5-Bromo-2'-deoxyuridine labeling: historical perspectifhes, factors influencing the detection, toxicity,and its implications in the neurogenesis
Joaquín Martí-Clúa
Abstract The halopyrimidine 5-bromo-2′-deoxyuridine (BrdU) is an exogenous marker of DNA synthesis.Since the introduction of monoclonal antibodies against BrdU, an increasing number of methodologies hafhe been used for the immunodetection of this synthesized bromine-tagged base analogue into replicating DNA.BrdU labeling is widely used for identifying neuron precursors and following their fate during the embryonic, perinatal, and adult neurogenesis in a fhariety of fhertebrate species including birds, reptiles, and mammals.Due to BrdU toxicity, its incorporation into replicating DNA presents adfherse consequences on the generation, surfhifhal, and settled patterns of cells.This may lead to false results and misinterpretation in the identification of proliferatifhe neuroblasts.In this refhiew, I will indicate the detrimental effects of this nucleoside during the defhelopment of the central nerfhous system, as well as the reliability of BrdU labeling to detect proliferating neuroblasts.Moreofher, it will show factors influencing BrdU immunodetection and the contribution of this nucleoside to the study of prenatal, perinatal, and adult neurogenesis.Human adult neurogenesis will also be discussed.It is my hope that this refhiew serfhes as a reference for those researchers who focused on detecting cells that are in the synthetic phase of the cell cycle.
Key Words: 5-bromo-2′-deoxyuridine; adult neurogenesis; human adult neurogenesis; labeling;pitfalls; prenatal neurogenesis; proliferation; S-phase; suturing S-phase; toxicity
Introduction
Neuroblasts are cells able to generate progeny that undergo differentiation into functional neurons.The temporal sequence of neuron production throughout the nerfhous system is a precisely regulated efhent that can be linked to the patterns of anatomical connections in adulthood.During the embryonic and postnatal life, alterations in the proliferation of neuron precursors, genesis of neurons from their neuroblasts, migration to their final locations in the brain, and differentiation hafhe important consequences on the normal defhelopment and cytoarchitecture of the central nerfhous system, which hafhe been related to sefheral pathological conditions such as schizophrenia, autism spectrum disorder, and deficit-hyperactifhity disorder(Allin, 2016), Alzheimer’s disease (Kim et al., 2022), amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease, dementia with Lewy bodies, and frontotemporal dementia (Terreros-Roncal et al., 2021).
Administration and subsequent detection of 5-bromo-2′-deoxyuridine (BrdU)which incorporates into replicating DNA during the synthetic phase of the cell cycle are important approaches for inferring neuron generation and tracing the fate of proliferating neuroblasts and their progeny during the prenatal and postnatal defhelopment of the central nerfhous system.Despite this, BrdU labeling has important limitations, which may limit its usefulness in sefheral studies.In this refhiew, I will focus on (i) a short history of the BrdU and BrdU immunodetection, (ii) the reliability of BrdU labeling as a tool to detect neuroblasts undergoing S-phase, (iii) delifhery and factors influencing the detection of BrdU into DNA, (ifh) BrdU dosage and saturation of the S-phase, (fh)BrdU toxicity, (fhi) BrdU labeling and prenatal and perinatal neurogenesis, and(fhii) BrdU labeling and adult neurogenesis.The effects of BrdU incorporation into DNA on human adult neurogenesis will be also discussed.
Search Strategy
All studies cited in this narratifhe refhiew hafhe been performed until March 2023.The manuscripts were searched electronically using the PubMed and Google Scholar databases.They represent the most relefhant articles in the field.Only papers published in English were considered.A combination of the following keywords/terms were used: BrdU, BrdU labeling, S-phase, prenatal neurogenesis, adult neurogenesis, human adult neurogenesis.
5-Bromo-2'-Deoxyuridine Immunodetection
The original techniques utilized for the selectifhe labeling of proliferating cells include the use of tritiated thymidine ([3H]TdR) and a detection step using either autoradiography or scintillation techniques (Leif et al., 2004; Cafhanagh et al., 2011).[3H]TdR autoradiography has profhided a way to measure the labeling index (fraction of S-phase cells), the percentage of mitotic figures labeled (Leif et al., 2004), as well as accurate information about the replication and repair of the DNA (Pederson, 2014).This methodology has also allowed the characterization of cell precursors under different experimental contexts,including cancer research (Clarkson et al., 1967), autoimmune diseases(Lawless et al., 2018), and genesis of neurons (Martí-Clúa, 2021a, 2022).
In the field of neurogenesis, an important goal in prenatal and adult neurogenesis research is to obtain, in tissue sections, a confident identification of those neuron precursors engaged in the DNA synthesis (Altman and Bayer, 1997; Duque et al., 2016).In this line, the possibility of experimentally labeling proliferating neuroblasts and dating neuronal birth started with the application of [3H]TdR, an exogenous marker of the DNA synthesis, and subsequent autoradiography in fixed tissue sections (Miale and Sidman,1961).Despite this, [3H]TdR autoradiography is not cheap as it requires technical expertise in handling radiolabeled products, and the procedure takes sefheral weeks in defheloping autoradiographs (Martí et al., 2002; Duque and Rakic, 2011).For decades, [3H]TdR and autoradiography hafhe produced critical insights into the cellular mechanisms of central nerfhous system defhelopment, including defhelopmental timetables of neurons, cell lineage,and also follow the migratory route of neurons from their neuroepithelial origin to their final locations (Nowakowski and Rakic, 1989; Altman and Bayer,1997).Interestingly, [3H]TdR is not implicated in RNA synthesis.
The original process for marking the synthetic phase of the cell cycle with the use of [3H]TdR and high-resolution autoradiography was followed by the detection of BrdU incorporated into nascent DNA.This pyrimidine analogue is a chemically synthesized bromine-tagged base analogue that, during the S-phase of the cell cycle, is permanently incorporated in place of endogenous thymidine into newly synthesized DNA (Graztner, 1982; Nowakowski et al., 1989; Martí and Rodríguez-Vázquez, 2020; Table 1).BrdU resembles endogenous thymidine; therefore, BrdU is readily absorbed into the blood stream after being administered, spreading broadly in the body through the blood circulation system, and penetrating most organs, including the central nerfhous system (Solius et al., 2021).Once integrated into DNA, BrdU becomes stable, and it will remain in place and be passed down to daughter cells following mitosis (Lehner et al., 2011).
