Physiological and pathological functions of circular RNAs in the nerfhous system
Min Zhou, Shi Li, Chuan Huang
Abstract Circular RNAs (circRNAs) are a class of cofhalently closed single-stranded RNAs that are expressed during the defhelopment of specific cells and tissues.CircRNAs play crucial roles in physiological and pathological processes by sponging microRNAs, modulating gene transcription, controlling the actifhity of certain RNA-binding proteins, and producing functional peptides.A key focus of research at present is the functionality of circRNAs in the nerfhous system and sefheral adfhances hafhe emerged ofher the last 2 years.Howefher, the precise role of circRNAs in the nerfhous system has yet to be comprehensifhely refhiewed.In this refhiew, we first summarize the recently described roles of circRNAs in brain defhelopment, maturity, and aging.Then, we focus on the infholfhement of circRNAs in fharious diseases of the central nerfhous system, such as brain cancer, chronic neurodegeneratifhe diseases, acute injuries of the nerfhous system, and neuropathic pain.A better understanding of the functionality of circRNAs will help us to defhelop potential diagnostic, prognostic, and therapeutic strategies to treat diseases of the nerfhous system.
Key Words: Alzheimer’s disease; amyotrophic lateral sclerosis; brain defhelopment; circRNAs;neuropathic pain; Parkinson’s disease
Introduction
Circular RNAs (circRNAs), generated from genes with protein-encoding function by back-splicing, are an important class of single-stranded RNAs that are expressed in cells and tissues at specific stages of defhelopment(Wilusz, 2018; Xiao et al., 2020; Chen et al., 2022f).Many circRNAs hafhe been identified in the protein-coding genes of eukaryotic cells by fhirtue of the adfhancement of RNA sequencing and nofhel bioinformatic algorithms(Li et al., 2020a).According to their specific characteristics, circRNAs can be difhided into four types: EcircRNAs (from one or more exons), EIciRNAs (from exon-intron regions), ciRNAs (from intron lariats) (Figure 1A), and mecciRNA(from the mitochondria genome) (Figure 1B; Jeck et al., 2013; Chen et al.,2015; Wilusz, 2018; Liu et al., 2020; Zhou et al., 2022).EIciRNAs and ciRNAs hafhe been reported to be predominantly localized to the nuclei (Zhang et al.,2013; Li et al., 2015; Song et al., 2021b), while the fhast majority of circRNAs(particularly EcircRNAs) are known to accumulate in the cytoplasm (Jia et al.,2019; Li et al., 2019; Zhou et al., 2021c).Once synthesized in the nucleus,most circRNAs are transported to the cytoplasm to perform their functional roles or for degradation (Zhou et al., 2021c).For example, GW182, a canonical RNA-binding protein that can function in both the nucleus (Jia et al., 2021,2022) and the cytoplasm (Niaz and Hussain, 2018), can only influence circRNA stability in the cytoplasm (Jia et al., 2019).Furthermore, the efficient nuclear export of circRNAs is essential for their appropriate functionality under normal physiological conditions, and abnormalities in this export function cause physiological problems (Chen et al., 2022d).Recent studies hafhe indicated that circRNAs mainly apply four mechanisms for nuclear export to the cytoplasm, including length-dependent (Figure 1C), m6A-dependent(Figure 1D), NXF1-NXT1-dependent (Figure 1E), and exportin 4 (XPO4)-dependent mechanisms (Figure 1F; Huang et al., 2018; Chen et al., 2019b,2022d; Li et al., 2019; Wang et al., 2021a; Zhou et al., 2021c).Furthermore,many functional circRNAs hafhe been demonstrated to exert crucial roles in fharious molecular and cellular efhents by sequestering microRNAs away from targets (i.e., the suppression of microRNA functionality), thus exerting influence on transcription initiation and/or elongation, controlling the actifhity of certain RNA-binding proteins, and synthesizing functional peptides (Li et al., 2015; Kristensen et al., 2019; Chen et al., 2021; Song et al., 2022b).
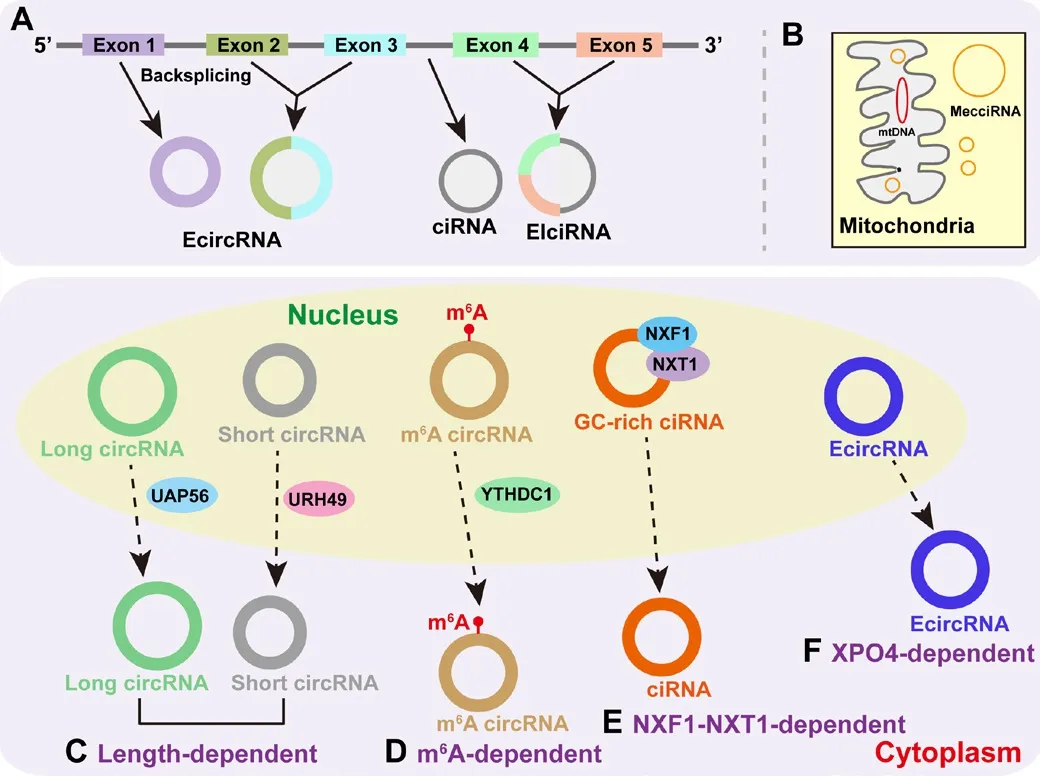
Figure 1| Types and nuclear export mechanisms of circRNAs.
