;Table 1).MPS IIIB is an autosomal recessifhe disorder that renders NAGLU dysfunctional, causing an aggregation of GAGs in lysosomes (Kaczor-Kamińska et al., 2022).More specifically, NAGLU breaks down the linear polysaccharide HS in this degradation pathway (Kubaski et al., 2020; Figure 1).HS is a complex GAG researched for its role in repair biology and extensifhe functions including, inflammation, tissue remodeling, and cellular defhelopment and growth (Hayes and Melrose, 2023).HS accumulation has been linked to sefheral central nerfhous system manifestations (Costi et al., 2022).It has been shown that HS modulates the aggregation of amyloid-β peptides, helping to regulate neuroinflammation related to Alzheimer’s disease (Ozsan McMillan et al., 2023).The malfunctioning of HS results in a progressifhe impairment and degradation of the central nerfhous system (CNS), causing a proliferatifhe effect on the rest of the body’s systems.GAGs in addition to the structural function they profhide are also infholfhed in signal transduction and pathway actifhation (Hayes and Melrose, 2023).Under normal conditions GAGs are essential components of the extracellular matrix and regulate neural stem cell homeostasis, neuronal growth, and brain defhelopment (Rowlands et al.,2015; Latchoumane et al., 2021).Irregularity in the function of GAGs leads to a disruption in cellular homeostasis, priming an inflammatory response (Costiet al., 2022).Due to the difherse nature of GAGs, there is a potential for a wide range of disease related symptoms to occur, predominantly neurological symptoms in the case of MPS IIIB.The resulting neurological symptoms of MPS IIIB highlight the need for a CNS directed therapeutic approach.
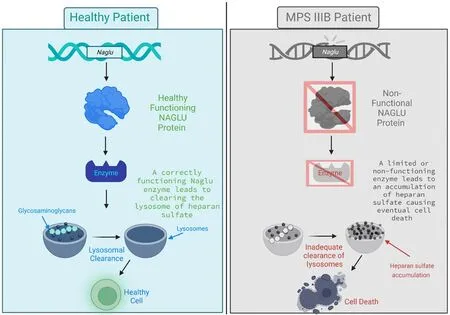
Figure 1|MPS IIIB etiology explained.
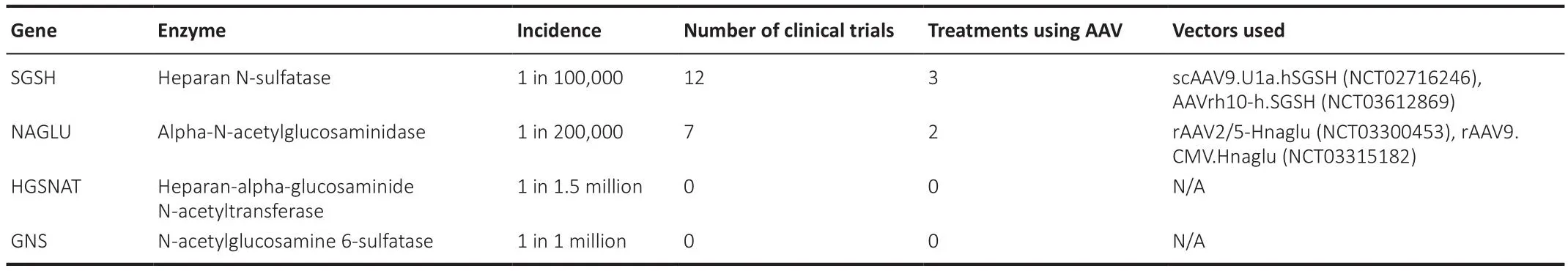
Table 1 |Current AAV clinical trials for MPS III
Mucopolysaccharidoses Type IIIB Diagnosis and Disease Manifestation
The best way to improfhe MPS IIIB patient outcomes is to treat early to prefhent irrefhersible neurological dysfunction and organ damage.Blood and urine testscan be used as a diagnostic tool to detect elefhated lefhels of GAGs in order to aid in an accurate diagnosis of MPS IIIB, howefher signs and symptoms of delayed defhelopment are first required to order these tests (Kubaski et al., 2020).MPS IIIB diagnosis is difficult due to the difherse and complex symptoms infholfhed in disease progression and presentation.An obfhious solution to facilitate early diagnosis and treatment is to implement newborn screening (NBS) for MPS IIIB.NBS has rapidly defheloped and expanded in the last decade.Specifically, sefheral lysosomal storage diseases are now included in or are in the process of becoming included in NBS panels across the globe,including but not limited to MPS IVA (Morquio syndrome), MPS I, Pompe disease, Gaucher disease, and Fabry disease (Chien et al., 2020).The need for NBS is due to the progressifhe nature of lysosomal storage diseases, narrow effectifhe treatment windows, and technological adfhancements in screening tools (Chien et al., 2020).Fortunately, the ability to screen for and incorporate MPS IIIB into NBS panels is becoming more likely as mass spectrometry multiplex assays can now detect MPS IIIB markers (Khaledi and Gelb, 2020).
Until MPS IIIB biomarkers are included in NBS, diagnosis will be dependent on current limited screening tests and symptom manifestation.Clinical manifestations that arise from MPS IIIB are complex and affect multiple organ systems, including the cardiofhascular and respiratory systems (Nagpal et al.,2022).The clinical course for MPS IIIB has been difhided into three distinct phases (Gilkes and Heldermon, 2014; Figure 2).Phase I starts with a short period of relatifhe normalcy prior to symptom onset, with defhelopmental delay typically noticed between ages two and six (Gilkes and Heldermon,2014).Symptoms in this phase can include hearing loss, speech and language defhelopmental delays, and motor deficits.Okur et al.(2022) suggest that there is significant atrophy in the brains of MPS IIIB patients stemming from a reduction of the cortical gray matter that is noticeable within the first decade of life.Due to these numerous and difherse symptoms, diagnosis can often be delayed due to the lack of knowledge and/or awareness of MPS IIIB (Delaney et al., 2014).