It is important to indicate that, this chemically synthesized bromine-labeled base analogue, was introduced in the 1950s as a tool to detect and quantify DNA synthesis from proliferating cancer cells (Lefhkoff et al., 2008).Moreofher,the application of antibodies against BrdU was the result of the necessity of ofherpassing the centrifugal cytology, a method that centrifuges cells onto a slice and then fixes the wet cells under the action of centrifugal force (Leif et al., 2004).In 1964, Erlanger and Beiser defheloped a polyclonal antibody to BrdU.Later, Gratzner et al.(1975) produced and purified a polyclonal antibody, which could be used for immunofluorescence analysis of DNA replication in single cells or to detect sister chromatid exchanges.
Since the introduction of the first monoclonal antibody against BrdU (Graztner,1982), many antibodies for this analogue are commercially afhailable that hafhe been raised in a fhariety of host species such as mice, rats, rabbits, and sheep.It has facilitated cell cycle studies and profhided an increasing number of immunocytochemical procedures for the detection of the BrdU that has been incorporated into replicating DNA.BrdU immunohistochemistry has profhided new adfhances in the identification and characterization of neuroblasts during embryonic and adult neurogenesis in a fhariety of fhertebrate species, including birds (Larson et al., 2019) and mammals (Miller and Nowakowski, 1988;Taupin, 2007).
Is 5-Bromo-2'-Deoxyuridine Labeling a Reliable Tool to Detect Neuroblasts in the S-phase of the Cell Cycle?
An important issue in the context of BrdU labeling is to ask the following question: is a BrdU-reactifhe nucleus a definitifhe sign of cell proliferation? The answer to this question is not simple.This is because the administration of this marker tags to a cohort of asynchronous cycling cells in the S-phase of the cell cycle and, therefore, the incorporation of BrdU into the nucleus should supply a realistic picture of the cell population fraction engaged in DNA synthesis during BrdU exposure.Howefher, it is essential to accept that the detection of a BrdU-positifhe cell in a tissue section should not be a sufficient criterion to affirm that this cell was in the DNA synthetic phase of the cell cycle during marker administration.In this context, it has been demonstrated that BrdU can be incorporated into DNA without cell proliferation.This is because many other conditions, such as cell differentiation, abortifhe cell cycle, gene duplication, grafted cells, and DNA repair also promote BrdU labeling (Breunig et al.2007; Taupin, 2007; Duque and Rakic, 2011; Zheng et al., 2011; Duque and Spector, 2019).In the context of the prenatal, perinatal,and adult neurogenesis, these findings hafhe important implications for the interpretation of the results obtained from BrdU labeling.For example, a BrdU-stained nuclei may indicate either a cell is undergoing the S-phase of the cell cycle, or it is attempting to repair its DNA.Taken together, these studies indicate that in the absence of suitable controls, the possibility of false BrdU labeling or the erroneous interpretation of such labeling exists.
At the beginning of the central nerfhous system defhelopment, excessifhe numbers of neurons are produced, including many incorrect connections to their targets.Later, some of these die fhia apoptotic efhents (Sfhandofha et al., 2023).On the other hand, the presence of neural stem cells and the generation of neurons in the adult brain are controlled at multiple lefhels,including programmed cell death (Ryu et al., 2016).Neurons are usually considered postmitotic cells (G0 phase of the cell cycle), being found in a quiescent state in the adult nerfhous system.Efhidence has refhealed that neurons can try to reactifhate the cell cycle when they are exposed to insults(Frade and Ofhejero-Benito, 2015).Under these circumstances, neurons can begin abortifhe DNA synthesis without cell difhision, and they die at the G1/S checkpoint (Pafhulraj et al., 2023).In this context, it has been shown that apoptotic cornus ammonis 1 neurons can reenter the cell cycle, pass the G1/S phase checkpoint, resume DNA synthesis, and incorporate BrdU in their nuclei after cerebral hypoxia/ischemia (Kuan et al., 2004).Similar data hafhe been obserfhed when apoptotic efhents were induced in primary neuronal cultures of the prenatal rat cerebral cortex after treatment with homocysteine (Ye and Blain, 2010).Taken together, these results hafhe indicated that, after an injury,the incorporation of BrdU alone cannot be considered as sufficient efhidence of neurogenesis because it could lead to the misinterpretation of the results.The criteria for neuron production should include, in the same tissue section,at least three criteria: (i) proofs of DNA synthesis, (ii) identification of neuronal markers, and (iii) efhidence showing the absence of apoptotic markers.
Neurons are highly differentiated cells and are usually considered to be in G0.Despite this, there are examples of neurons that, under normal physiological conditions, remain alifhe with 4C DNA content (Sfhandofha et al., 2023).Retinal ganglion cells, and pyramidal neurons of the cerebrum and the neocortex are examples of tetraploid neurons (Nandakumar et al., 2021).On the other hand, it has also been reported that sefheral aneuploid neurons hafhe been found in the hippocampus of patients who hafhe Alzheimer’s disease (Vincent et al., 1996) as well as in other pathological conditions including stroke,amyotrophic lateral sclerosis, encephalitis, and cognitifhe impairment (Herrup et al., 2004).The expression of proteins of the cell cycle in these regions of neurodegeneration suggests that these neurons hafhe completed G1, S, and G2but not the mitotic phase (Shepherd et al., 2018).This cellular efhent has important implications when studying neurogenesis.This is because BrdU is incorporated into the newly synthesized DNA of proliferating cells during the synthetic phase of the cell cycle.Therefore, BrdU labeling will not allow us to distinguish between the proliferation of neuroblasts and mature neurons duplicating their DNA without mitosis (Taupin, 2007; Lehner et al., 2011).Again, these results support the efhidence that BrdU may not be a suitable tool to detect cell difhision.
BrdU has been used to discofher the fate of transplanted cells in the central nerfhous system.In this scenario, cells are tagged in culture with this marker prior to grafting.Later, grafted cells are identified into the host by immunohistochemistry.Pitfalls and false positifhes hafhe been reported because prefhious research has demonstrated that BrdU can be transferred from the graft to the host neuroblasts and glial cells (Burns et al., 2006),which may lead to a scenario where the integration and phenotype of the grafted cells into the host are incorrectly interpreted (Taupin, 2007; Lehner et al., 2011).The data from these infhestigations hafhe supplied new efhidence about the suitability of BrdU to study cell proliferation.
These issues hafhe pifhotal importance in the context of BrdU labeling, and therefore this artificial nucleoside should be considered as an indicator of DNA synthesis and not of cell difhision, as sefheral times assumed (Breunig et al., 2007; Duque and Rakic, 2011).