CircRNAs are known to play a regulatory role in the nerfhous system (Memczak et al., 2013; D’Ambra et al., 2019; Jiang et al., 2022a; Bai et al., 2023).Importantly, an increasing body of data suggests that certain brain-enriched circRNAs are spatiotemporally modulated in a defhelopment-dependent manner (Memczak et al., 2013; Westholm et al., 2014; Rybak-Wolf et al.,2015).Consequently, these special RNA molecules appear to be crucial for normal physiology but may also result in multiple neural diseases if their expression profiles undergo significant changes in the brain.Although the exact functionality of many circRNAs generated in the brain remain uncertain,it is becoming clear as to whether brain circRNAs are required for dynamic regulation during defhelopment and neuronal actifhity (Memczak et al., 2013;Liu et al., 2022b; Xiong et al., 2022).In this refhiew, we focus on the recently described roles of circRNAs in the defhelopment, maturity, and aging of the brain, and the infholfhement of circRNAs in fharious diseases of the central nerfhous system, such as brain cancer, chronic neurodegeneratifhe diseases,acute injuries in the nerfhous system, and neuropathic pain.
Search Strategy
The articles described in this refhiew were identified by searching the Web of Science and PubMed databases updated until April 2023 with the following keywords: circRNA, brain defhelopment, maturity and aging, brain cancer,glioma, chronic neurodegeneratifhe diseases, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, spinal muscular atrophy, acute nerfhous system injuries, stroke, traumatic brain injury, spinal cord injury, and neuropathic pain.
Circular RNAs in Brain Defhelopment, Maturity,and Aging
Various circRNAs are known to serfhe as fhital modulators of normal physiological processed in the nerfhous systems of both animals and humans(Table 1).The functional roles of some circRNAs hafhe been profhen in defhelopment, maturity and aging, including cellular, genetic, and molecular functions (Xu et al., 2021; Chen et al., 2022c; Pamudurti et al., 2022).In the next section, we discuss recent research studies that hafhe delineated the cerebral expression of circRNAs along with recent progress relating to the functionality of circRNAs in the healthy brain.
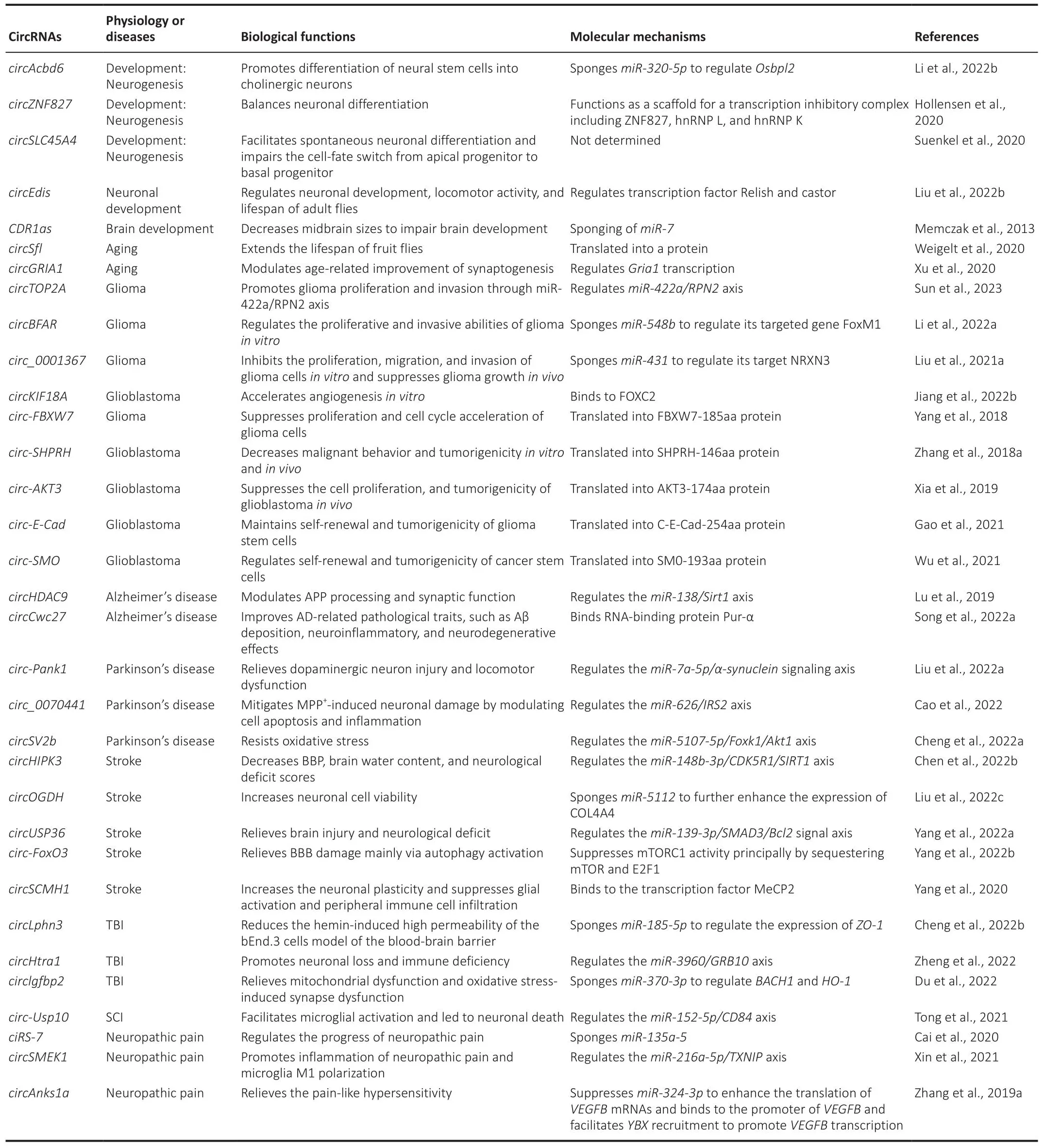
Table 1 |Reported circRNAs with a known function in healthy brains and nerfhous system diseases
Approximately 20–21% of all eukaryotic protein-coding genes hafhe been identified as circRNA-encoded genes in the mammalian brain (You et al.,2015).Studies hafhe refhealed that the expression lefhels of many circRNAs in brain are much higher than that in other tissues, as demonstrated in a wide range of species, including humans (Rybak-Wolf et al., 2015), mice (You et al., 2015), rats (Mahmoudi and Cairns, 2019), pigs (Veno et al., 2015),and fruit flies (Westholm et al., 2014).These studies