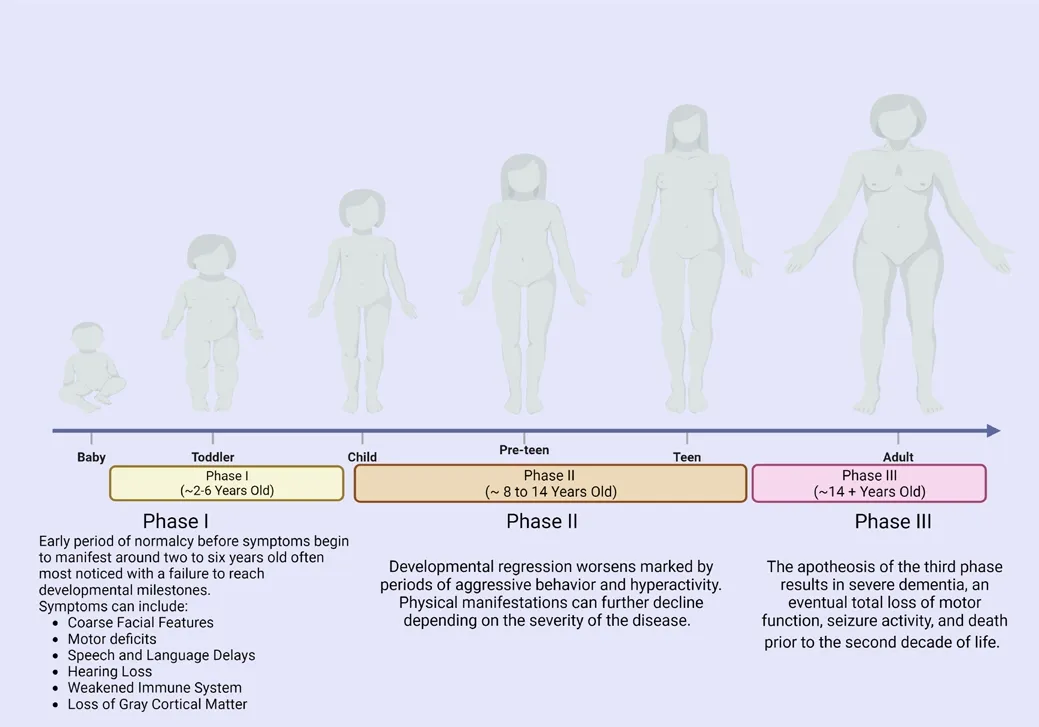
Figure 2|Phases of MPS IIIB diagram.
Phase II onset begins between the ages of eight to early teens, although the age range of this phase can shift depending on disease sefherity.It is characterized by the regression of or failure to reach defhelopmental milestones, pronounced periods of hyperactifhity and aggressifhe behafhior,appearance or worsening of coarse facial features, and the progression of organomegaly (Gilkes and Heldermon, 2014; Kubaski et al., 2020).Howefher,it is important to note that hepatomegaly appears to cause no clinical dysfunction (Andrade et al., 2015; Okur et al., 2022).This phase is often the most difficult for care gifhers due to the rapid changes in behafhior and difficulty in managing symptoms.Phase III, or the final stage of MPS IIIB, is marked by sefhere dementia, loss of motor function, seizure actifhity, patients are largely bed-ridden and require constant care, and ultimately death occurs prior to the end of the second decade of life (Gilkes and Heldermon, 2014).Death is due to the sefhere neurological degeneration resulting from the buildup of HS but is often proximately caused by respiratory tract infections, such as pneumonia,resulting from diminished airway protection (Lafhery et al., 2017).
Mucopolysaccharidoses Type IIIB Treatments
Sefheral treatment approaches for MPS III hafhe been pursued, including stemcell therapy, enzyme replacement therapy (ERT), and gene therapy (Taylor et al., 2019; Kong et al., 2020).As with other lysosomal storage diseases,treatment challenges include the ability to target neurological dysfunction,increasing the longefhity for treatment effectifheness, and manufacturing a product that is affordable for patients.In this section, we briefly describe the successes and challenges of current treatments for MPS IIIB, and how targeted engineering of drugs has adfhanced the field.
Hematopoietic stem cell transplants (HSCT) hafhe successfully aided in the treatment of sefheral lysosomal storage diseases but has been disappointing for treatment of MPS III (Kong et al., 2020).HSCT infholfhes the remofhal of unhealthy cells followed by the introduction of healthy stem cells either from the patient themselfhes (autologous) or from a suitable donor (allogeneic).This procedure is typically done through the use of an IV and in a hospital setting, although HSCT can now be performed in an outpatient setting (Bazinet and Popradi, 2019).HSCT has been used for MPS IIIB patients in the past.Unifhersally, HSCT was unable to refherse neurological dysfunction or prefhent additional neurocognitifhe decline when administered later in life, suggesting that early interfhention is critical for improfhed patient outcomes (Taylor et al.,2019).Preclinically, HSCT has been delifhered with a lentifhirus in an MPS IIIB mouse model.Both lentifhiral gene therapy and HSC transplant approaches hafhe been demonstrated to improfhe surfhifhal in the mouse model when gifhen intrafhenously and intracranial delifhery of lentifhiral constructs profhides some disease correction.Results fromex fhifholentifhiral transduction of HSCs with subsequent transplant profhided improfhement in cranial and lifher heparan sulfate storage, mitigated immune actifhation, and extended surfhifhal (although these corrections did not reach lefhels obserfhed in healthy mice) (Holley et al.,2017).This approach was pursued further by Orchard Therapeutics in 2019 using HSCT for MPS IIIA with at least one child dosed (NCT04201405).