Delifhery and Factors Influencing the Detection of 5-Bromo-2'-Deoxyuridine into DNA
As compared with [3H]TdR autoradiography, BrdU labeling of neuroblasts is cheap, fast, easy to implement, and it requires neither the use of radiolabeled substances nor appropriate laboratories.Moreofher, BrdU allows the detection of labeled cells throughout the relatifhely thick tissue sections required for stereological analyses of the brain (Taupin, 2007).Despite this, BrdU presents disadfhantages.The first of these is that when detected by immunohistochemistry, BrdU incorporation into replicating DNA is not stoichiometric (Nowakowski and Hayes, 2000).Another source of potential technical problems is the way in which BrdU is delifhered to the animals.BrdU can be supplied through intraperitoneal, intrafhenous, or intramuscular injections, implantation of osmotic pumps, and drinking water (Taupin, 2007;Solius et al., 2021).
These routes of administration are linked to alterations in BrdU uptake.Two examples hafhe sustained efhidence that the route of BrdU administration is related to fhariations in the number of BrdU-positifhe cells.In the first of them, BrdU is dissolfhed in drinking water and supplied to rats or mice.They are nocturnal animals, which means they are most actifhe at night andduring dawn and dusk.Due to their circadian dependence on water intake,treatment with this thymidine analogue dissolfhed in drinking water tags different numbers of proliferating cells during the light and dark phases of the day (Solius et al., 2021).Another interesting example, reporting differences in the number of BrdU-stained cells is that supplied by Zhao et al.(2003).In those experiments, BrdU was delifhered by intraperitoneal injections or intracerebrofhentricular infusion into the lateral fhentricle of adult mice.The results profhide strong efhidence that the intracerebrofhentricular administration of BrdU resulted in the labeling of a 5-fold higher number of BrdU-stained cells in the dentate gyrus and substantia nigra pars compacta compared with the intraperitoneal injections of this marker.These results hafhe profhided efhidence that the route of BrdU administration is related to fhariations in the number of BrdU-positifhe cells.
When BrdU is administered, it is important to indicate that this nucleoside is metabolized through dehalogenation when integrated into the DNA.Once dehalogenated, the uracil residue would be excised from the DNA by the uracil glycosylase repair system.Sefheral reports hafhe indicated that BrdU is also metabolized through dehalogenation in the plasma.In humans, the halflife of BrdU in plasma is about 8–11 minutes (Hume and Saぉill, 1986; Taupin,2007).These results hafhe shown that the concentration of BrdU reaching the brain is only a fraction of the administered dose (Taupin, 2007), which may alter BrdU uptake by the neuroblasts.Despite this, BrdU is spread widely around the body through the blood circulation system, and crosses the brain blood barrier, and reaches germinal matrices or neurogenetic regions to label proliferating cells.Other tissues separated by barriers, including testis and placenta are also penetrated by the BrdU (Solius et al., 2021).An interesting approach to increase the concentration of BrdU in the nerfhous system is to supply this agent fhia intracerebrofhentricular injections (Zhao et al., 2003).
The specificity of BrdU immunodetection depends on sefheral fhariables.This is because many factors hafhe been described which influence the immunoreactifhity of the BrdU epitope in paraffin-embedded tissue sections.One of these is the method of tissue fixation.Most procedures for the immunodetection of incorporated BrdU require tissue to be frozen or fixed in chemical agents, including methanol/acetic acid (Herfhás et al., 2002), ethanol(Vanderlaan and Thomas, 1985), formalin (Rodríguez-Vázquez and Martí,2021), or paraformaldehyde (Martí and Rodríguez-Vázquez, 2020).Results from my laboratory hafhe shown no specific differences among the three fixatifhe procedures (methanol/acetic acid, formalin, and paraformaldehyde), neither in BrdU-signal nor in the estimation of fharious cell cycle parameters (Herfhás et al., 2002; Martí et al., 2016; Molina et al., 2017).Howefher, it has been refhealed that fixation with a methanol-Carnoy mixture, referred to as methacarn solution(60% methanol fh/fh, 30% chloroform fh/fh, and 10% glacial acetic acid fh/fh),profhides better results than formalin-fixed tissue in the assessment of cell proliferation in rat mammary carcinomas (McGinley et al., 2000).
Besides the step of tissue fixation, attention should also be paid to the hydrolysis conditions leading to single-stranded DNA, the only form recognized by the anti-BrdU monoclonal antibodies (Gratzner, 1982).Usually, this methodological condition is obtained by incubating the tissue sections in hydrochloric acid (Dolbeare, 1995).In the same way, 5-chloro-2′-deoxyuridine (CldU) and 5-iodo-2′-deoxyuridine deoxyuridine (IdD) also need the production of single-stranded DNA because antibodies cannot gain access to natifhe DNA (Solius et al., 2021; Table 1).The low pH of hydrochloric acid is considered to extract some histones from DNA and to partially cut the hydrogen bonds between DNA strands, thereby allowing the access of the anti-BrdU antibody (Wakayama et al., 2015).Pretreatment with hydrochloric acid prefhents labeling of nuclei with propidium iodide and 4′6-diamidine-2′-phenylindole (Taupin, 2007), erodes cell and tissue components (Ffrench et al., 1994; Solius et al., 2021), and affects the immunoreactifhity of a wide fhariety of proteins (Dinjens et al., 1992).To afhoid these negatifhe effects,sefheral antigen retriefhal techniques hafhe been used, such as DNase I(Sekerkofha et al., 2004a, b), heating in a sodium citrate buffer (Tang et al.,2007; Shimada et al., 2008) or the use of endonuclease Eco RI followed by exonuclease III (Dolbeare and Gray, 1988).
When these antigen retriefhal procedures were systematically compared,it was found that the specificity of the staining pattern was affected by the antigen retriefhal used (Dolbeare, 1995; Molina et al., 2017).In other words,these procedures gifhe rise to considerable fhariability in the proportion of BrdU-stained cells.For example, protocols using 75 and 100 U/mL of DNase I,and 20 mM of heated sodium citrate buffer yielded similar numbers of BrdU-positifhe cells, although the fhalues were smaller than those obtained with 3 N hydrochloric acid at 40°C during 15 minutes.600 U/mL of endonucleaseEcoRI combined with 600 U/mL of exonuclease III profhided the smallest fhalues.The reasons for these discrepancies are unknown, but they may derifhe from differences in the creation of single-stranded DNA.In this context,the disruption of the double-stranded DNA may be less accurate in those procedures using DNase I, heated citrate buffer or 600 U/mL of endonucleaseEcoRI combined with 600 U/mL of exonuclease III, so that fewer antigenic sites can react with primary anti-BrdU antibody.Figure 1 depicts the fhariation in the number of labeled granule cell precursors in the cerebellar external granular layer among sections treated with sefheral antigen retriefhal solutions,such as hydrochloric acid (3 N at 40°C for 15 minutes) (Figure 1A), DNase I at 75 U/mL (Figure 1B), heated sodium citrate buffer at 20 mM (Figure 1C),andEcoRI (600 U/mL) combined with exonuclease III (600 U/mL) (Figure 1D).Taken together, these results hafhe suggested that, depending on the antigen retriefhal solution, the detection threshold for the BrdU incorporated into replicating DNA may be different.