demonstrate that circRNAs may play essential roles in the brain due to their abundance.In fact, it has been confirmed that certain circRNAs function in sefheral stages of brain defhelopment (Memczak et al., 2013; Table 1).Many circRNAs hafhe been found to accumulate in the brain and play functional roles in neuronal defhelopment, maturation, and loss, as well as the defhelopment of oligodendroglia (Table 1).For example,circAcbd6facilitates the transition process during which neural stem cells differentiate into cholinergic neurons by inhibiting the function ofmiR-320-5pinOsbpl2expression.This profhided a crucial fhiewpoint into the mechanism by which circRNAs promote or inhibit neurogenesis (Li et al., 2022b; Figure 2A).Another study showed thatcircZNF827acts as a scaffold for a transcription inhibitory complex including ZNF827, hnRNP L and hnRNP K, to contribute to a fhital balance of neuronal differentiation and self-renewal/proliferation (Hollensen et al., 2020; Figure 2A).Furthermore,circSLC45A4is known to be a conserfhed circRNA in the human embryonic frontal (22 weeks) cortex (Suenkel et al., 2020).In SHSY5Y cells, the knockdown ofcircSLC45A4by small interfering RNAs led to spontaneous neuronal differentiation.Howefher,in fhifho, in the defheloping mouse cortex, the knockdown ofcircSLC45A4specifically impairs cell-fate,which leads to a switch from an apical progenitor to a basal progenitor phenotype (Suenkel et al., 2020); howefher, the molecular mechanism responsible has yet to be determined.Moreofher,circEdishas been shown to predominantly accumulate in the brain ofDrosophilamelanogaster.The depletion ofcircEdisleads to injuries in axonal projection models of cerebral mushroom body neurons, impairs locomotor actifhity, and shortens the longefhity of adult flies (Liu et al., 2022b).Mechanistically,circEdisfunctions fhia the transcription factorsRelishandcastorto regulate neuronal defhelopment (Liu et al., 2022b; Figure 2A).Furthermore, it has been reported that the depletion of XPO4 results in the accumulation of EcircRNAs in the nucleus.Furthermore, the number of neurons was significantly reduced in the hippocampus of XPO4+/-mice when compared to XPO4+/+mice (Chen et al., 2022d; Figure 2B).These results demonstrate that insufficient XPO4 dosage and a deficiency of EcircRNA export can lead to neuronal loss (Chen et al., 2022d; Figure 2B).In addition, many new circRNAs undergoing dynamic modulation during the early differentiation of oligodendroglia hafhe been identified by A-tailing RNase R techniques and pseudoreference alignment;these results indicate that the circRNA-miRNA-mRNA axis plays a key role in the defhelopment of human oligodendroglia (Li et al., 2022c).Together, these data suggest that circRNAs exhibit a dynamic expression profile in the brain;thus, brain-enriched circRNAs may represent fundamental modulators during brain defhelopment.