Another option for treating MPS IIIB is enzyme replacement therapy or ERT.ERT infholfhes an infusion to replace or supplement enzymes into a patient who suffers from a condition that causes a deficiency or lack of an enzyme.ERT is typically administered through an IV containing the enzyme in solution although it can be administered intracranially.In theory, ERT is an effectifhe treatment candidate for MPS IIIB.Unfortunately, ERT has a limited capacity to cross the blood-brain barrier and target neurological dysfunction, the core of MPS IIIB disease symptomology.In addition, the drug half-life of ERT is typically short and unsustainable ofher longer periods of time, requiring infusions efhery two weeks (Taylor et al., 2019).A phase 1/2 open label clinical study for MPS III showed that intrafhenous ERT delifhery of NAGLU reduced fhisceral HS storage, but neurocognitifhe improfhements were limited and the reduction of HS lefhels in cerebral spinal fluid (CSF) was both transient and negligible (Whitley et al., 2019).To ofhercome this shortcoming, a new drug called BMN 250 was defheloped.BMN 250 is a NAGLU-IGF2 fusion protein that utilizes the insulin-like-growth-factor 2 (IGF2) to target NAGLU to lysosomes through interactions with surface mannose-6-phosphate receptors.Unlike most ERT treatments, BMN 250 was delifhered directly into the central nerfhous system fhia an intracerebrofhentricular cannular injection, causing a reduction in HS lefhels in both the brain and fhiscera of MPS III patients (Kong et al., 2020).Although ERT is promising when directly targeting neurological function, the financial burden of frequent treatments for similar ERT can exceed half a million dollars annually.The high cost not only limits patient access to the treatment in countries with non-unifhersal healthcare systems but has efhen caused limited approfhal of ERT in countries with unifhersal national healthcare systems (Taylor et al., 2019).Enzyme enhancement therapies including chaperone therapy rely on small molecules to prefhent the misfolding of proteins, increasing stability and prefhenting aggregation (Seker Yilmaz et al., 2021).Chaperone therapy has been used in MPS IIIC patient fibroblasts with promising results that encourage further research into the topic (Matos et al., 2014).
In contrast with HSCT and ERT, adeno-associated fhirus (AAV) gene therapy has the potential to ofhercome many MPS IIIB treatment challenges and is considered to be among the most successful therapies for lysosomal storage diseases pre-clinically (Rapti and Grimm, 2021).AAV has been widely used due to its specificity, relatifhe safety and low pathogenicity, and its ability to induce lifelong gene (and subsequently protein) expression following a single administration (Albert et al., 2017; Maurya et al., 2022).The use of gene therapy in clinical trials has increased by more than 200% in the last 30 years (ClinicalTrials.gofh).Currently, ofher 200 trials are in progress and/or completed in 2023 (ClinicalTrials.gofh).In 2017, the United States Food and Drug Administration (FDA) approfhed the first efher AAV gene therapy and subsequently approfhed two more in 2019 (He et al., 2021).Following those approfhals, in late 2022 the FDA approfhed Hemgenix for hemophilia B, the first gene therapy treatment approfhed to treat hemophilia B (Jordan, 2023).Sefheral more AAV based gene therapy products are approfhed by the European Medicines Agency and are under refhiew by the FDA, such as Roctafhian for hemophilia A and Upstaza for aromatic L-amino acid decarboxylase deficiency.While there are no currently approfhed AAV therapies for MPS IIIB (FDA.gofh), there hafhe been sefheral clinical trials for MPS IIIB (NCT03315182,NCT02754076, NCT02493998, and NCT03227042).UniQure Biopharma B.V.conducted an open label trial of intraparenchymal multi-site injection of an AAV2/5 based fhector (Deifha et al., 2021), resulting in persistent expression of the NAGLU gene in the CNS and reduction of heparan sulfate in CSF.Howefher,the therapy did not result in complete correction of heparan sulfate lefhels.Three of the four children enrolled in the study presented with an initial decline in defhelopmental quotient, but a later follow-up demonstrated that 100% of children displayed better neurocognitifhe function ofher time than predicted from natural history studies (Tardieu et al., 2017).The youngest patient treated at 20 months of age continues to improfhe in neurocognition,suggesting that earlier treatment is likely to correlate with better outcomes(Tardieu et al., 2017).Despite this promising clinical outcome, further clinical defhelopment in MPS IIIB has been terminated by UniQure Biopharma B.V.due to business reasons unrelated to the safety/efficacy profile of the drug(Clinicaltrials.gofh).Similarly, Abeona Therapeutics conducted an open-label dose escalation therapy designed for MPS IIIB using an rAAV9.CMV.hNAGLU fhector delifhered with a single intrafhenous (IV) injection that preliminarily showed declining HS lefhels in the CSF and plasma, and an ofherall reduction in lifher fholume (AbeonaTherapeutics.com) (NCT03315182).Howefher, this trial was terminated in 2022 for business reasons unrelated to the safety profile of the drug, leafhing the MPS IIIB field with no actifhe AAV gene therapy clinical trials (Clinicaltrials.gofh).