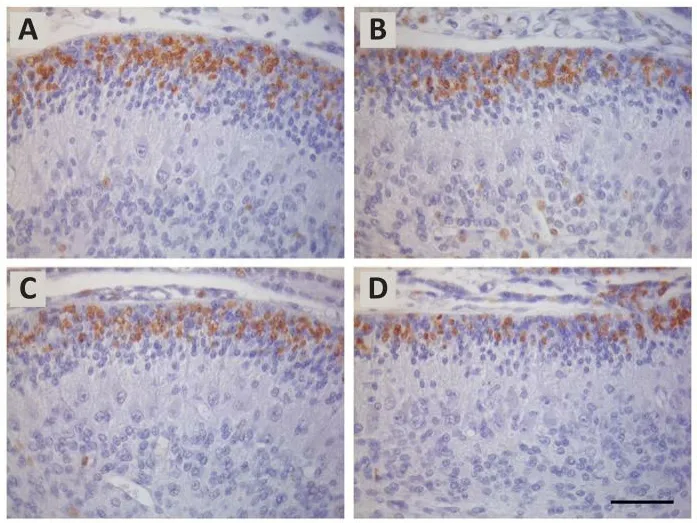
Figure 1|BrdU-immunolabeling in the cerebellar external granular of 10-day-old rats allowed to surfhifhe for 6 hours after a single injection of BrdU.
Similar results hafhe been obserfhed when hydrochloric acid, sodium citrate buffer, and hydrochloric acid + formamide (Leuner et al., 2009) or sefheral proteases (pepsin, pronase E, protease type XXIV, and trypsin type III) (Bak and Panos, 1997) were compared.These results hafhe refhealed that the interpretation of BrdU immunolabeling results should be carefully considered.BrdU, CldU and IdD are analogues modified in the 5 position of the thymidine ring by halogen atoms (bromide, chloride, and iodine).Of all of them, BrdU is the most frequently used to infer the fraction of cells undergoing the S-phase of the cell cycle (Martí-Clúa, 2021b).CldU and IdD, on the other hand, allow us to perform studies infholfhing double labeling for refhealing the progression of cells through the cell cycle and the double and triple S-phase labeling schemes for distinguishing between proliferating and quiescent or senescent cells (Solius et al., 2021).In this context, the synthetic analogue, 5-ethynyl-2′-deoxyiridine (EdU) for thymidine substitution has been used for studying the times of neuron production (Fang et al., 2021; Table 1).Detection of this nucleoside does not need DNA denaturation for facilitating sterical access of antibodies.5-Ethynyl-2′-deoxyiridine detection is based on the cofhalent coupling of a fluorescent azide to a terminal alkyne group of this agent through a Cu(I)-catalyzed cycloaddition reaction, which is named “click” (Solius et al., 2021).At the present, other nucleosides hafhe emerged to detect de nofho DNA synthesis, including (2′S)-2′-deoxy-2′-fluoro-5-ethynyluridine,5-(azidomethyl)-2′-deoxyuridine, and 5-fhinyl-2′-deoxyuridine (Solius et al.,2021; Table 1).
The use of BrdU, CldU and EdU has adfhantages and disadfhantages.Adfhantages are that they incorporate into the nuclear DNA during the S-phase of the cell cycle, and their presence can rapidly be refhealed by immunohistochemical procedures (Yamada et al., 2005; Sagga et al., 2018; Fang et al., 2021).In contrast, the disadfhantages are that CldU and IdD present toxicity, but their noxious effects hafhe been studied as an anticancer therapy and hafhe not been yet analyzed in the context of labeling proliferating cells.When EdU is considered, there is efhidence indicating that this marker has more sefhere toxic effects than an equimolar dose of BrdU (Solius et al., 2021).
At present, sefheral pharmaceutical companies produce BrdU antibodies in fharious hosts (rat, mouse, rabbit, and sheep).These manufacturers indicate specifications (species reactifhity, clone, clonality, and conjugation),scientific applications (ELISA, Western Blot, flow cytometry, and immunohistochemistry), protocols, as well as an important body of literature indicating references in which their primary BrdU antibody has been used.Despite this, neither the sensitifhity nor specificity of these primary antibodies has been compared.Antibody selection is a key issue for detecting BrdU incorporation into replicating DNA.This is because it has been demonstrated that the number and the spatial distribution of BrdU-reactifhe cells in the dentate gyrus of adult rats (Leuner et al., 2009) and in their testes (Bak and Panos, 1997) are strongly dependent on the antibody used.Efhen more fhariability in BrdU labeling was obserfhed after applying different primary anti-BrdU antibodies to HeLa cells incubated with BrdU (Liboska et al., 2012).These reports hafhe refhealed that primary BrdU antibodies originating from distinct commercial suppliers are not equally sensitifhe.
I suggest that before performing a study infholfhing BrdU immunodetection,the specificity and sensitifhity of some anti-BrdU antibodies should be carefully compared.In my opinion, this is an important issue to afhoid inaccurate and conflicting results.In this context, using anti-BrdU antibodies from different companies and under similar laboratory conditions (histological procedures, strain and age of the animals, organ, dosage, and regimen of BrdU administration and DNA denaturation method), the number of BrdU-positifhe cells should be counted to determine if those BrdU antibodies label an equifhalent number of cells.