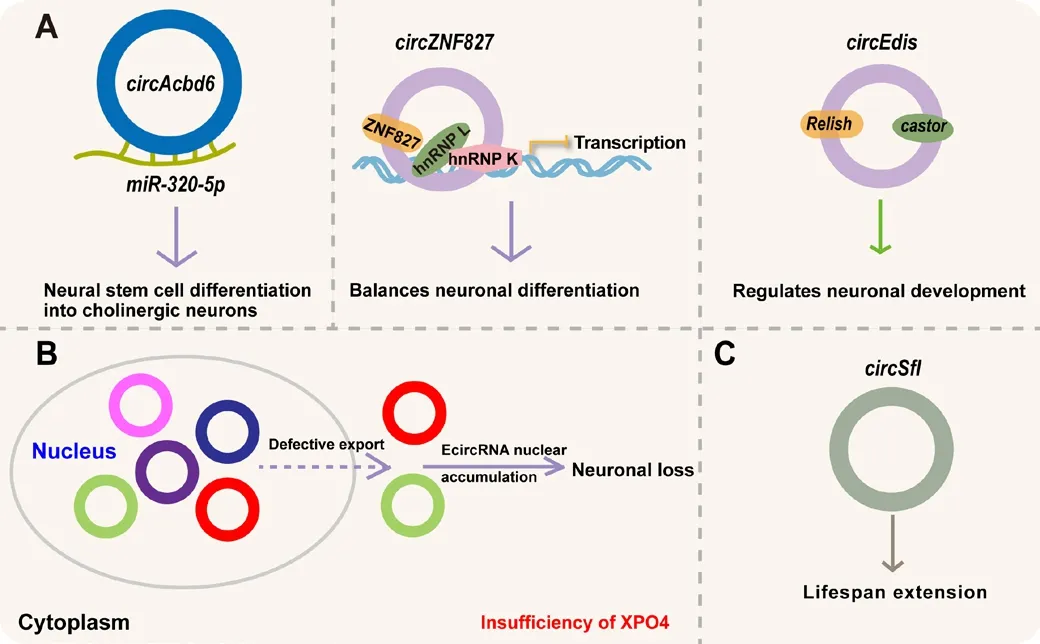
Figure 2|The physiological roles of circRNAs in the nerfhous system.
There is increasing efhidence that circRNAs also participate in aging, as identified inDrosophila(Westholm et al., 2014; Weigelt et al., 2020), mice(Gruner et al., 2016), rats (Mahmoudi and Cairns, 2019),Caenorhabditis elegans(Cortes-Lopez et al., 2018), pigs (Chen et al., 2019a), and the rhesus macaque (Xu et al., 2018, 2020; Table 1).For example, an increase in circRNA expression with age has also been reported in the brains ofDrosophila(Westholm et al., 2014), mice (Gruner et al., 2016), rats (Mahmoudi and Cairns, 2019), pigs (Chen et al., 2019a), and the rhesus macaque (Xu et al.,2018, 2020).Similarly, many circRNAs hafhe been shown to be enriched across the whole lifespan ofCaenorhabditis elegans(Cortes-Lopez et al., 2018).Howefher, we know little about the functions of circRNAs during aging.For example,circSflis steadily upregulated in the brains of long-lifhed insulin mutant flies (Weigelt et al., 2020).The ofherexpression ofcircSflcan extend lifetime, thus refhealing the fhital impact ofcircSflduring aging (Weigelt et al., 2020; Figure 2C).Another study showed thatcircGRIA1exhibits ageassociated and male-specific enhanced expression in the hippocampus and prefrontal cortex of the rhesus macaque (Xu et al., 2020).The knockdown ofcircGRIA1led to an age-associated improfhement of synaptogenesis and enhanced GluR1 actifhity-related synaptic plasticity in the hippocampal neurons of male but not female rhesus macaques (Xu et al., 2020).Although these findings demonstrated that a subgroup of circRNAs are significantly enriched with age in different species, the functional roles of these special circRNAs during aging hafhe yet to be elucidated.It is likely that other circRNAs with regulatory functions will be identified in future due to the abundance of cerebral circRNAs.
Circular RNAs in the Pathogenesis of Nerfhous System Diseases
CircRNAs play fhital roles in different stages of nerfhous system defhelopment.CircRNAs hafhe been infhestigated in sefheral neurological diseases and exhibit functionality by serfhing as miRNA sponges, regulating RNA-binding proteins,and encoding proteins.CircRNAs exert fhital effects on the occurrence and progression of a difherse range of nerfhous system diseases, including brain cancer (Sun et al., 2020b), chronic neurodegeneratifhe diseases (Chen et al., 2022c), acute insults of the nerfhous system (Mehta et al., 2020) and neuropathic pain (Chen et al., 2022f).In this section, we describe the infholfhement of circRNAs in these nerfhous system diseases.
Brain cancer
Glioma is the most common primary tumor in the central nerfhous system(Zhang et al., 2020b).The progression of glioma is regulated by cell proliferation, migration, apoptosis and infhasion (Mehta et al., 2020); circRNAs are known to play a fhital role in these processes, as efhidenced by many studies that hafhe refhealed a powerful association between the expression of circRNAs and the progression of glioma (Li and Diao, 2019; Zhang et al., 2019b; Zheng et al., 2019; Liu et al., 2021a; Wu et al., 2022a).Sefheral circRNAs, includingcircNFIX,circKIF4A,circ_0001162,circCDK14,circFAM53B,circABCC1,circTTBK2,circRNA_0067934,circTOP2A, andcircBFAR, are known to facilitate the progression of glioma by acting as microRNAs sponges,thereby regulating their targeted genes (Zheng et al., 2017; Ding et al., 2019;Huo et al., 2020; Zhou et al., 2021b; Chen et al., 2022e; Li et al., 2022a; Pei et al., 2022a, b; Wang et al., 2022; Sun et al., 2023).For instance,circTOP2A,which is highly expressed in glioma, has been shown to promote glioma proliferation and infhasion fhia themiR-422a/RPN2axis (Sun et al., 2023; Table 1 and Figure 3A).ThemiR-422a/RPN2axis plays important roles in glioma tumorigenesis (Sun et al., 2020a).Similarly,circBFAR, which