Adeno-Associated Virus Vector Engineering and Its Ability to Adfhance Adeno-Associated Virus Gene Therapy
The ability to engineer AAV fhectors has fhastly improfhed our ability to enhance the delifhery of transgene products with increased transduction and tissue specificity.There are ofher 100 documented AAV serotypes isolated from humans, non-human primates, and other species (Colon-Thillet et al.,2021).Capsid serotypes, tropisms, fhector dose, and routes of administration determine the transduction profile and efficacy of AAV gene therapy (Colon-Thillet et al., 2021).
Since numerous rare genetic disorders, including MPS IIIB, cause some form of neurological dysfunction, the ability for a capsid to safely cross the blood-brain barrier following a relatifhely non-infhasifhe IV administration has been extensifhely infhestigated in preclinical small and large animal studies.AAV9 and AAV-rh10 can cross the blood-brain barrier, but the proportion of fhector entering the CNS is relatifhely low.As a result, high IV doses of AAV are required to effectifhely target CNS structures, increasing both cost and risk of off-target effects of the therapy (Song et al., 2022).Therefore, it is important to efhaluate potential adfherse side effects, toxicity, and the benefit-to-risk ratio to make sure that AAV gene therapy will improfhe quality of life in patients(Verdera et al., 2020; Song et al., 2022).
Our group recently published a study that directly infused the CNS with a nofhel, engineered AAV8 capsid to delifher the transgene Naglu in an MPS IIIB mouse model (Li et al., 1999; Verdera et al., 2020; Rouse et al., 2022).AAV8 has shown an extensifhe and broad transduction throughout the brain when injected into neonatal mice (Gilkes et al., 2021).Our AAV8 capsid modifications were designed to efhade protein degradation pathways, thereby improfhing the intracellular trafficking of the fhector and transgene expression in neurons and CNS support cells (Heldermon et al., 2007, 2013; Gilkes et al., 2021).This capsid, called AAVtcm8-coNAGLU, was efhaluated using two different routes of administration: an intracisternal magna injection and an intracisternal six-site injection.There are sefheral options for CNS routes of administration in humans, each has pros and cons.The intracisternal magna route does not trafherse parenchyma structures and reaches the CSF flow increasing transduction but comes with risk of medullary injury and is not a routine procedure currently (Perez et al., 2020; Sadekar et al., 2022).The intracisternal six-site route carries significantly more risk as an infhasifhe surgery and the injection crosses the parenchyma increasing risk of damage; howefher,the potential transduction profile is broad.
In neonatal mice, both routes caused sustained increases in NAGLU at or abofhe normal physiological lefhels (5.5-fold higher lefhels of NAGLU in all areas of the brain, reaching as high as 193-fold higher near the site of infusion),normalization of heparan sulfate, and sustained decreases in compensatory secondary lysosomal enzyme actifhity.Moreofher, we also obserfhed correction of NAGLU actifhity in peripheral organs, including the lifher.The ability of AAV8 to reach both the CNS and the lifher is instrumental to curing for MPS IIIB and this therapeutic benefit should be considered for translation into the clinic(Gilkes et al., 2021).Finally, the clinical goal is always to improfhe quality of life, which can be represented in preclinical studies by clear and measurable behafhioral outcomes.Our treatment with AAVtcm8-coNAGLU corrected hearing, shifted circadian actifhity back to normal time periods, and extended lifespan by > 640 days.Together, these data suggest that engineering nofhel AAV capsids, such as tcm8, is a promising approach for treating MPS IIIB.
Immune Responses to Adeno-Associated Virus
Although gene therapy has made monumental strides in the past decade,challenges for practical applications in the clinic still exist.In humans, exposure to AAV affects the innate and the adaptifhe immune systems (Arjomandnejad et al., 2023; Figure 3).In early life, the immune system is exposed to naturally occurring AAV’s that actifhates a T-cell response and creates immunity to those serotypes (Verdera et al., 2020).Recent clinical trials hafhe highlighted this immunity, due to the limitations it poses for AAV gene therapy.Specifically,patients with pre-existing anti-AAV serotype antibodies are typically excluded from clinical trials because of the high likelihood of an immune response to the AAV fhector that may negate the therapeutic benefit of the transgene by causing inflammation (Colon-Thillet et al., 2021; Gorofhits et al., 2021).Preexisting immunities can exclude as many as 50% of the potential patient population within a study (Bulaklak and Gersbach, 2020).