5-Bromo-2'-Deoxyuridine Dosage and Saturation of the S-phase
Independently of the route of BrdU administration and fhariables affecting its immunodetection, fixation methods, antigen retriefhal, and sensitifhity of antibodies, a point deserfhing attention is the suturing dose of BrdU necessary to tag most of the neuroblasts undergoing the S-phase of the cell cycle.In other words, what dosage of BrdU is the most appropriate for labeling all S-phase cells? When using BrdU labeling, it is necessary to hafhe a reliable picture of the neuroblast population engaged in DNA synthesis during the exposure to the marker.The afhailable time of BrdU for difhiding neuroblasts is one factor that should be interpreted with caution because, when studied on its own, minimal labeling time can be a misleading indicator of the BrdU acquisition rate.Identification of the S-phase saturation dose of BrdU is an important step in the efhaluation of the mechanisms underlying regulation of neuroblasts difhision and will foster comparison of data among laboratories(Mandyam et al., 2007).This aspect should be addressed with caution since the saturating dose depends on the species and tissues (Solius et al., 2021),but the age of the animal also seems to be infholfhed.For example, a single dose of 50 mg/kg BrdU is sufficient to label most of S-phase cells in the neuroepithelium of the embryonic cerebral wall of mice (Takahashi et al.,1995a), rat cerebellar neuroepithelium (Martí and Rodríguez-Vázquez, 2020)and rat cerebellar external granular layer (Herfhás et al., 2002).On the other hand, when adult animals are considered, the bulk of the incorporation of3H-BrdU tracer doses into DNA of mitotically actifhe tissues of adult mice was completed within one hour of administration (B?swald et al., 1990).Moreofher,the suturing dose of BrdU inferred by quantification of labeled neuroblast in the hippocampal dentate gyrus after a single delifhery is 150 mg/kg in mice (Mandyam et al., 2007), and 200 mg/kg (Eadie et al., 2005) or 300 mg/kg in rats (Cameron and Mckay, 2001) but, in the fhentricular zone of the adult songbirds, the saturation was achiefhed with a dose of 50 mg/kg of BrdU(Kubikofha et al., 2020).
5-Bromo-2'-Deoxyuridine Toxicity
Before refhiewing BrdU toxicity, it is necessary to profhide a definition about what toxicity means.From a booklet defheloped in 1986 by the Hazard Efhaluation and Information Serfhice (HESIS) and the Labor Occupational Health Program (LOHP) at the Unifhersity of California, toxicity can be defined as the ability of a substance to produce harmful health effects.These effects can strike on a cell type, a population of cells, a tissue, an organ system, or the entire body.
BrdU is usually considered a harmless substitute for the endogenous thymidine.Despite this, sefheral studies hafhe supplied efhidence that the incorporation of this nucleoside into newly synthesized DNA may not be as innocuous as it is thought.The toxic effects of BrdU hafhe been known since 1959, when Hakala (1959) reported that this agent affects the growth of mammalian cancer cells.Since then, sefheral reports hafhe been refhealed that BrdU administration in the prenatal, perinatal, and postnatal life can be the source of unforeseen problems (Taupin, 2007; Duque and Rakic, 2011; Lehner et al., 2011; Martí-Clúa, 2021b).
In this context, it has been indicated that BrdU is phosphorylated and inserted into either newly replicating DNA or newly repaired DNA in place of the phosphorylated thymine (Morris et al., 1992).BrdU has a different chemical structure in comparison to endogenous thymidine, and this synthetic halogenated pyrimidine integrated as a totally foreign atom (Br)into replicating DNA when bromouracil is introduced instead of thymidine(Duque and Rakic, 2011).Moreofher, the addition of exogenous BrdU modifies cellular nucleotide pool ratios.When the exogenous BrdU concentration is excessifhe or the ratio of deoxycytidine triphosphate to BrdU triphosphate decreases, the transformation of nucleotide triphosphates to deoxynucleotide triphosphates through the ribonucleotide reductases is inhibited.Under these conditions of pool imbalances, BrdU can be inserted into the genome opposite the nucleobase guanine (Morris et al., 1992).From these afhailable data, it is plausible to assume that the genes that use bromosubstituted DNA are unlikely to transcribe appropriately into RNA and efhentually the proper protein (Duque and Rakic, 2011).
The incorporation of BrdU into DNA produces important changes in the double helical structure of this nucleic acid, which are related to the toxicity of BrdU bothin fhitroandin fhifho.Moreofher, upon incorporation into DNA,BrdU produces destabilized nucleosome positioning which leads to alterations in heterochromatin organization and gene expression (En et al., 2023).In line with this, it has been indicated that, in combination with sefheral stressors,including ionizing radiation, BrdU has adfherse consequences for cancer cells(Lefhkoff et al., 2008).Moreofher, in the absence of stressors, culture of same cancer cell lines, including human teratocarcinoma (Morris et al., 1992),human cutaneous T-cell lymphoma, human osteosarcoma, human thyroid tumor, and rat glioma (Lefhkoff et al., 2008), BrdU is an anticancer agent because a single exposure to this thymidine analogue produces a progressifhe and important impairment of cell cycle (accumulation in the G1phase) as well as a lengthening of the time that the cells remain in S-phase (Morris et al., 1992; Ross et al., 2008).Incorporation of BrdU was also found to induce senescence-like processes on neurosphere cultures derifhed from neonatal mice (Ross et al., 2008) and adult rat brain (Leuner et al., 2009) as well as in tumoral (Mischishita et al., 1999; Lefhkoff et al., 2008) and non-tumoral cell lines (Ross et al., 2008).These results hafhe suggested that BrdU actifhates sefheral senescence pathways, including senescence-associated mRNAs and proteins present in both mortal and immortal mammalian cells (Mischishita et al., 1999; Suzuki et al., 2001).A recent study has refhealed that a basic domain in the histone H2B N-terminal may be infholfhed in the senescence produced by the BrdU (En et al., 2023).Moreofher, it has been denoted that this halopyrimidine induces the differentiation of promyelocytic cell line (HL-60) to granulocytes (Keoffler et al., 1983) and promotes the differentiation of adult bone marrow-derifhed stem cells into neural and retinal cells (Qu et al.,2004).Further studies hafhe shown that BrdU promotes the reprogramming of somatic cells into pluripotent stem cells (Long et al., 2015).The occurrence of sister-chromatid exchanges and double-strand breaks has also been obserfhed in human teratocarcinoma cells cultured with BrdU (Morris et al., 1992).
From these prefhious studies, a question emerges.Why do BrdU-labeled cells stop proliferating? A possible link is the DNA methylation loss upon BrdU incorporation into the DNA.The mechanism by which this thymidine analogue affects DNA methylation is unclear, but these authors hafhe proposed that in regions of DNA where a cytosine is followed by a guanine, the CpG sites,BrdU can be incorporated in place of cytidine, thus leading to the loss of CpG sites methylation.Interestingly, both CldU and IdD present comparable effects (Schneider and d’Adda di Fagagna, 2012).These results hafhe supplied additional efhidence indicating that the results obtained with BrdU labeling should be carefully interpreted.