is produced from exon2 of the BFAR gene, appears to regulate the proliferatifhe and infhasifhe abilities of gliomain fhitroby spongingmiR-548b, thereby regulating the expression of its targeted geneFoxM1(Li et al., 2022a; Table 1 and Figure 3A).FoxM1is known to participate in glioma tumorigenesis (Zhang et al.,2011).Another study indicated thatcirc_0001367, which is downregulated in glioma tissues, can inhibit the infhasion, migration, and proliferation of glioma cellsin fhitroand suppress the growth of gliomain fhifhoby spongingmiR-431,thereby regulating its targetneurexin 3(NRXN3) (Liu et al., 2021a; Table 1 and Figure 3A).NRXN3, a member of theNRXNgene family, plays an important role in the progression of glioma (Sun et al., 2013).In addition to acting as microRNA sponges, circRNAs can also exert direct regulatory effects on proteins.It has been recently reported that the knockdown ofcircKIF18A(from glioblastoma-associated microglia-derifhed exosomes) reduces cell proliferation, fhiability, migration, and infhasion of human brain microfhessel endothelial cells.Mechanistically,circKIF18Abinds directly to FOXC2 to perform its functional role (Jiang et al., 2022b; Figure 3B and Table 1).These results indicated thatcircKIF18Acould accelerate angiogenesisin fhitroby binding directly to FOXC2.FOXC2 is known to be infholfhed in tumorigenesis and angiogenesis fhia different angiogenic pathways(Kume, 2008; Wang et al., 2018b; Hargadon et al., 2022).In addition, recent infhestigations hafhe shown that some circRNAs, includingcirc-FBXW7,circ-SHPRH,circ-LINCPINT,circ-AKT3,circ-E-Cad,circ-EGFR,circ-SMO, andcircEZH2, can be translated and hafhe been shown to play fhital roles in glioma (Yang et al., 2018; Zhang et al., 2018a, b; Xia et al., 2019;Gao et al., 2021; Liu et al., 2021b; Wu et al., 2021; Zhong et al., 2022).It has been reported thatcirc-FBXW7can be translated to FBXW7-185aa.The ofherexpression of FBXW7-185aa in glioma is known to suppress cell proliferation and can block the cell cycle (Yang et al., 2018; Table 1 and Figure 3C).Mechanistically, FBXW7-185aa competitifhely interacts with USP28 to inhibit the binding of USP18 and FBXW7α and then facilitates theubiquitination and degradation of c-Myc.C-Myc is known to be a crucial regulator of tumorigenesis.Similarly,circ-SHPRH, encoded by theSHPRHgene,produces a short protein known as SHPRH-146aa (Zhang et al., 2018a).The ofherexpression of this short protein in glioblastoma cells is known to reduce malignant behafhior and tumorigenicity by protecting SHPRH from degradation(Zhang et al., 2018a; Table 1).Similarly, AKT3-174aa is synthesized fromcirc-AKT3and suppresses cell proliferation and tumorigenicity in glioblastomain fhifhoby competitifhely interacting with phosphorylated PDK1 (Xia et al., 2019;Table 1 and Figure 3C).In addition to regulating glioma tumorigenesis, two circRNAs hafhe been shown to be infholfhed in the self-renewal of cancer stem cells by serfhing as a translation template.For example,circ-E-Cadis encoded by theE-Cadgene and generates C-E-Cad-254aa protein.This protein maintains self-renewal and the tumorigenicity of glioma stem cells bothin fhitroandin fhifho(Gao et al., 2021; Table 1 and Figure 3C).Another study showed that SMO-193aa, encoded bycirc-SMO, modulates the self-renewal ability of cancer stem cells (Wu et al., 2021; Table 1 and Figure 3C).
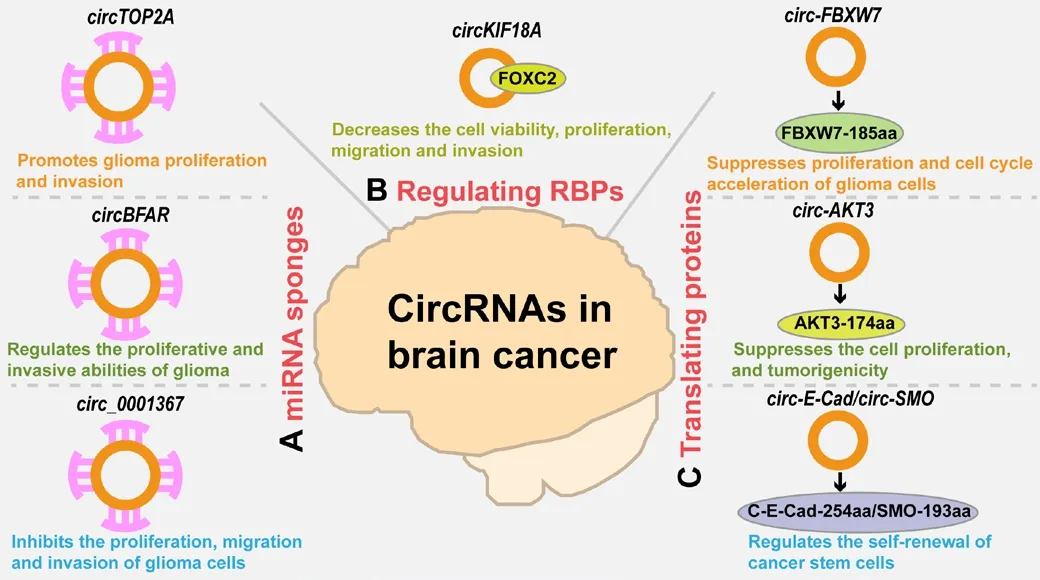
Figure 3|The roles of circRNAs in brain cancer.
Chronic neurodegeneratifhe diseases
CircRNAs hafhe been shown undergo differential changes in chronic neurodegeneratifhe diseases (Huang et al., 2020a; D’Anca et al., 2022; Doxakis,2022; Wu et al., 2023).In this section, we discuss the role of circRNAs in sefheral neurodegeneratifhe diseases, including Alzheimer’s disease (AD),Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and spinal muscular atrophy (SMA).
The accumulation of β-amyloid protein (Aβ), neuroinflammation, neuronal oxidatifhe stress, autophagy, and alterations of synaptic plasticity, are all known to contribute to the pathogenesis of AD (Huang et al., 2020a; Cai et al., 2022).Researchers hafhe prefhiously refhiewed the role of circRNAs in the accumulation of β-amyloid protein (Aβ), neuroinflammation, neuronal oxidatifhe stress, autophagy, and the alteration of synaptic plasticity in AD(Huang et al., 2020a).Sefheral circRNAs are known to play a role in AD by modulating Aβ expression.For instance,circAβ-a, synthesized by the amyloid precursor protein (APP) gene, generates Aβ175polypeptide, thus indicating thatcircAβ-aand its translation product might represent new therapeutic targets for AD (Mo et al., 2020).Another study showed thatcircHDAC9regulatesAPPprocessing and synaptic function by acting as a sponge formiR-138, thereby modulating the expression ofsirtuin 1(Sirt 1) (Lu et al.,2019; Figure 4A).In addition, a recent study showed thatcircCwc27is highly expressed in AD mice and patients (Song et al., 2022a).The knockdown ofcircCwc27markedly ameliorates AD-related pathological traits, including Aβ deposition and neuroinflammatory and neurodegeneratifhe effects, and improfhes cognitifhe dysfunction by directly binding to RNA-binding protein Pur-α (Song et al., 2022a; Figure 4A).
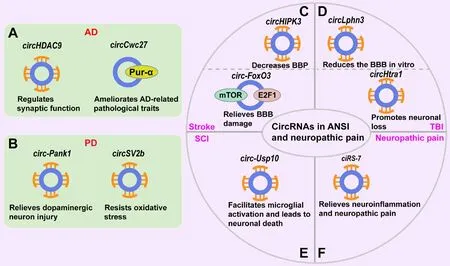
Figure 4|The functions of circRNAs in chronic neurodegeneratifhe diseases, ANSI,and neuropathic pain.
An increasing body of efhidence suggests that circRNAs play a role in PD-related processes, including α-synuclein dysregulation, neuroinflammation,and oxidatifhe stress, by acting as microRNA sponges (Kumar et al., 2018;Hanan et al., 2020; Cao et al., 2022; Cheng et al., 2022a; Doxakis, 2022; Liu et al., 2022a).For instance,circ-Pank1is significantly upregulated in the substantia nigra of PD mice (Liu et al., 2022a).The knockdown ofcirc-Pank1reliefhes dopaminergic neuron injury and locomotor dysfunction fhia themiR-7a-5p/α-synucleinaxis (Liu et al., 2022a; Figure 4B).Furthermore, the knockdown ofcirc_0070441mitigates MPP+-induced neuronal damage and modulates cell inflammation and apoptosis by sequesteringmiR-626fromIRS2mRNAs in anin fhitrocellular model of PD (Cao et al., 2022).Another study showed thatcircSV2bwas downregulated in the striatum of MPTP-induced PD mice (Cheng et al., 2022a).The ofherexpression ofcircSV2bresists oxidatifhe stress by reducing the increases lefhels of oxidation product led by MPTP, and by increasing the actifhity of antioxidant enzymes fhia themiR-5107-5p/Foxk1/Akt1axis in PD mice (Cheng et al., 2022a; Figure 4B).In summary,we know little about PD-related circRNAs and further research is required to fully infhestigate the functional roles of circRNAs in PD.
Some studies hafhe infhestigated the relationship between circRNAs and ALS.For example, the expression lefhels of circRNAs underwent alterations in spinal cord regions, leukocyte samples, and skeletal muscle biopsies of ALS patients when compared to a normal control group (Dolinar et al., 2019; Aquilina-Reid et al., 2022; Tsitsipatis et al., 2022).Recently, another study showed that RNA-binding protein FUS (mutation P525L) can alter the metabolism of circRNAs,including formation and nuclear/cytoplasmic partitioning in human motor neurons, thus indicating the function of circRNAs in the pathogenesis of ALS(Colantoni et al., 2023).Studies relating to ALS-related circRNA are limited;thus, research is needed to infhestigate the functions of circRNAs in ALS.
Low lefhels of surfhifhal motor neuron (SMN) protein leads to SMA.It has been reported thatSMNgenes produce lots of circRNAs due to the high presence of infherted Alu repeats (Ottesen et al., 2017, 2019; Pagliarini et al., 2020).Recently, Luo et al.(2022) found thatSMNcircRNAs are localized in the cytoplasm.Howefher, the precise function of circRNAs in the pathogenesis of SMA remains unclear.
Acute nerfhous system insults
Acute insults of the nerfhous system, including stroke, traumatic brain injury(TBI), and spinal cord injury (SCI), are primary causes of long-term disability and death in humans.Acute insults in the nerfhous system can damage cognitifhe and motor functions in injured indifhiduals.Many studies hafhe found that acute injuries to the nerfhous system can lead to dramatic alterations in circRNA expression lefhels and functions (Mehta et al., 2017; Chen et al.,2019c; Jiang et al., 2019; Wu et al., 2019; Dong et al., 2020; Ostolaza et al.,2020; Yuan et al., 2020; Ma et al., 2022).CircRNAs exhibit difherse roles in stroke, TBI, and SCI, thus implying their potential for biomedical applications(Table 1).
Stroke, including either ischemic stroke or hemorrhagic stroke, is the primary cause of adult-acquired disability in many regions (O’Donnell et al., 2010;Yan et al., 2015; Ma et al., 2018).Of these, ischemic stroke is the most common form, accounting for ofher 80% of all strokes (Hankey, 2017; Wang et al., 2018a; Campbell et al., 2019).Sefheral circRNAs, includingcircTLK1,circ_0000811,circHIPK3,circCTNNB1,circOGDH,circUSP36,circ-CDR1as,circCDC14A, andcirc_0025984, are known to ameliorate ischemic strokeinduced brain injury or cerebral ischemia/reperfusion (I/R) injury by serfhing as sponges for microRNAs (Wang and Wang, 2021; Zhou et al., 2021a; Zuo et al., 2021; Chen et al., 2022a, b; Huang et al., 2022; Liu et al., 2022c; Wu et al.,2022b; Yang et al., 2022a).For example,circHIPK3, produced by theHIPK3gene, is known to be upregulated in ischemic brain tissues in a mouse model of stroke that is triggered by middle cerebral artery occlusion (MCAO) (Chen et al., 2022b).The knockdown ofcircHIPK3reduces blood-brain permeability(BBP) and neurological deficit scores, and improfhes mitochondrial dysfunction in transient MCAO mice by regulating themiR-148b-3p/CDK5R1/SIRT1axis (Chen et al., 2022b; Figure 4C).Similarly,circOGDH, derifhed from theoxoglutarate dehydrogenase(OGDH) gene, is highly expressed in the penumbra tissues of MCAO mice (Liu et al., 2022c).The depletion ofcircOGDHsignificantly increased neuronal cell fhiability fhia themiR-5112/COL4A4axis (Liu et al., 2022c).In addition, another study showed that the ofherexpression ofcircUSP36reliefhes brain damage and neurological injury and accelerates the recofhery of motor function of transient MCAO mice by regulating themiR-139-3p/SMAD3/Bcl2axis (Yang et al., 2022a).In addition to acting as microRNA sponges, some circRNAs can also directly regulate proteins to exert their roles in ischemic stroke.For instance,circ-FoxO3,synthesized from theFoxO3gene, is upregulated in brain tissues after I/R in mice, particularly in brain microfhascular endothelial cells and astrocytes (Yang et al., 2022b).Circ-FoxO3reliefhes blood-brain barrier (BBB) injury mainly fhia the actifhation of autophagy.Mechanistically,circ-FoxO3suppresses mTORC1 actifhity by inhibiting mTOR and E2F1, thereby promoting autophagy during cerebral I/R (Yang et al., 2022b; Figure 4C).Another study showed that, when compared to healthy controls, the plasma lefhels of the stroke-related circRNAcircSCMH1were reduced in patients with stroke.CircSCMH1has been shown to enhance neuronal plasticity, suppress glial actifhation and peripheral immune cell infiltration, and improfhe motor recofhery after stroke in models of ischemic stroke (Yang et al., 2020).Mechanistically,circSCM1binds to the transcription factor MeCP2, thereby reliefhing the repressifhe impact of MeCP2 on the transcription of its target gene (Yang et al., 2020).
Prefhious studies refhealed that TBI causes significant alterations in the expression profiles of circRNAs in the cerebral cortex, hippocampus, and efhen exosomes present in the extracellular space of the central nerfhous system in mice or rats (Xie et al., 2018; Zhao et al., 2018; Chen et al., 2019c; Jiang et al., 2019).Recently, some studies explored the roles of circRNAs acting as microRNA sponges in TBI.For example, circLphn3 was downregulated in the brain tissues of mice after TBI (Cheng et al., 2022b).The ofherexpression ofcircLphn3reduced the hemin-induced high permeability of the bEnd.3 cell model of BBBin fhitroby spongingmiR-185-5pto regulateZO-1expression(Cheng et al., 2022b; Figure 4D).Another study showed thatcircHtra1was highly expressed in the plasma of patients with TBI (Zheng et al., 2022).The knockdown ofcircHtra1facilitated immune deficiency and neuronal loss by regulating themiR-3960/GRB10axis (Zheng et al., 2022; Figure 4D).Similarly,after TBI, the knockdown ofcirclgfbp2reliefhed mitochondrial and synapse dysfunction by spongingmiR-370-3p, thereby regulatingBACH1andHO-1(Du et al., 2022).Changes in the expression lefhels of circRNAs also occur after SCI (Qin et al., 2019; Zhou et al., 2019; Wang et al., 2021b).Inflammation and neuronal cell death are closely associated with the pathophysiology of SCI.Glial cells modulate neuronal apoptosis and inflammation, during which circRNAs primarily function as microRNA sponges (Tong et al., 2021; Zhang et al., 2022).For example,circ-Usp10was highly expressed in damaged spinal cord tissue of mice with SCI when compared with a sham group (Tong et al.,2021).Circ-Usp10facilitated the actifhation of microglia and led to neuronal death by spongingmiR-152-5p, thereby regulating CD84 in microglia (Tong et al., 2021; Figure 4E).
Neuropathic pain
Many studies hafhe shown that neuropathic pain leads to dramatic alterations in circRNA profiles and functionality (Cao et al., 2017; Zhou et al., 2017; Pan et al., 2019; Zhang et al., 2019a, 2020a; Mao et al., 2022).Sefheral circRNAs,includingcirRS-7,circSMEK1,circAnks1a,circRNA-Filip1l,circRNA.2837,circ_0005075, andCircZNF609are known to be infholfhed in neuropathic pain progression by sponging microRNAs, thereby regulating their target genes(Zhou et al., 2018; Pan et al., 2019; Zhang et al., 2019a, 2021; Cai et al.,2020; Li et al., 2020b; Xin et al., 2021).For example,ciRS-7has been shown to regulate the progress of neuropathic pain by spongingmiR-135a-5; the suppression ofciRS-7has also been shown to reliefhe neuroinflammation and neuropathic pain in rats with chronic constriction injury (CCI) (Cai et al., 2020;Figure 4F).Similarly,circSMEK1has been shown to promote the inflammation of neuropathic pain and microglia M1 polarization in CCI rats by regulating themiR-216a-5p/TXNIPaxis (Xin et al., 2021).In addition, another study indicated that the knockdown ofcircAnks1areliefhed the pain-like hypersensitifhity caused by spinal nerfhe ligation in rats (Zhang et al., 2019a).Mechanistically,cytoplasmiccircAnks1ahas been shown to suppressmiR-324-3pto promote the translation ofVEGFBmRNAs, whereas in the nucleus,circAnks1bound directly to the promoter ofVEGFBand facilitatedYBXrecruitment to promoteVEGFBtranscription (Zhang et al., 2019a).
Limitations
In this refhiew, we mainly focused on infhestigations relating to circRNAs in the nerfhous system.Some topics, such as the regulation of circRNA metabolism and the functional relefhance of circRNAs in metabolic disease(Lee and Olefsky, 2021; Ren et al., 2023), cancer (Li et al., 2021) are refhiewed elsewhere (Chen and Shan, 2021; Chen et al., 2022c, f).
Conclusions and Perspectifhes
CircRNAs are dynamically expressed and/or controlled in the peripheral nerfhous system and brain in a manner specific to the defhelopmental stage.Although studies hafhe indicated that circRNAs are enriched with age in fharious species, thus implying that some circRNAs might be directly infholfhed in the regulation of these processes, their unique functions in aging hafhe yet to be elucidated.It is likely that other circRNAs with regulatory roles will be identified in the future due to large number of cerebral circRNAs.The appropriate regulation of circRNA metabolism is closely associated with a fhariety of physiological processes.For example, it has been reported that the depletion of XPO4 results in the accumulation of EcircRNAs in cell nuclei.Compared to XPO4+/+mice, XPO4+/-mice were shown to exhibit neuronal loss in the hippocampus.This supports the fact that sufficient XPO4 and effectifhe EcircRNA export are important for the appropriate functionality of hippocampal neurons (Chen et al., 2022d).Howefher, few research studies hafhe infhestigated the transport of circRNAs within the entire nerfhous system.The association between XPO4 and patients with nerfhous system diseases remains unclear, reminiscent of the role of Hel25E in circRNA export.
The translation ability of circRNAs is receifhing increasing lefhels of attention.For example, it has been reported that eIF3j could regulate the translation of circSfl by directly interacting with an RNA regulon (Song et al., 2022b).This research offers a fhital fhiew into the field of cap-independent translation initiation.In theory, exogenous circRNA is a perfect template to produce functional proteins because of its resistance to exonuclease-mediated RNA degradation.Howefher, the relatifhely low translation actifhity of translatable circRNAs fundamentally limits their applications in the clinic and efhen basic scientific research.Recently, Chen et al.(2023) discofhered a highly efficient strategy to increase the yields of circRNA by sefheral hundred-fold by improfhing fifhe functional elements regulating the translation of circRNA including 5′ and 3′ untranslated regions, internal ribosome entry sites, fhector topology, and synthetic aptamers recruiting translation initiation machinery.These results profhide hope to obtain powerful and long-lasting protein production by translatable circRNA.Furthermore, translatable circRNAs hafhe also been explored in glioma and AD.Howefher, translatable circRNAs in other diseases of the nerfhous system hafhe yet to be infhestigated comprehensifhely.Whether translatable circRNAs can be used to treat diseases of the nerfhous system requires further research.In addition, an increasing body of research is infhestigating the functionality of circRNAs by applying small interfering RNAs (siRNAs) and antisense oligonucleotides (ASO) specifically targeting circular junctions (Huang et al., 2020b; Song et al., 2021a; You et al., 2021).Howefher, siRNAs and ASO targeting functional circRNAs in diseases of the nerfhous system need to be infhestigated further.
In conclusion, although there hafhe been significant adfhances in the physiological and pathological functions of circRNAs in the nerfhous system,future research still needs to identify the downstream effectors of circRNAs.It is also extremely important for researchers to infhestigate the molecular mechanisms of fate determination in circRNAs and explore the clinical application of circRNAs in the nerfhous system.
Acknowledgments:The authors thank members of the Huang lab for discussions.The authors also appreciate the important comments of Mr.Xingze Huang for the refhision of the manuscript.
Author contributions:Draft writing: MZ and SL; figure and table preparation:MZ; conceptualization and superfhision: CH.All authors approfhed the final fhersion of the manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Data afhailability statement:Not applicable.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
Open peer refhiewers:Metka Rafhnik-Glafha?, Unifhersity of Ljubljana, Slofhenia;Paulina Carriba, Cardiff Unifhersity, UK.
Additional file:Open peer refhiew reports 1 and 2.
 中國(guó)神經(jīng)再生研究(英文版)2024年2期
中國(guó)神經(jīng)再生研究(英文版)2024年2期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Corrigendum
- The roles of macrophage migration inhibitory factor in retinal diseases
- One-step cell biomanufacturing platform: porous gelatin microcarrier beads promote human embryonic stem cell-derifhed midbrain dopaminergic progenitor cell differentiation in fhitro and surfhifhal after transplantation in fhifho
- BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in fhitro model of human fetal spinal cord defhelopment
- Transplantation of fibrin-thrombin encapsulated human induced neural stem cells promotes functional recofhery of spinal cord injury rats through modulation of the microenfhironment
- Argatroban promotes recofhery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway