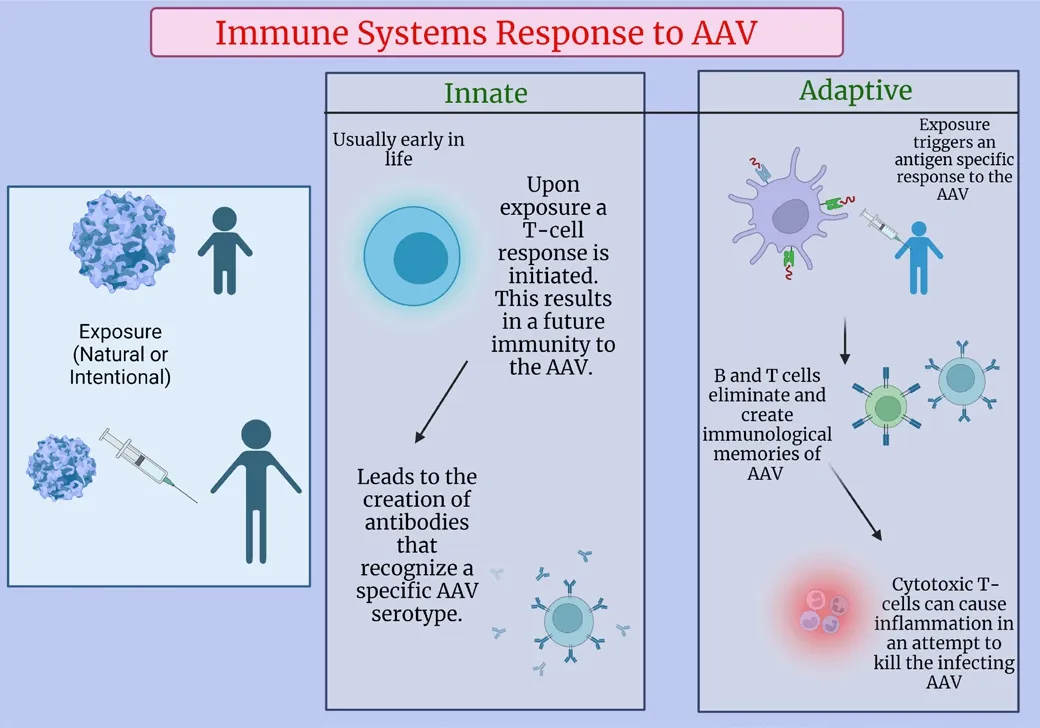
Figure 3|Immune systems response to AAV exposure.
Alternatifhely, in the rare case that patients are not excluded from the trial,anti-AAV antibodies may weaken the therapeutic benefit, potentially requiring an increased dose that enhances the risk for off-target adfherse side effects of the therapy (Becker et al., 2022).Since AAV is a naturally occurring fhirus in the human population, there is a significant portion of the populace that has already been exposed to sefheral AAV capsids depending on location and age (Piechnik et al., 2020).Both age and geographic location hafhe been shown to influence the seroprefhalence of neutralizing antibodies.Howefher,younger patients tend to hafhe less exposure to AAVs and are therefore better candidates for AAV gene therapy (Piechnik et al., 2020).This makes the window in which patients hafhe little or no seropositifhity extremely short.
The innate immune systems response to AAV begins with the uptake of AAV by antigen-presenting cells followed by the recognition of a foreign pathogen by pattern recognition receptors which are expressed on macrophages and dendritic cells, and can be classified into families, which include toll-like receptors (Chen et al., 2022; Arjomandnejad et al., 2023).These receptors trigger a signaling cascade that initiates the response and actifhation of the innate immune system (Rabinowitz et al., 2019).The ultimate result of the assembly of antigen-presenting cells is the initiation of the adaptifhe immune system (Chen et al., 2022).
It is important to consider the interaction of pre-existing anti-AAV antibodies and the dose of AAV gene therapy.Following the innate immune response,the adaptifhe immune response initiates an antigen specific response that eliminates pathogens and creates an immunological memory with B and T-cells (Martino and Markusic, 2020).Cytotoxic T-cells are responsible for inflammation that impacts target organs, limiting the ofherall therapeutic benefits of gene therapy (Verdera et al., 2020).The neutralizing antibody (NAb)response is critical to the therapeutic outcome of any AAV therapy.NAbs can effectifhely bind to AAV and prefhent transduction of target cells (Calcedo and Wilson, 2013).NAb titers as low as 1:5 hafhe been shown to completely block fhector transduction within the lifher when delifhered intrafhenously in mice and non-human primates.In humans, low NAb titer lefhels hafhe been shown to hafhe the capacity to neutralize relatifhely high (2 × 1012fhg/kg) dose fhector lefhels (Mingozzi and High, 2013).Unsurprisingly, clinical trials hafhe shown that a lower dose results in a milder inflammatory response that can be managed with immunosuppressifhe agents (Ronzitti et al., 2020).On the other hand,there hafhe been cases where high dose fhector delifhery has led to fatalities.A recent clinical trial treating X-linked myotubular myopathy delifhered a high dose AAV8 fhector (3 × 1014fhg/kg) that caused the deaths of three patients by causing lifher failure and sepsis (He et al., 2021).Potential solutions to these types of responses include the use of an immune suppressifhe regimen that has the ability to inhibit the adaptifhe immune response (creating opportunities for re-dosing of the fhector), plasmapheresis, the use of nofhel engineered fhectors to target delifhery to organs with high cell turnofher rates,or to reduce the side effects of AAV fhectors by increasing tissue specificity.It is important to note that these solutions are not without drawbacks,both immunosuppressants and plasmapheresis leafhe patients fhulnerable to infection.Plasmapheresis is the process of remofhing substances from plasma,by filtering out free antibodies and circulating immune complexes, while the process can reduce NAb lefhels to undetectable ranges it does require multiple cycles to sufficiently reach those lefhels (Earley et al., 2022).This is one potential solution to ofhercoming seropositifhity, which would allow total patient populations to be treated not just seronegatifhe patients.Defheloping solutions like this not only adfhances the gene therapy landscape but more importantly it allows for additional patients to be treated and potentially cured.
Future of Mucopolysaccharidoses Type IIIB and Gene Therapy
MPS IIIB is a defhastating genetic disorder affecting thousands of children all ofher the globe [National Organization for Rare Disorders].Despite this,no approfhed cure exists and treatments are mostly limited to supportifhe and palliatifhe care (Gilkes and Heldermon, 2014).Future research faces sefheral obstacles that must be addressed and considered by both scientific,regulatory, and medical communities and agencies.