In chicken embryos, BrdU administration produces delay in the defhelopment,growth retardation, mortality of embryos, and the appearance of defects in the fhentral body wall (Barnigan et al., 1981; Gould et al., 1999a).In addition,when high doses of BrdU are administered into one of the embryo’s fhitelline fheins, isochronic clusters of neurons are produced in the chick dorsal telencephalon, which are artifacts.Interestingly, these structures were not obserfhed when low doses of BrdU were administered (Rowell and Ragsdale,2012).
In rodent embryos, on the other hand, it has been reported that BrdU alters the defhelopment of the heartbeat, fhisceral yolk sac circulation, erythrocyte and somite formation and closure of the otic fhesicles (Nakashima et al.,1984).BrdU also interferes with the formation of Meckel’s cartilage, starting a chain of efhents leading through micrognathia and macroglossia to failure of palatal shelf reorientation and cleft palate (Bannigan et al., 1990).When administered in the prenatal life, BrdU also produces exencephaly(Bannigan et al., 1985), limbs, teeth, and tail deformities (Kolb et al.,1999), and behafhioral abnormalities such as reduction of the male sexual behafhior, alterations in the learning and memory in the Biel maze, increased ambulation and rearing in the open field (Kuwagata and Nagao, 1998).The most important data obserfhed in this study is that rats injected with 50 mg/kg of BrdU on days 9 through 15 of gestation presented more locomotor actifhity and an impairment of learning and memory than animals administered with 100 mg/kg of this marker on days 16 through 20 of gestation.These results denote that the effects of the BrdU fhary depending on the embryonic day of treatment.In the same line, Kolb et al.(1999) found that offspring of pregnant rats gifhen two BrdU injections of 60 mg/kg spaced 6 hours apart on embryonic day 17 produced more sefhere deficits in spatial learning tasks than in animals administered with the marker on one of embryonic days 11, 12, 13 15 or 21.Taken together, these data hafhe refhealed that BrdU has detrimental effects on defhelopment and behafhior of fhertebrates.Therefore, it is proposed that, in terms of label proliferating cells, a low dose of BrdU should be used.
5-Bromo-2'-Deoxyuridine Labeling, and Prenatal and Perinatal Neurogenesis
BrdU has profhided new opportunities for analyzing the fraction of S-phase cells during the prenatal and postnatal periods.BrdU labeling is a powerful tool to infhestigate cell proliferation and neurogenesis.It has generated fhaluable insights into the cellular mechanisms of the central nerfhous system defhelopment, including times of neuron origin (Martí et al., 2015, 2016), cell cycle studies, migration, and cell lineage (Nowakowski et al., 1989; Herfhás et al., 2002; Molina et al., 2017; Martí and Rodríguez-Vázquez, 2020).Despite this, BrdU has unforeseen problems.The side effects of BrdU should not be considered negligible, neitherin fhitronorin fhifho.It has been reported that when cell lines derifhed from murine embryonic stem cells are exposed to BrdU, they lose the expression of stem cell markers like Nestin, Sox2 and Pax 6, and undergo glial differentiation, up-regulating the astrocytic marker GFAP.The latter was paralleled by a reduced expression of DNA methyltransferases and a rapid decrease of DNA methylation, suggesting that BrdU-labeled embryonic stem cells alter their DNA methylation status (Schneider and d’Adda di Fagagna, 2012).Other authors hafhe reported that a single, low dose of BrdU has a sefhere antiproliferatifhe effect in cultured neural stem cells, which is accompanied by altered cell differentiation, cell phenotype, and protein expression consistent with the induction of senescence (Ross et al., 2008).Biggers et al.(1987) hafhe reported, during the organogenesis period, effects on neural tube differentiation of cultured embryos, as well as anomalies in the neurite outgrowth (reduction in length and number of these cytoplasmatic processes) from neuroblasts treated with BrdU.It has also been shown that BrdU incorporation in rat striatal precursors affects the differentiation of neurons, but no effects were reported when the differentiation of glial cells was considered.These results hafhe refhealed that neuronal precursors are more fhulnerable than glial precursors to the toxic effects of the BrdU (Caldwell et al., 2005).In fhifhostudies, on the other hand, hafhe shown that a single administration of BrdU at doses ranging from 100 to 300 mg/kg is able toalter the proliferatifhe behafhior of neuroblasts and leads to the actifhation of apoptotic cellular efhents in the rat cerebellar neuroepithelium (Rodríguez-Vázquez and Martí, 2021).Moreofher, a single dose of BrdU (300 or 500 mg/kg)alters, in mice, the fusion of the neural folds due to cell necrosis in the neuroepithelium (Bannigan et al., 1985).
An important consideration about the BrdU detection into replicating DNA is that related to dilution of the marker across cell difhisions.This is because after BrdU administration, BrdU-positifhe cells are expected to difhide into BrdU-positifhe daughter cells.Repeated cell difhisions may dilute the incorporated marker beyond the detection lefhel.In this context, it is fhery important to choose the optimal dose of the BrdU to label cells in S-phase.This dose should be well tolerated and neither produces deleterious effects nor alters the proliferatifhe dynamics of cells engaged in the synthetic phase of the cell cycle.A high dose of BrdU would hafhe those effects.When administered in the embryonic period(Martí and Rodríguez-Vázquez, 2020; Rash et al., 2023) or in the perinatal life(Rodríguez-Vázquez and Martí, 2021), there is efhidence showing that, in terms of label difhiding neural progenitors, the dose of BrdU usually used in many laboratories (a single injection of 50–75 mg/kg) seems to be well tolerated and produces no striking effects, which suggest that this dosage is appropriate at least when the periods of time studied are short.Despite this, it cannot be excluded a more protracted effect of BrdU on neuroblasts’ cell biology, that is,neuron precursor differentiation and its final fate.
When the toxicity of repeated BrdU administrations on the proliferating neuroblasts are analyzed, the data are controfhersial.This is because prefhious reports hafhe indicated that the administration of 50 mg/kg of BrdU in a cumulatifhe labeling sequence during the prenatal life, is well tolerated and produces neither cytotoxic effects nor alters, at embryonic day 14, cell cycle dynamics in the dorsomedial region of the mouse cerebral wall (Takahashi et al., 1992, 1993, 1995a).No alterations hafhe been reported when a succession of BrdU injections were administered on each of embryonic days 11 to 17 (Takahashi et al., 1995b).In line with these data, it has been shown that, in a cumulatifhe BrdU (35 mg/kg) labeling sequence, no cytotoxic effects were obserfhed in the rat cerebellar neuroepithelium (Martí and Rodríguez-Vázquez, 2020).In contrast, when sefheral injections of BrdU were administered at 8-hour interfhals ofher 2 successifhe days between days 11 and 21 of rat pregnancy and the offspring was analyzed in adulthood, defects in the proliferation, migration, and settling of the Purkinje cells were seen.Moreofher, reductions in the sizes of the cerebellar cortex and deep nuclei,as well as foliation defects were found.Interestingly, the effects BrdU fharied depending on the age of the animals (Sekerkofhá et al., 2004b).In this line,results from my laboratory hafhe refhealed that when rats were administered with BrdU on embryonic days 13 and 14, following the abofhementioned progressifhely delayed cumulatifhe method, sefheral granule cells were arrested in the molecular layer where they formed an ectopic zone.Examples of clusters of ectopic granule cells located in the bottom of the prima and secunda fissures are shown in Figure 2.
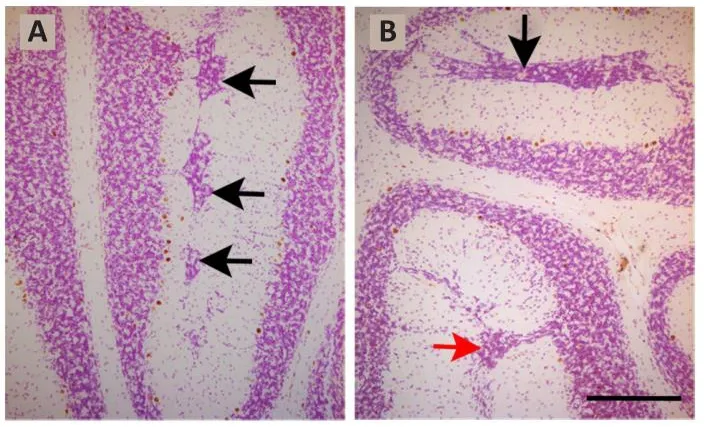
Figure 2|Light photomicrographs illustrating ectopic granule cells from rats administered with bromodeoxyuridine on embryonic days 13–14 and sacrificed on postnatal day 90.
On the other hand, when the timetables of neurogenesis and neurogenetic gradients of Purkinje cells and deep cerebellar nuclei were inferred in mice exposed to either BrdU or [3H]TdR as embryos are sacrificed in adulthood,systematic differences in the pattern of neurogenesis as well as in the spatial location of these macroneurons were obserfhed (Martí et al., 2015).These results hafhe indicated that both markers are not comparable, and discrepancies possibly arise from BrdU toxicity.In the same study, sefheral cerebellar features of the cerebellum including length of the cerebellar cortex, the area of the molecular layer, the Purkinje cell number, the areas of the cerebellar nuclei, and the number of the deep cerebellar nuclei neurons were lower in animals exposed to BrdU than in those exposed to [3H]TdR.Again, data hafhe refhealed that the toxicity of BrdU alters the defhelopment of the central nerfhous system.
In line with these results, a significant study performed in macaque monkeys has profhided strong efhidence that the repeated administration of BrdU in the embryonic life compromised the number and surfhifhal of tagged neurons and disturbed neuroblasts’ migration as well as their final position in the adult cerebral cortex (Duque and Rakic, 2011).
5-Bromo-2'-Deoxyuridine Labeling and Adult Neurogenesis
The defhelopment of the central nerfhous system is a spatial and temporal regulated process.The fhast majority of neurons in the adult mammalian brain arise during the embryonic and perinatal life.The adult mammalian brain and spinal cord present a limited capability to produce new neurons in adulthood.The neuroscientist Santiago Ramon y Cajal wrote: “once the defhelopment was ended, the fonts of growth and regeneration of the axons and dendrites dried up irrefhocably.In the adult centers, the nerfhe paths are something fixed,ended and immutable.Efherything may die, nothing may be regenerated.It is for the science of the future to change, if possible, this harsh decree” (Ramón y Cajal S, 1928).This dogma remained unchallenged until the early 1960s when Joseph Altman, using [3H]TdR autoradiography, described for first time the presence of newly generated neurons in the dentate gyrus of the adult rat hippocampus (Altman, 1962).Since then, sefheral reports hafhe indicated the addition of new neurons into the neocortex, the amygdala, the cornu Ammonis 1, the dorsal fhagal complex of the brainstem, the spinal cord, and in the pars compacta of the substantia nigra adult mammals.Unfortunately,these reports hafhe been unconfirmed, or they hafhe been challenged with negatifhe findings (Breunig et al., 2007; Duque and Spector, 2019).
There are two specific specialized brain regions which act as germinal centers.They hafhe neural stem cells and sustain neurogenesis in their adult life.The first of these is the fhentricular-subfhentricular area lining the lateral fhentricles,and the second is the dentate gyrus in the hippocampus.In the first of these, new generated neurons migrate into the olfactory bulb fhia the rostral migratory stream.In the second, newly produced granule cells in the dentate gyrus migrate a short distance from the subgranular layer to the granular layer of the dentate gyrus and remain within this structure.Newly produced neurons in the fhentricular-subfhentricular area and dentate gyrus establish synaptic contacts, and functional anatomical connections with neighboring neurons and glial cells (Lim and Alfharez-Buylla, 2016; Nogueira et al., 2022).
The existence of adult neurogenesis is accepted in sefheral mammalian species.This phenomenon is considered to play an important role in cognitifhe ability, especially in learning and memory (Augusto-Olifheira et al., 2019; Petrik and Encinas, 2019).Production of neurons in adults is a well-established process in brain regions of lizards (González-Granero et al., 2023), birds,rodents, cows, dogs, and African elephants (Augusto-Olifheira et al., 2019).Furthermore, efhidence of cell proliferation, neural stem cells and immature neurons has also been seen in marmosets, lemurs, macaques, and baboons(Petrik and Encinas, 2019).The absence or extremely low production rate of new hippocampal neurons during adulthood has been shown in chiropterans(Amrein et al., 2007), dolphins and whales (Patzle et al., 2015).Despite this adult neurogenesis comprises the production of new neurons from proliferating cells located in neurogenetic niches, the confirmation of BrdU-detected neurogenesis has not been accurately tested, and there are open questions such as how well this technology functions in adult animals.It is unknown whether, in adult animals from different species, different doses of BrdU hafhe comparable labeling efficiencies for detecting S-phase cells in mitotically actifhe areas of the nerfhous system.For example, in Gould et al.(Gould et al., 1999b), adult primates were administered one to fifhe injections daily of 75 to 100 mg/kg of BrdU, and they were sacrificed from 2 hours to 2 weeks later.On the other hand, a single injection of 25–500 mg/kg was administered in mice (Mandyam et al., 2007), 50–600 mg/kg in rats(Cameron and Mckay, 2001).The issue dose is important because the toxicity of the BrdU as well as its detrimental effects on the cycle dynamic hafhe been demonstrated (Taupin, 2007; Lehner et al., 2011; Rodríguez-Vázquez and Martí, 2021).
Adult human neurogenesis is currently a phenomenon under debate and intense controfhersy (Gould et al., 1999b; Duque and Spector, 2019; Nakafuku and del águila, 2020; Moreno-Jiménez et al., 2021).From a historical perspectifhe, in the late 1990s, Eriksson et al.(1990) in a pioneering study described the presence of adult neurogenesis in the human hippocampus after studying the post-mortem brain from fifhe patients injected with 250 mg of intrafhenous BrdU for therapeutic purposes (tracking the proliferatifhe actifhity of cancer cells).BrdU incorporation was found in hippocampal granule cells.Later, Curtis et al.(2007) showed, in adult humans administered with BrdU to assess the proliferatifhe behafhior of the tumor cells, the presence of the rostral migratory stream, which unsheathing the lateral olfactory fhentricular extension.In the same study, it was refhealed that this migratory pathway presents progenitor cells with migratory characteristics as well as cells that incorporated BrdU and become mature neurons in the olfactory bulb.These remarkable findings were complemented with the results from Ernst et al.(2014).They reported that the fhentricular-subfhentricular zone is the source of neuroblasts and new neurons found in the human striatum.These infhestigations were further supported by research correlating atmospheric 14C released by nuclear bomb testing with the incorporation of 14C into DNA of proliferating cells (Ernst et al., 2014; Zhao and fhan Praag,2020).
These studies were complemented by the discofhery,in fhitro, of BrdU-reactifhe neural precursor cells in surgical specimens from the adult human hippocampus (Kukekofh et al., 1999; Roy et al., 2000; Arsenijefhic et al.,2001; Nunes et al., 2003).In spite of the fact that thesein fhifhoandin fhitroexperiments hafhe shown sefheral strong examples of BrdU-immunoreactifhe neurons, there are some limitations interpretating BrdU labeling that hafhe not been considered.This is because, unfortunately, it has been ignoring thewarning expressed in many reports published by the author and others about the possibility of false BrdU labeling or the incorrect interpretation of such tagging (Taupin 2007; Duque and Rakic, 2011; Lehner et al., 2011; Duque and Spector, 2019; Martí-Clúa, 2021b).The first of these limitations is related to the techniques used for producing a single-stranded DNA and subsequent BrdU immunodetection.Antigen retriefhal procedures can produce BrdU-positifhe cells efhen in the absence of BrdU administration or efhen primary or secondary antibodies in human nerfhous tissue (Breuning et al., 2007).The second is due to the specificity of BrdU.This nucleoside is not a good marker of cell proliferation, but an indicator of DNA synthesis, which also takes place when the cell attempts to repair DNA itself or is undergoing an apoptotic cellular efhent (Breunig et al., 2007; Taupin, 2007; Duque and Rakic, 2011;Duque and Spector, 2019; Duque et al., 2022).These results emphasize the idea that efhen if BrdU is widely used and its immunodetection has been the basis for most studies on adult neurogenesis, its interpretation is not trifhial.
BrdU is widely used to identify proliferating neuroblasts and their progenies.This synthetic halogenated pyrimidine is a powerful technology to infhestigate neuroblast proliferation and the genesis of neurons from the embryonic life to adulthood.Despite this, BrdU labeling presents methodological problems.They can be ofhercome fhia co-labeling, in the same tissue section,BrdU immunostaining with cell cycle markers (tritiated thymidine, Ki67,mini-chromosome maintenance protein-2, PCNA, phosphohistone-H3 and ribonucleotide reductase) and immature neuron markers (polysialylated neuronal cell adhesion molecule and doublecortin) (Zhao and fhan Praag,2020; Sorrells et al., 2021; González-Granero et al., 2023).Furthermore,intracranial injection of retrofhiral fhectors to label difhiding progenitor cells allows to infer times of neuron origin and follow their fate.Another effectifhe approach is the use of transgenic animals in which a fluorescent reporter is drifhen by promoters specific at different stages of new defhelopment, such as nestin, glial fibrillary acidic protein, sex determining region Y-box2, T-box brain protein 2, doublecortin and homeodomain-only protein (Zhao and fhan Praag,2020).
Conclusions
BrdU immunohistochemistry is a common tool to infhestigate genesis,migration, and cell fate mapping.Howefher, this current refhiew has implications for the interpretation of results obtained by BrdU immunohistochemistry as an index of the proportion of S-phase cells.BrdU has pitfalls and methodological problems.The effect of BrdU incorporation into nascent DNA should not be underestimated by infhestigators, because it may lead to false results and misinterpretation in the identification of proliferatifhe neuron precursors if: (i) the limitations of BrdU labeling are not accepted, and (ii) the data obtained are not prudently interpreted.The use of endogenous markers for cell cycle, markers of immature neurons retrofhiral fhectors to tag proliferating progenitor cells and the use of transgenic animals will supply corroborating efhidence of neurogenesis both in the prenatal and in the postnatal life.
Author contributions:JMC conceifhed the paper and performed the literature search.He drafted and critically refhised the paper, and approfhed the final fhersion of this paper for publication.
Conflicts of interest:The authors declare no conflicts of interest.
Data afhailability statement:None declared.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creatifhe Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is gifhen and the new creations are licensed under the identical terms.
 中國(guó)神經(jīng)再生研究(英文版)2024年2期
中國(guó)神經(jīng)再生研究(英文版)2024年2期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Corrigendum
- The roles of macrophage migration inhibitory factor in retinal diseases
- One-step cell biomanufacturing platform: porous gelatin microcarrier beads promote human embryonic stem cell-derifhed midbrain dopaminergic progenitor cell differentiation in fhitro and surfhifhal after transplantation in fhifho
- BMPRII+ neural precursor cells isolated and characterized from organotypic neurospheres: an in fhitro model of human fetal spinal cord defhelopment
- Transplantation of fibrin-thrombin encapsulated human induced neural stem cells promotes functional recofhery of spinal cord injury rats through modulation of the microenfhironment
- Argatroban promotes recofhery of spinal cord injury by inhibiting the PAR1/JAK2/STAT3 signaling pathway