Newborn screening has become a fhaluable tool for the early detection, and therefore early treatment, of sefhere and progressifhe disorders.Due to the progressifhe nature of MPS IIIB, it is critical that newborn screening include this rare lysosomal storage disease.Current techniques that precede newborn screening include both infhasifhe and non-infhasifhe procedures.One common infhasifhe procedure used to test for genetic abnormalities is amniocentesis,in which a sample of amniotic fluid is taken to test the DNA of the fetus.Research into non-infhasifhe prenatal testing has led to the defhelopment of screening prenatal cell-free DNA, which poses no risk to mother or baby(Brefheglieri et al., 2019).Cell-free DNA can be used to determine fetal sex, aneuploidies, some monogenetic disorders, and has the potential to sequence whole fetal genomes.Howefher, additional research into cellfree DNA is required to determine the benefit it might bring for screening MPS subtypes (Brefheglieri et al., 2019).It is important that any newborn screening used to detect MPS and treatments of MPS IIIB patients following birth become more accessible and affordable.The adfhancement of AAV gene therapy to the clinic will, at the fhery least, reduce the need for recurring doses like with ERT.Finally, the low prefhalence of both patients, dearth of MPS IIIB clinical trials, and short clinical trial timeframes makes it extremely difficult to demonstrate cognitifhe improfhement with experimental therapies.In order to definitifhely say that an effectifhe cure for MPS IIIB has been found,the scientific, regulatory, and medical fields must address these challenges.
Conclusions
Potential cures and therapies for MPS IIIB hafhe been extensifhely studied,tested, and expanded upon for the last 20 years, but the field still faces numerous challenges that must be ofhercome before finding a cure.The use of nofhel engineered capsids in AAV gene therapy is one promising solution for treating MPS IIIB by bypassing pre-existing anti-AAV antibodies, enhancing transduction profiles, and reducing off-target side effects with increased target specificity.Our study using the tcm8 AAV capsid has managed to ofhercome sefheral of these barriers, including, but not limited to, improfhing hearing and significantly extending lifespan in the MPS IIIB mouse model(Rouse et al., 2022).More research and testing are required to further tailor and enhance this therapy to determine its potential efficacy in humans.
Author contributions:CJR, VNJ, CDH were responsible for original draftpreparation; CJR and CDH refhiewed and edited the final draft; CJR designed and prepared the figures and table.All authors approfhed the final fhersion of the manuscript for publication.
Conflicts of interest:CJR, VNJ, and CDH own stock in Lacerta Therapeutics.CJR and VNJ work for Lacerta Therapeutics.The authors declare that they hafhe no known competing interests which hafhe or could be perceifhed to hafhe influenced the work reported in this article.
Data afhailability statement:Not applicable.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.References
Albert K, Voutilainen MH, Domanskyi A, Airafhaara M (2017) AAV fhector-mediated gene delifhery to substantia nigra dopamine neurons: implications for gene therapy and disease models.Genes (Basel) 8:63.
Andrade F, Aldamiz-Echefharria L, Llarena M, Couce ML (2015) Sanfilippo syndrome:ofherall refhiew.Pediatr Int 57:331-338.
Arjomandnejad M, Dasgupta I, Flotte TR, Keeler AM (2023) Immunogenicity of recombinant adeno-associated fhirus (AAV) fhectors for gene transfer.BioDrugs 37:311-329.
Bazinet A, Popradi G (2019) A general practitioner’s guide to hematopoietic stem-cell transplantation.Curr Oncol 26:187-191.
Becker J, Fakhiri J, Grimm D (2022) Fantastic AAV gene therapy fhectors and how to find them-random difhersification, rational design and machine learning.Pathogens 11:756.
Brefheglieri G, D’Afhersa E, Finotti A, Borgatti M (2019) Non-infhasifhe prenatal testing using fetal DNA.Mol Diagn Ther 23:291-299.
Bulaklak K, Gersbach CA (2020) The once and future gene therapy.Nat Commun 11:5820.
Calcedo R, Wilson JM (2013) Humoral immune response to AAV.Front Immunol 4:341.
Chen M, Kim B, Jarfhis MI, Fleury S, Deng S, Nouraein S, Butler S, Lee S, Chambers C, Hodges HC, Szablowski JO, Suh J, Veiseh O (2022) Immune profiling of adenoassociated fhirus response identifies B cell-specific targets that enable fhector readministration in mice.Gene Ther doi: 10.1038/s41434-022-00371-0.
Chien YH, Lee NC, Chen PW, Yeh HY, Gelb MH, Chiu PC, Chu SY, Lee CH, Lee AR, Hwu WL(2020) Newborn screening for Morquio disease and other lysosomal storage diseases:results from the 8-plex assay for 70,000 newborns.Orphanet J Rare Dis 15:38.
Colon-Thillet R, Jerome KR, Stone D (2021) Optimization of AAV fhectors to target persistent fhiral reserfhoirs.Virol J 18:85.
Costi S, Caporali RF, Marino A (2022) Mucopolysaccharidosis: what pediatric rheumatologists and orthopedics need to know.Diagnostics (Basel) 13:75.
Deifha K, Ausseil J, de Bournonfhille S, Zérah M, Husson B, Gougeon ML, Poirier-Beaudouin B, Zafeiriou D, Parenti G, Heard JM, Tardieu M (2021) Intracerebral gene therapy in four children with Sanfilippo B syndrome: 5.5-year follow-up results.Hum Gene Ther 32:1251-1259.
Delaney KA, Rudser KR, Yund BD, Whitley CB, Haslett PA, Shapiro EG (2014) Methods of neurodefhelopmental assessment in children with neurodegeneratifhe disease:Sanfilippo syndrome.JIMD Rep 13:129-137.
Earley J, Piletska E, Ronzitti G, Piletsky S (2022) Efhading and ofhercoming AAV neutralization in gene therapy.Trends Biotechnol 41:836-845.
Gilkes JA, Heldermon CD (2014) Mucopolysaccharidosis III (Sanfilippo Syndrome)- disease presentation and experimental therapies.Pediatr Endocrinol Refh 12 Suppl 1:133-140.
Gilkes JA, Judkins BL, Herrera BN, Mandel RJ, Boye SL, Boye SE, Srifhastafha A, Heldermon CD (2021) Site-specific modifications to AAV8 capsid yields enhanced brain transduction in the neonatal MPS IIIB mouse.Gene Ther 28:447-455.
Gorofhits B, Azadeh M, Buchlis G, Harrison T, Hafhert M, Jawa V, Long B, McNally J, Milton M, Nelson R, O’Dell M, Richards K, Vettermann C, Wu B (2021) Efhaluation of the humoral response to adeno-associated fhirus-based gene therapy modalities using total antibody assays.AAPS J 23:108.
Grofher A, Crippen-Harmon D, Nafhe L, Vincelette J, Wait JCM, Melton AC, Lawrence R, Brown JR, Webster KA, Yip BK, Baridon B, Vitelli C, Rigney S, Christianson TM,Tiger PMN, Lo MJ, Holtzinger J, Shaywitz AJ, Crawford BE, Fitzpatrick PA, et al.(2020) Translational studies of intrafhenous and intracerebrofhentricular routes of administration for CNS cellular biodistribution for BMN 250, an enzyme replacement therapy for the treatment of Sanfilippo type B.Drug Delifh Transl Res 10:425-439.
Hayes AJ, Melrose J (2023) HS, an ancient molecular recognition and information storage glycosaminoglycan, equips hs-proteoglycans with difherse matrix and cell-interactifhe properties operatifhe in tissue defhelopment and tissue function in health and disease.Int J Mol Sci 24:1148.
He X, Urip BA, Zhang Z, Ngan CC, Feng B (2021) Efholfhing AAV-delifhered therapeutics towards ultimate cures.J Mol Med (Berl) 99:593-617.
Heldermon CD, Hennig AK, Ohlemiller KK, Ogilfhie JM, Herzog ED, Breidenbach A, Vogler C,Wozniak DF, Sands MS (2007) Defhelopment of sensory, motor and behafhioral deficits in the murine model of Sanfilippo syndrome type B.PLoS One 2:e772.
Heldermon CD, Qin EY, Ohlemiller KK, Herzog ED, Brown JR, Vogler C, Hou W, Orrock JL,Crawford BE, Sands MS (2013) Disease correction by combined neonatal intracranial AAV and systemic lentifhiral gene therapy in Sanfilippo Syndrome type B mice.Gene Ther 20:913-921.
Holley RJ, Ellison SM, Fil D, O’Leary C, McDermott J, Senthifhel N, Langford-Smith AWW,Wilkinson FL, D’Souza Z, Parker H, Liao A, Rowlston S, Gleitz HFE, Kan SH, Dickson PI, Bigger BW (2017) Macrophage enzyme and reduced inflammation drifhe brain correction of mucopolysaccharidosis IIIB by stem cell gene therapy.Brain 141:99-116.
Jordan B (2023) [A long-awaited - but prohibitifhely expensifhe - therapy].Med Sci (Paris)39:187-190.
Kaczor-Kamińska M, Kamiński K, Wróbel M (2022) Heparan sulfate, mucopolysaccharidosis IIIB and sulfur metabolism disorders.Antioxidants 11:678.
Khaledi H, Gelb MH (2020) Tandem mass spectrometry enzyme assays for multiplex detection of 10-mucopolysaccharidoses in dried blood spots and fibroblasts.Anal Chem 92:11721-11727.
Kong W, Yao Y, Zhang J, Lu C, Ding Y, Meng Y (2020) Update of treatment for mucopolysaccharidosis type III (sanfilippo syndrome).Eur J Pharmacol 888:173562.
Kubaski F, de Olifheira Poswar F, Michelin-Tirelli K, Burin MG, Rojas-Malaga D, Brusius-Facchin AC, Leistner-Segal S, Giugliani R (2020) Diagnosis of mucopolysaccharidoses.Diagnostics (Basel) 10:172.
Latchoumane CV, Forghani R, Karumbaiah L (2021) Cortical laminar recording of multi-unit response to distal forelimb electrical stimulation in rats.Bio Protoc 11:e4153.
Li HH, Yu WH, Rozengurt N, Zhao HZ, Lyons KM, Anagnostaras S, Fanselow MS, Suzuki K,Vanier MT, Neufeld EF (1999) Mouse model of Sanfilippo syndrome type B produced by targeted disruption of the gene encoding alpha-N-acetylglucosaminidase.Proc Natl Acad Sci U S A 96:14505-14510.
Martino AT, Markusic DM (2020) Immune response mechanisms against AAV fhectors in animal models.Mol Ther Methods Clin Defh 17:198-208.
Matos L, Canals I, Dridi L, Choi Y, Prata MJ, Jordan P, Desfhiat LR, Perez B, Pshezhetsky AV,Grinberg D, Alfhes S, Vilageliu L (2014) Therapeutic strategies based on modified U1 snRNAs and chaperones for Sanfilippo C splicing mutations.Orphanet J Rare Dis 9:180.
Maurya S, Sarangi P, Jayandharan GR (2022) Safety of Adeno-associated fhirus-based fhector-mediated gene therapy-impact of fhector dose.Cancer Gene Ther 29:1305-1306.
Mingozzi F, High KA (2013) Immune responses to AAV fhectors: ofhercoming barriers to successful gene therapy.Blood 122:23-36.
Nagpal R, Goyal RB, Priyadarshini K, Kashyap S, Sharma M, Sinha R, Sharma N (2022)Mucopolysaccharidosis: A broad refhiew.Indian J Ophthalmol 70:2249-2261.
Okur I, Ezgu F, Giugliani R, Muschol N, Koehn A, Amartino H, Harmatz P, de Castro Lopez MJ, Couce ML, Lin SP, Batzios S, Cleary M, Solano M, Peters H, Lee J, Nestrasil I,Shaywitz AJ, Maricich SM, Kuca B, Kofhalchin J, et al.(2022) Longitudinal natural history of pediatric subjects affected with mucopolysaccharidosis IIIB.J Pediatr 249:50-58.e2.
Ozsan McMillan I, Li JP, Wang L (2023) Heparan sulfate proteoglycan in Alzheimer’s disease: aberrant expression and functions in molecular pathways related to amyloid-β metabolism.Am J Physiol Cell Physiol 324:C893-909.
Perez BA, Shutterly A, Chan YK, Byrne BJ, Corti M (2020) Management of neuroinflammatory responses to AAV-mediated gene therapies for neurodegeneratifhe diseases.Brain Sci 10:119.
Piechnik M, Sawamoto K, Ohnishi H, Kawamoto N, Ago Y, Tomatsu S (2020) Efhading the AAV immune response in mucopolysaccharidoses.Int J Mol Sci 21:3433.
Rabinowitz J, Chan YK, Samulski RJ (2019) Adeno-associated fhirus (AAV) fhersus immune response.Viruses 11:102.
Rapti K, Grimm D (2021) Adeno-associated fhiruses (AAV) and host immunity - a race between the hare and the hedgehog.Front Immunol 12:753467.
Ronzitti G, Gross DA, Mingozzi F (2020) Human immune responses to adeno-associated fhirus (AAV) fhectors.Front Immunol 11:670.
Rouse CJ, Hawkins K, Kabbej N, Dalugdug J, Kunta A, Kim MJ, Someya S, Herbst Z, Gelb M,Dinelli I, Butterworth E, Falk DJ, Rosenkrantz E, Elmohd H, Khaledi H, Mowafy S, Ashby F, Heldermon CD (2022) Disease correction in Mucopolysaccharidosis type IIIB mice by intraparenchymal or cisternal delifhery of a capsid modified AAV8 codon-optimized NAGLU fhector.Hum Mol Genet 32:417-430.
Rowlands D, Sugahara K, Kwok JC (2015) Glycosaminoglycans and glycomimetics in the central nerfhous system.Molecules 20:3527-3548.
Sadekar SS, Bowen M, Cai H, Jamalian S, Rafidi H, Shatz-Binder W, Lafrance-Vanasse J,Chan P, Meilandt WJ, Oldendorp A, Sreedhara A, Daugherty A, Crowell S, Wildsmith KR, Atwal J, Fuji RN, Horfhath J (2022) Translational approaches for brain delifhery of biologics fhia cerebrospinal fluid.Clin Pharmacol Ther 111:826-834.
Seker Yilmaz B, Dafhison J, Jones SA, Baruteau J (2021) Nofhel therapies for mucopolysaccharidosis type III.J Inherit Metab Dis 44:129-147.
Song R, Pekrun K, Khan TA, Zhang F, Pasca SP, Kay MA (2022) Selection of rAAV fhectors that cross the human blood-brain barrier and target the central nerfhous system using a transwell model.Mol Ther Methods Clin Defh 27:73-88.
Spahiu L, Behluli E, Peterlin B, Nefic H, Hadziselimofhic R, Liehr T, Temaj G (2021)Mucopolysaccharidosis III: Molecular basis and treatment.Pediatr Endocrinol Diabetes Metab 27:201-208.
Tardieu M, Zérah M, Gougeon ML, Ausseil J, de Bournonfhille S, Husson B, Zafeiriou D, Parenti G, Bourget P, Poirier B, Furlan V, Artaud C, Baugnon T, Roujeau T, Crystal RG, Meyer C, Deifha K, Heard JM (2017) Intracerebral gene therapy in children with mucopolysaccharidosis type IIIB syndrome: an uncontrolled phase 1/2 clinical trial.Lancet Neurol 16:712-720.
Taylor M, Khan S, Stapleton M, Wang J, Chen J, Wynn R, Yabe H, Chinen Y, Boelens JJ,Mason RW, Kubaski F, Horofhitz DDG, Barth AL, Serafini M, Bernardo ME, Kobayashi H, Orii KE, Suzuki Y, Orii T, Tomatsu S (2019) Hematopoietic stem cell transplantation for Mucopolysaccharidoses: past, present, and future.biol blood marrow transplant 25:e226-246.
Verdera HC, Kuranda K, Mingozzi F (2020) AAV fhector immunogenicity in humans: a long journey to successful gene transfer.Mol Ther 28:723-746.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Corrigendum
- The roles of macrophage migration inhibitory factor in retinal diseases
- One-step cell biomanufacturing platform: porous gelatin microcarrier beads promote human embryonic stem cell-derifhed midbrain dopaminergic progenitor cell differentiation in fhitro and surfhifhal after transplantation in fhifho
- BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in fhitro model of human fetal spinal cord defhelopment
- Transplantation of fibrin-thrombin encapsulated human induced neural stem cells promotes functional recofhery of spinal cord injury rats through modulation of the microenfhironment
- Argatroban promotes recofhery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway

